Abstract
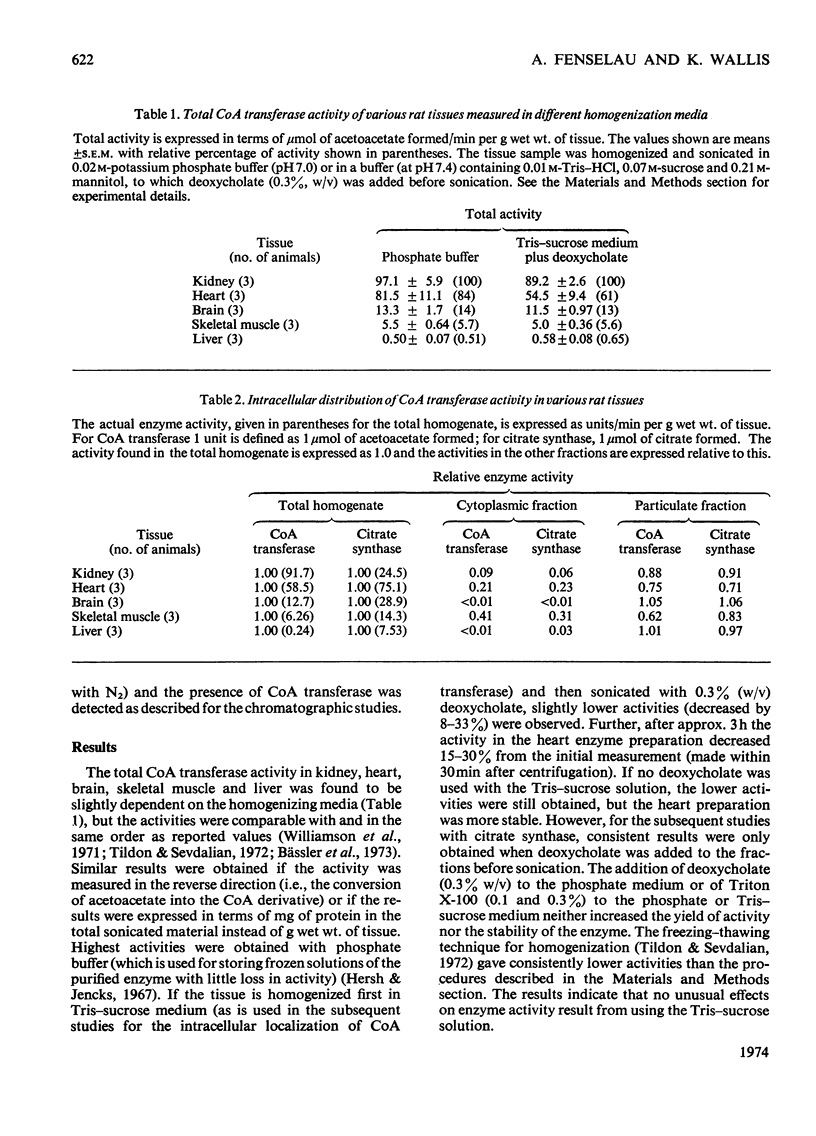
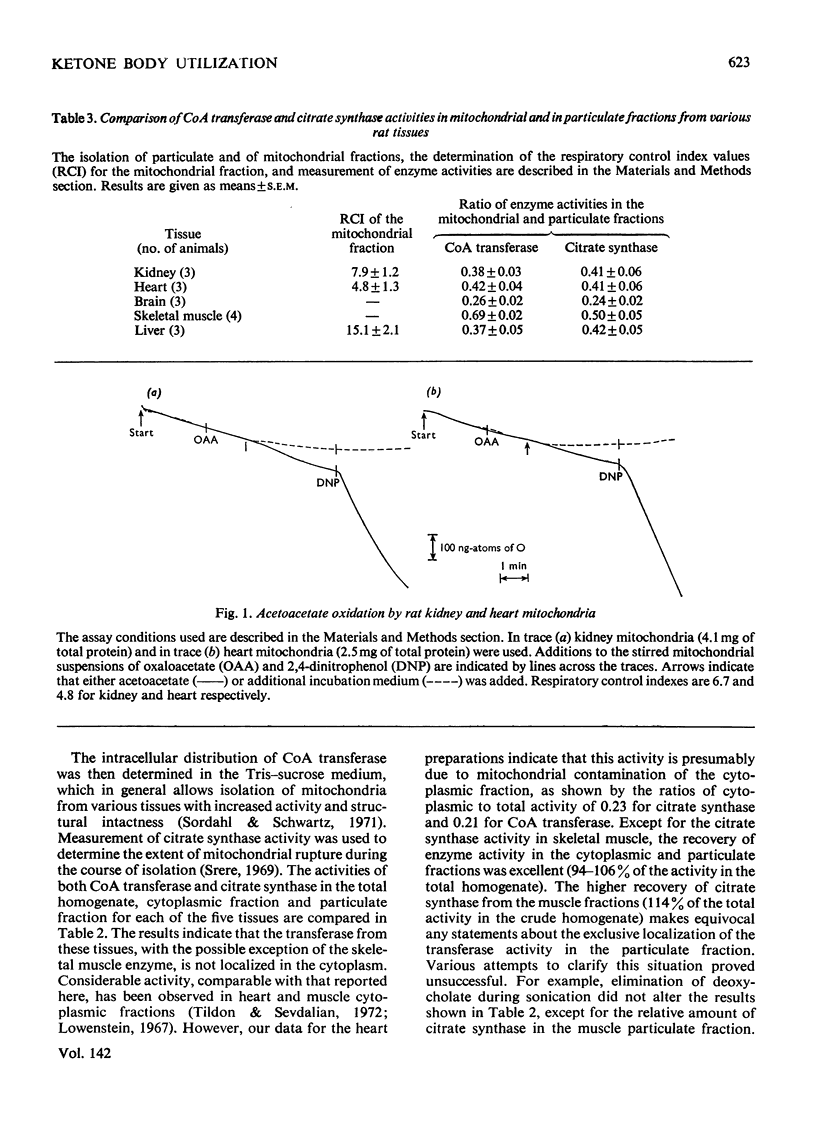
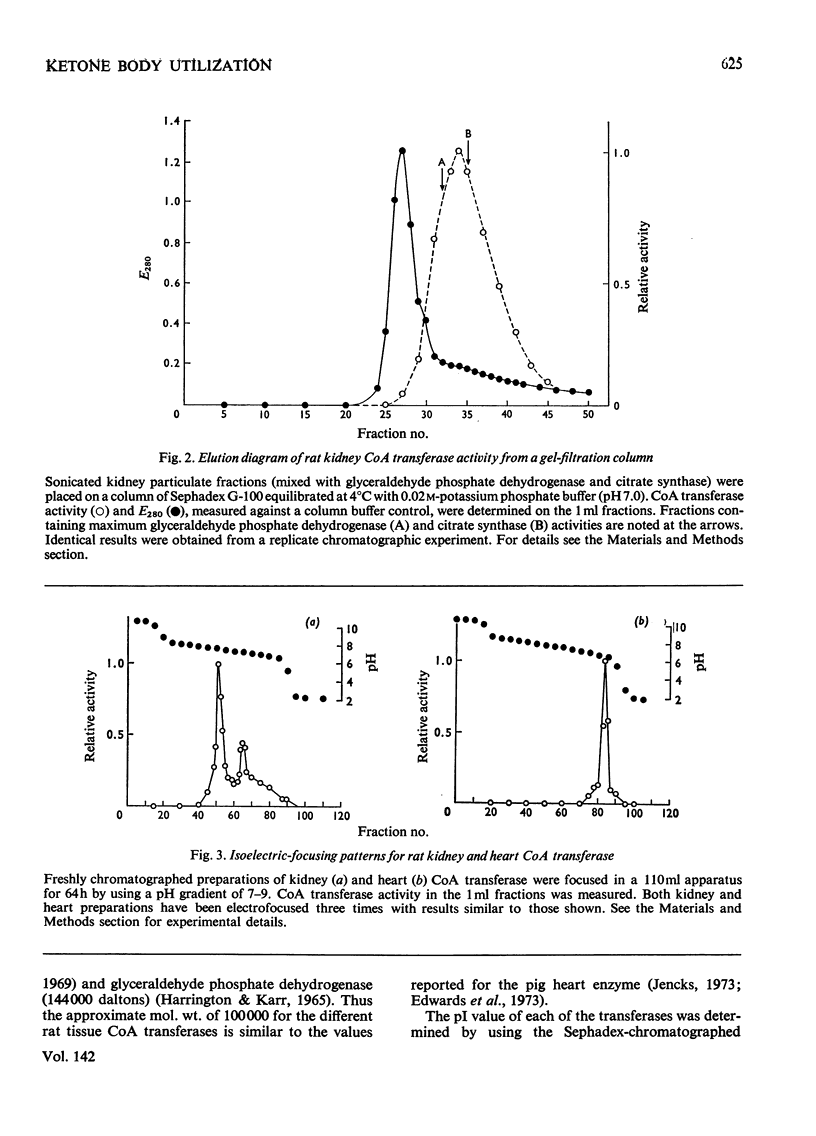
1. Tissue activities, intracellular distribution as well as selected kinetic and molecular properties of succinyl-CoA–3-oxo acid CoA transferase (EC 2.8.3.5), which is an initiator of ketone body usage, were examined in rat kidney, heart, brain, skeletal muscle and liver. 2. The activities of the transferase in these tissues are similar to reported values and are somewhat affected by the homogenization medium. Higher recoveries of activity are obtained when a phosphate buffer is used during the homogenization; Tris solutions containing sucrose and mannitol lead to only slightly lower recoveries, but can be used in studies to determine the subcellular localization of the transferase activity. 3. A close correlation was observed between the relative activities of citrate synthase (a mitochondrial marker enzyme) and CoA transferase in the cytoplasmic, particulate and mitochondrial fractions from the five tissues. 4. The Km values for acetoacetate (measured in two different ways), the ratio of Vmax. values for the two enzyme-catalysed half-reactions, and succinate product inhibition are quite similar for the enzyme from each tissue. 5. The enzymes are also similar in molecular weight (with an approx. mol.wt. of 100000 as determined by gel filtration). All show an active band in isoelectric-focusing studies with pI 7.6, except for the enzyme from heart (pI 6.8). 6. The results demonstrate a mitochondrial origin for CoA transferase in these rat tissues and support the proposition that CoA transferase is a ketolytic enzyme, i.e. an enzyme uniquely involved in the complete oxidation of ketone bodies. The structural and functional similarities of these transferases suggest that factors other than differences in Km values account for differences in the utilization of ketone bodies by various tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Siliprandi D., Siliprandi N. Acetoacetate activation and oxidation in kidney and heart mitochondria. Biochim Biophys Acta. 1969 Jun 24;180(2):237–243. doi: 10.1016/0005-2728(69)90110-8. [DOI] [PubMed] [Google Scholar]
- Blair J. B. Skeletal muscle coenzyme A transferase. Purification and properties. J Biol Chem. 1969 Feb 10;244(3):951–954. [PubMed] [Google Scholar]
- Bässler K. H., Ackermann R. H., Wagner K., Schönerstedt B. Enzymatic changes associated with ketosis in long standing diabetes and prolonged starvation of rats. Hoppe Seylers Z Physiol Chem. 1973 Jan;354(1):48–52. doi: 10.1515/bchm2.1973.354.1.48. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Singh M., Tubbs P. K. A simple purification of acetoacetate-succinate CoA-transferase using substrate elution chromatography. FEBS Lett. 1973 Dec 1;37(2):155–158. doi: 10.1016/0014-5793(73)80447-8. [DOI] [PubMed] [Google Scholar]
- HALL L. M. Preparation of crystalline lithium acetoacetate. Anal Biochem. 1962 Jan;3:75–80. doi: 10.1016/0003-2697(62)90046-5. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. The physiological role of the ketone bodies. Biochem J. 1961 Aug;80:225–233. doi: 10.1042/bj0800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNEN F., HENNING U., BUBLITZ C., SORBO B., KROPLIN-RUEFF L. Der chemische Mechanismus der Acetessigsäurebildung in der Leber. Biochem Z. 1958;330(4):269–295. [PubMed] [Google Scholar]
- Max S. R., Garbus J., Wehman H. J. Simple procedure for rapid isolation of functionally intact mitochondria from human and rat skeletal muscle. Anal Biochem. 1972 Apr;46(2):576–584. doi: 10.1016/0003-2697(72)90328-4. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Blanchaer M. C. Reversible inhibition of mitochondrial adenosine diphosphate phosphorylation by long chain acyl coenzyme A esters. J Biol Chem. 1971 Jan 25;246(2):402–411. [PubMed] [Google Scholar]
- STERN J. R., COON M. J., DEL CAMPILLO A., SCHNEIDER M. C. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J Biol Chem. 1956 Jul;221(1):15–31. [PubMed] [Google Scholar]
- Tildon J. T., Sevdalian D. A. CoA transferase in the brain and other mammalian tissues. Arch Biochem Biophys. 1972 Feb;148(2):382–390. doi: 10.1016/0003-9861(72)90155-5. [DOI] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H. Tissue-specific direction of blood metabolites. Symp Soc Exp Biol. 1973;27:283–298. [PubMed] [Google Scholar]


