Abstract
Objective
Angiotropism/perivascular invasion (PVI) is an emerging topic in various types of cancer, with studies primarily focusing on melanoma. However, limited data are available on the significance of PVI in breast cancer. This study aimed to assess the prognostic significance of PVI in breast cancer and its correlation with traditional clinicopathological prognostic parameters.
Methods
A total of 150 patients with breast cancer diagnosed between July 2020 and January 2022 were included. Clinicopathological data were retrieved from the hospital records. The presence of PVI was evaluated on hematoxylin&eosin stained slides, and the association between PVI and clinicopathological parameters was statistically analyzed. A p-value of <0.05 was regarded as statistically significant.
Results
All patients were female. The mean age was 54.0±13.6 years (range 26-97 years). PVI was significantly more common in patients with ≥2.5 cm tumors and the absence of PVI showed a significant correlation with a lower histologic grade (p=0.004 and p=0.040, respectively). Lymphovascular invasion (LVI) and perineural invasion (PNI) were also significantly more frequent in tumors with PVI (p=0.001 and 0.02, respectively). There was a statistically significant association between the absence of both PVI and extranodal extension (ENE) (p=0.035).
Conclusions
The specific role of PVI in different types of cancer has not yet been clarified. Our findings showed that PVI was significantly associated with tumor size, histological grade, LVI, PNI, and ENE, all of which are well-known negative prognostic factors in breast cancer. The presence of PVI is a promising topic in breast cancer research, and the PVI status in pathology reports may help oncologists perform better risk assessments for patients with breast carcinoma.
Keywords: Breast, cancer, perivascular invasion, angiotropism, prognosis
Abstract
Amaç
Anjiyotropizm/perivasküler invazyon (PVI), çeşitli kanser türlerinde yeni ortaya çıkan bir konudur ve çalışmalar çoğunlukla melanoma odaklanmaktadır. Ancak meme kanserinde PVI’nın önemi hakkında sınırlı miktarda veri bulunmaktadır. Bu çalışmada PVI’nın meme kanserinde prognostik önemini ve geleneksel klinikopatolojik prognostik parametrelerle ilişkisini değerlendirmeyi amaçladık.
Yöntemler
Temmuz 2020-Ocak 2022 tarihleri arasında meme kanseri tanısı alan toplam 150 hasta dahil edildi. Klinikopatolojik veriler hastane kayıtlarından elde edildi. Hematoksilen-eozin boyalı lamlarda PVI varlığı değerlendirildi ve PVI ile klinikopatolojik parametreler arasındaki ilişki istatistiksel olarak analiz edildi. İstatistiksel anlamlılık olarak p değerinin <0,05 olduğu kabul edildi.
Bulgular
Hastaların tamamı kadındı. Ortalama yaş 54.0±13,6 (dağılım 26-97) idi. PVI, 2,5 cm’den büyük tümörleri olan hastalarda anlamlı derecede daha yaygındı ve PVI yokluğu anlamlı derecede daha düşük histolojik derece ile ilişkiliydi (sırasıyla p=0,004 ve 0,040). Lenfovasküler invazyon (LVI) ve perinöral invazyon (PNI)da PVI’lı tümörlerde anlamlı olarak daha sık görüldü (sırasıyla p=0,001 ve 0,02). PVI ile ekstranodal uzanım (ENU) yokluğu arasında istatistiksel olarak anlamlı ilişki vardı (p=0,035).
Sonuçlar
PVI’nın çeşitli kanser tiplerindeki spesifik rolü henüz netleştirilmemiştir. Ancak bulgularımız, PVI’nın, meme kanserinde iyi bilinen negatif prognostik faktörler olan tümör boyutu, histolojik derece, LVI, PNI ve ENU ile anlamlı şekilde ilişkili olduğunu göstermektedir. Bu nedenle PVI varlığı meme kanseri açısından ümit verici bir konudur ve patoloji raporunda PVI durumunun yer alması onkoloğa meme karsinomu olan hastalarda daha iyi risk değerlendirmesi yapmada yardımcı olabilir.
Keywords: Meme, kanser, perivasküler invazyon, anjiyotropizm, prognoz
INTRODUCTION
The passive spread of tumor cells through vascular structures is considered most common mechanism of metastasis in patients with cancer. Additionally, tumor cells can actively disseminate along neural structures and the abluminal surface of vascular spaces1. Pericytic angiotropism, also known as perivascular invasion (PVI), was first described in melanomas2. The term describes the movement of tumor cells along the external surface of blood vessels. Some studies have identified this mechanism as being associated with an elevated risk of local recurrence and metastasis in melanoma3, 4. The concept of “PVI” is now being increasingly investigated in other tumors, including pancreatic and prostatic adenocarcinomas, well-differentiated liposarcomas, endometrial and tubo-ovarian carcinosarcomas, and sarcomatoid carcinoma of the vulva5, 6, 7, 8.
Breast cancer is the primary cause of cancer-related mortality in women9. When examining the relationship between PVI and breast cancer is poorly understood. A previous study reported that PVI was correlated with infiltrative tumor behavior and occult lymph node involvement in invasive lobular breast carcinoma10. Additionally, periarterial and perivenous invasion in invasive breast carcinoma of the no special type (NST) was significantly correlated with lymph node metastasis, as demonstrated in a study by Shioya et al11.
Investigating the relationship between vascular structures and tumor cells, specifically cancerous invasion around vessels, could provide a new method for detecting LVI and linking it to lymph node metastases. Therefore, our study focused on detecting PVI in breast cancer and examining its association with key clinicopathological prognostic factors, as well as LVI and lymph node metastasis.
MATERIALS and METHODS
Ethical Approval
This retrospective study protocol and informed consent form were approved by the Clinical Research Ethics Committee of Başakşehir Çam and Sakura City Hospital (decision no: 8, date:31.03.2021). This study was conducted in compliance with the 2013 Declaration of Helsinki. Patients provided informed consent for the use of histopathological images. This study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines12.
Patient Selection
In total, 178 consecutive mastectomy specimens from patients who underwent breast cancer surgery were retrospectively evaluated in the Department of Pathology at our institution between July 2020 and January 2022. The assessment of the required sample size was based on the total patient population who received treatment in the defined period. Follow-up intervals were documented for the overall survival analysis. Twelve patients were lost to follow-up; therefore, phone calls were organized to collect missing follow-up data for these individuals.
Clinical Information and Histopathological Evaluation
The clinical and histopathological parameters, encompassing age, multifocality, tumor size, histologic type and grade, presence of LVI, presence of perineural invasion (PNI), tumor stage (pT), nodal status (pN), presence of extranodal extension (ENE), and receptor status [estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2) status, and Ki67 labeling index] through immunohistochemical staining), was retrieved from the hospital’s digital medical record system and corresponding pathology reports.
Patients’ ages were categorized into two groups: <50 years and ≥50 years old. Tumor sizes were grouped as <2.5 cm and ≥2.5 cm. The histological types were invasive carcinoma of NST, invasive lobular carcinoma, and others. Histologic grading was performed using the Nottingham histologic score, which was classified as Grade 1, 2, or 313. The presence or absence of multifocality, LVI, PNI, and ENE was noted. The stages for pT and pN were determined based on both pathological reports and clinical information. pTs were classified into stages 1, 2, 3, and 4, whereas nodal stages (pN) were categorized as 0, 1, 2, or 3, in accordance with the guidelines established by the College of American Pathologists (CAP)13.
Immunohistochemical assessment of ER, PgR, and HER2 was carried out in line with the CAP/American Society of Clinical Oncology (ASCO) Guidelines13, 14. HER2 positivity was determined by the presence of complete and circumferential immunohistochemical expression in >10% of tumor cells. Equivocal HER2 positivity was further analyzed by silver-enhanced in situ hybridization in accordance with the ASCO/CAP guidelines13. The proportion of tumor cells positive for Ki67 was assessed in hot spots, with a cut-off value of 30% based on the latest international consensus study15. Tumors were classified into five molecular based on the St Gallen International Expert Consensus 2013: Luminal A (ER and PgR-positive, HER2-negative, and Ki67 ≤14%); luminal B1 (ER-positive, HER2-negative, and either PgR<10% or negative or Ki67 >14%); luminal B2 (ER-positive, HER2-overexpressed or amplified, any PgR, any Ki67); HER2-positive (non-luminal) (HER2-overexpressed or amplified, ER-negative and PgR-negative) and triple-negative breast cancer (ER-negative and PgR-negative, HER2-negative)16.
Evaluation of PVI
PVI was defined as tumor cells encircling the outer walls of blood vessels or lymphatics, within 1-2 mm of the leading edge of the primary tumor, as previously described17. The presence of PVI at the edges or periphery of the tumor was evaluated by using hematoxylin&eosin (H&E) staining alone. All tumor slides, including those stained with H&E, were reviewed for the presence of PVI.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0 (Chicago, IL). Distributional characteristics, numerical parametric data as mean±standard deviation, whereas median and interquartile range were used to describe non-parametric data. Categorical data are expressed as frequencies. The comparison of categorical variables was carried out using Pearson’s chi-square test, Fisher’s exact test, and the Freeman-Halton test. The t-test was used to analyze independent numerical variables with parametric data, whereas the Mann-Whitney U test was used for non-parametric data. A complete-case analysis strategy was used to address missing data. Multivariate logistic regression was applied to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between clinical and pathologic parameters and presence of PVI. Statistical significance was set at a p-value threshold of <0.05.
RESULTS
The study involved 150 patients in total. Twenty-eight patients who received neoadjuvant chemotherapy were excluded from the study. Sentinel lymph node dissection was performed in all patients, and axillary lymph node dissection was performed in 64 patients based on the results of frozen section analysis or clinical considerations.
The study included female patients with an average age of 54.0±13.6 years (range: 26-97 years). Among the patients, 66% (n=84) were ³50 years old. Tumors were multifocal in 27% (n=40) of the patients. The mean diameter of the tumor was 2.7±1.9 cm (ranging from 0.2 to 15 cm), and 54% (n=81) of the patients had a tumor diameter of <2.5 cm.
The most common histologic type was invasive carcinoma of NST (78%, n=117), followed by invasive lobular carcinoma (9%, n=13), and other histologic subtypes (13%, n=20) [apocrine carcinoma (n=1), mucinous carcinoma (n=5), tubular carcinoma (n=3), cribriform carcinoma (n=2), micropapillary carcinoma (n=1), solid papillary carcinoma with invasion (n=2), invasive carcinoma with mixed ductal and lobular features (n=6)].
The most common histologic grade of the tumors was Grade 2 (52%, n=78). LVI was present in 49% (n=73) of the patients, whereas PNI was detected in 37% (n=56). Metastatic nodal disease (pN1, pN2, and pN3) was observed in 50% (n=75) of the patients. Among the molecular subtypes, the most common was the luminal B1 subtype, accounting for 50% (n=75) of the patients. The demographic and clinicopathological data for all patients are provided in Table 1.
Table 1. Demographic and clinicopathological characteristics of all patients.
|
Patient count (n=150) |
||
|
Age |
<50 years old |
66 (44%) |
|
³50 years old |
84 (66%) |
|
|
Multifocality |
Present |
40 (27%) |
|
Absent |
110 (73%) |
|
|
Tumor size |
<2.5 cm |
81 (54%) |
|
≥2.5 cm |
69 (46%) |
|
|
Histologic type |
Invasive carcinoma of the NST |
117 (78%) |
|
Invasive lobular carcinoma |
13 (9%) |
|
|
Others |
20 (13%) |
|
|
Histologic grade |
1 |
22 (15%) |
|
2 |
78 (52%) |
|
|
3 |
50 (33%) |
|
|
Lymphovascular invasion |
Present |
73 (49%) |
|
Absent |
77 (51%) |
|
|
Perineural invasion |
Present |
56 (37%) |
|
Absent |
91 (63%) |
|
|
Tumor stage (pT) |
1 |
53 (35%) |
|
2 |
79 (53%) |
|
|
3 |
10 (7%) |
|
|
4 |
8 (5%) |
|
|
Nodal stage (pN) |
0 |
75 (50%) |
|
1 |
53 (36%) |
|
|
2 |
11 (7%) |
|
|
3 |
11 (7%) |
|
|
Extranodal extension |
Present |
50 (33%) |
|
Absent |
100 (67%) |
|
|
Molecular subtypes |
Luminal A |
34 (23%) |
|
Luminal B1 |
75 (50%) |
|
|
Luminal B2 |
23 (15%) |
|
|
HER2 (+) nonluminal |
5 (3%) |
|
|
Triple negative |
13 (9%) |
|
NST: No special type, HER2: Human epidermal growth factor receptor 2
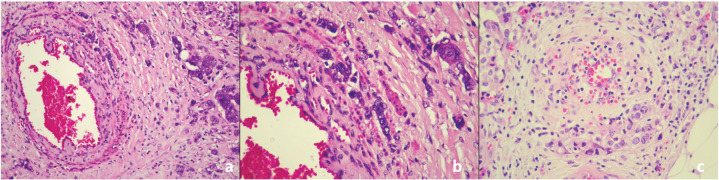
PVI was detected in 30% (n=45) of the patients. Among these, PVI involved capillaries in 47% (n=21) of the patients, while 53% (n=24) showed involvement of medium- to large-caliber vessels. The histopathological appearance of PVI in invasive carcinoma of NST is shown in Figure 1.
Figure 1.
Perivascular invasion and infiltration of tumor cells surrounding vascular structures. a: Invasive carcinoma of no special type (H&E, x200), b: Invasive carcinoma of no special type (H&E, x400), c: Invasive carcinoma of no special type (H&E, x400).
H&E: Hematoxylin&eosin
PVI was significantly more common in patients with tumors ³2.5 cm (p=0.004). A significant statistical relationship was observed between the absence of PVI and histologic grade 1 tumors (p=0.040). Furthermore, the presence of LVI and PNI was significantly more common in tumors with PVI (p=0.001 and p=0.020, respectively).
The absence of PVI was significantly more common in tumors without ENE (p=0.035). No statistically significant correlation was observed between PVI and molecular subtypes (p>0.05). The results of the multivariate logistic regression analysis indicated that patients with LVI had a threefold higher risk of PVI (OR=3.226, 95% CI=1.308–7.957, p=0.011).
Table 2 presents the univariate and binary logistic regression analyses of clinicopathological parameters in relation to PVI status.
Table 2. Univariate and binary logistic regression analyses of clinicopathological parameters according to perivascular invasion status.
|
Patient and tumor characteristics |
Perivascular invasion |
Univariate analysis |
Binary logistic regression analysis |
|||
|
Present (n=45) |
Absent (n=105) |
p-value |
p-value |
Odds ratio |
||
|
Tumor size (cm) |
<2.5 |
16 (11%) |
65 (43%) |
0.0041* |
- |
|
|
≥2.5 |
29 (19%) |
40 (27%) |
||||
|
Histologic grade |
1 |
3 (2%) |
19 (13%) |
0.0402* |
0.4433 |
1.096 |
|
2 |
30 (20%) |
48 (32%) |
||||
|
3 |
12 (8%) |
38 (25%) |
||||
|
Lymphovascular invasion |
Present |
32 (21%) |
41 (27%) |
0.0011* |
0.0113* |
3.226 |
|
Absent |
13 (9%) |
64 (43%) |
||||
|
Perineural invasion |
Present |
23 (15%) |
34 (23%) |
0.0201* |
0.0923 |
0.471 |
|
Absent |
22 (15%) |
71 (47%) |
||||
|
Extranodal extension |
Present |
21 (14%) |
29 (19%) |
0.035*1 |
0.3323 |
0.645 |
|
Absent |
24 (16%) |
76 (51%) |
||||
*p< 0.05, 1: Pearson’s chi-square test, 2: Fisher’s freeman’s Halton test, 3: Binary logistic regression analysis.
Follow-up
The average follow-up time was 28.0±6.2 months, with a median of 27 months (ranging from 2 to 39 months) for overall survival. Eight patients died because of the disease. None of these patients had PVI; however, they presented with other negative prognostic indicators, including advanced histologic grade, later-stage tumor, LVI, and metastatic nodal disease.
DISCUSSION
This study examined the presence and prognostic value of PVI in patients with breast cancer. In addition to the strong association between PVI and LVI, we observed a correlation between PVI and other poor prognostic factors, including higher histological grade, PNI, and ENE. The main limitations of this study include the limited sample size and the short follow-up duration, which hindered a thorough survival analysis.
In our study, we observed that larger tumors more frequently exhibited PVI. This may be attributed to the irregular, infiltrative growth pattern, which is typical of larger malignant tumors. Igawa et al. recently reported that PVI was associated with infiltrative tumor growth in invasive lobular carcinoma of the breast10. Additionally, a study on gastric cancer found cancer cells in the periarterial tissues of patients with advanced gastric cancer patients18.
In the current study, a significant relationship was observed between tumor grade and PVI. Although the majority of our patients had grade 2 tumors, PVI was significantly less common in those with lower grades. There are currently no studies in the literature that examined the relationship between PVI and tumor grade, and our findings contribute to the existing literature in this regard. While this observation warrants verification in larger study groups, it likely reflects the epithelial-to-mesenchymal transition process, as high-grade tumors tend to migrate more easily to perivascular areas due to the loss of adhesion molecules.
Shioya et al.11 demonstrated that PVI could be highly sensitive and prevalent (95.5 %) in invasive carcinoma of NST with lymph node metastases. In their study, the authors also reported that PVI was observed in 75.4 % of tumors without lymph node metastasis. The authors concluded that lymphatic invasion could occur prior to lymph node metastasis. A study on PVI in invasive lobular carcinoma found that PVI is associated with lymph node metastasis in the absence of lymphadenopathy, which can be challenging to detect even with imaging techniques10. In light of the existing literature, our findings also suggest a strong relationship between PVI and LVI. We observed PVI in half of the patients with lymph node metastasis, which is consistent with the expectations based on prior research. However, our study did not support the hypothesis that PVI occurs before lymph node metastasis, as we found a PVI rate of only 13% in patients without lymph node metastasis, a rate lower than that reported in the literature.
PVI appears to be a consequence of talk between tumor cells and blood vessels, potentially representing an early phase of LVI, as the significant association between PVI and LVI persisted in multivariate analysis. However, further studies, particularly those using in vitro techniques, are necessary to test these theories.
In addition to LVI, PNI was found to be correlated with PVI. Fedda et al. reported PVI in a patient with recurrent acantholytic squamous cell carcinoma of the scalp, which was characterized by poor differentiation and significant PNI19. They stated that the concept of PVI is similar to that of PNI, which is consistent with our findings.
The association between ENE and poor prognosis in breast cancer has been demonstrated in various studies20, 21. Although the data on this issue remain conflicting, most a negative prognostic effect of ENE in patients with breast cancer. We found that the absence of ENE was significantly associated with the absence of PVI. As far as we know, our study is the first to reveal this association, which may be particularly important for exploring different mechanisms of metastasis to lymph nodes. We believe this aspect warrants further investigation.
The molecular subtypes of breast cancer are an evolving topic that provides crucial insights into the mechanisms underlying the disease. Each tumor type has a distinct prognosis and various heterogeneous features22. In a recent study, patients with triple-negative breast cancer exhibited higher microvascular density; however, this finding did not directly support the presence of PVI23. In our study, PVI was not found to be specific to any molecular subtype of breast cancer, likely due to tumor heterogeneity.
Although the mechanism of PVI can be described as the movement of tumor cells along the abluminal side of blood vessels, there is a lack of standardized definitions for evaluating tumor cells associated with vessels with varying wall thicknesses24. PVI can affect capillaries and larger vessels. Although most blood vessels affected by PVI in melanoma are typically capillaries, carcinoma often involves medium- to large-caliber vessels25, 26. In our study of breast carcinomas, we observed slightly higher involvement of larger caliber vessels compared with capillaries.
CONCLUSION
In conclusion, our study provides new insights into the relationship between PVI and clinicopathological features of breast cancer. Our findings suggest that the presence of PVI may raise the suspicion for the existence of LVI and/or PNI in breast carcinomas, particularly in large or high-grade tumors. The incorporation of PVI status into pathology reports could enhance oncologists’ understanding and improve prognostic predictions. Tumor spread is a critical concern in breast cancer, and we believe our findings contribute valuable insights into this important topic.
Ethics
Ethics Committee Approval: This retrospective study protocol and informed consent form were approved by the Clinical Research Ethics Committee of Başakşehir Çam and Sakura City Hospital (decision no: 8, date:31.03.2021).
Informed Consent: This study was conducted in compliance with the 2013 Declaration of Helsinki. Patients provided informed consent for the use of histopathological images.
Footnotes
Author Contributions
Surgical and Medical Practices: BÇ.G., T.S.A., H.E., E.Ş., B.P., Concept: BÇ.G., T.S.A., B.P., Design: BÇ.G., T.S.A., B.P., Data Collection and/or Processing: BÇ.G., T.S.A., H.E., H.İ.Ö., E.Ş., Analysis and/or Interpretation: BÇ.G., T.S.A., H.E., H.İ.Ö., Literature Search: BÇ.G., T.S.A., H.İ.Ö., B.P., Writing: BÇ.G., T.S.A., B.P.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Lugassy C, Zadran S, Bentolila LA, et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron. 2014;7:139–52. doi: 10.1007/s12307-014-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnhill RL, Lugassy C. Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology. 2004;36(5):485–90. doi: 10.1080/00313020412331282708. [DOI] [PubMed] [Google Scholar]
- 3.Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA. Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol. 2008;32:1396–403. doi: 10.1097/PAS.0b013e3181753a8e. [DOI] [PubMed] [Google Scholar]
- 4.Wilmott J, Haydu L, Bagot M, et al. Angiotropism is an independent predictor of microscopic satellites in primary cutaneous melanoma. Histopathology. 2012;61(5):889–98. doi: 10.1111/j.1365-2559.2012.04279.x. [DOI] [PubMed] [Google Scholar]
- 5.Lugassy C, Vernon SE, Warner JW, et al. Angiotropism of human prostate cancer cells: implications for extravascular migratory metastasis. BJU Int. 2005;95(7):1099–103. doi: 10.1111/j.1464-410x.2005.05474.x. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJ, Gleeson FC, Zhang L. Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(2):246–8. doi: 10.1016/j.cgh.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Dyke JM, Crook ML, Platten M, Stewart CJ. Extravascular migratory metastasis in gynaecological carcinosarcoma. Histopathology. 2014;65(3):363–70. doi: 10.1111/his.12395. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Shrestha S, Rao PN, et al. Pericytic mimicry in well-differentiated liposarcoma/atypical lipomatous tumor. Hum Pathol. 2018;78:188. doi: 10.1016/j.humpath.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 10.Igawa A, Mizukami H, Kudoh K, et al. Perivascular infiltration reflects subclinical lymph node metastasis in invasive lobular carcinoma. Virchows Arch. 2022;481:533–43. doi: 10.1007/s00428-022-03391-8. [DOI] [PubMed] [Google Scholar]
- 11.Shioya A, Takata M, Kumagai M, et al. Periarterial or perivenous invasion is an independent indicator of lymph node metastasis in invasive breast carcinoma of no special type. Pathol Res Pract. 2024;260:155407. doi: 10.1016/j.prp.2024.155407. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative, et al. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–8. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–82. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 14.Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2021;113:808–19. doi: 10.1093/jnci/djaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moy AP, Duncan LM, Muzikansky A, Kraft S. Angiotropism in primary cutaneous melanoma is associated with disease progression and distant metastases: a retrospective study of 179 cases. J Cutan Pathol. 2019;46:498–507. doi: 10.1111/cup.13461. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H, Murata S, Kaida S, et al. Presence of cancer cells in the periarterial tissues of patients with advanced gastric cancer. Oncol Lett. 2018;16:1226–30. doi: 10.3892/ol.2018.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedda F, Migden MR, Curry JL, et al. Angiotropism in recurrent cutaneous squamous cell carcinoma: Implications for regional tumor recurrence and extravascular migratory spread. J Cutan Pathol. 2019;46:152–8. doi: 10.1111/cup.13388. [DOI] [PubMed] [Google Scholar]
- 20.Tang P, Moravek M, Oprea-Ilies G, Mon KS, Pambuccian SE. Extranodal extension, an international survey on its evaluation and reporting in breast cancer patients. Pathol Res Pract. 2022;237:154070. doi: 10.1016/j.prp.2022.154070. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Yang X, Yang W, Shui R. Prognostic value of extranodal extension in axillary lymph node-positive breast cancer. Sci Rep. 2021;11:9534. doi: 10.1038/s41598-021-88716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarean Shahraki S, Azizmohammad Looha M, Mohammadi Kazaj P, et al. Time-related survival prediction in molecular subtypes of breast cancer using time-to-event deep-learning-based models. Front Oncol. 2023;13:1147604. doi: 10.3389/fonc.2023.1147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaath H, Elango R, Alajez NM. Molecular classification of breast cancer utilizing long non-coding rna (lncRNA) transcriptomes identifies novel diagnostic lncRNA panel for triple-negative breast cancer. Cancers (Basel) 2021;13(21):5350. doi: 10.3390/cancers13215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravin R, Suarez-Meade P, Busse B, et al. Perivascular invasion of primary human glioblastoma cells in organotypic human brain slices: human cells migrating in human brain. J Neurooncol. 2023;164:43–54. doi: 10.1007/s11060-023-04349-9. [DOI] [PubMed] [Google Scholar]
- 25.Lugassy C, Lazar V, Dessen P, et al. Gene expression profiling of human angiotropic primary melanoma: selection of 15 differentially expressed genes potentially involved in extravascular migratory metastasis. Eur J Cancer. 2011;47:1267–75. doi: 10.1016/j.ejca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, de Sampaio PC, Lundy DM, et al. Heterogeneous perivascular cell coverage affects breast cancer metastasis and response to chemotherapy. JCI Insight. 2016;1:e90733. doi: 10.1172/jci.insight.90733. [DOI] [PMC free article] [PubMed] [Google Scholar]