Summary
Background
India, with the largest population and second-highest type 2 diabetes mellitus (T2DM) prevalence, presents a unique genetic landscape. This study explores the genetic profiling of T2DM, aiming to bridge gaps in existing research and provide insights for further explorations.
Methods
We conducted a systematic review and meta-analysis of literature published up to September 2024 using databases like PubMed, Web of Science, Scopus, and Google Scholar to identify SNPs associated with T2DM in case–control studies within the Indian population. Data extraction followed a rigorously designed checklist independently verified by two reviewers. The quality of the studies assessed by utilizing Newcastle Ottawa scale, and heterogeneity through Cochran's Q, τ2, H2 and I2 statistics. Fixed effect and random effect model was employed for meta-analysis based on heterogeneity, and publication bias was assessed by funnel plot analysis, Egger's and Begg's statistical test. In SNPs with adequate studies meta-regression was used to assess source of heterogeneity. Statistical analyses were performed using Stata 18.0 software.
Findings
Our search identified 1309 articles, with 67 included in the systematic review and 35 in the meta-analysis. These 67 case–control studies, involving 33,407 cases and 30,762 controls, analyzed 167 SNPs across 61 genes. Of these, 89 SNPs mapped to 46 genes showed significant associations with T2DM risk (P < 0.05), including 67 linked to increased risk and 16 with protective effects. Geographical analysis highlighted inter- and intra-regional variations. Meta-analysis of 25 SNPs revealed 12 SNPs with high T2DM risk compatibility. TCF7L2 gene exhibited a strong compatibility with an overall OR of 1.44 (95% CI 1.36–1.52) and S-value 112.41, while TCF7L2 variants rs7903146 and rs12255372, with OR 1.56 (95% CI 1.43–1.66) and S-value 89.036, OR of 1.36 (95% CI 1.17–1.35) with an S-value of 15.45 respectively.
Interpretation
Our study highlights the importance of considering the diverse ethnic groups of India for development of targeted and effective T2DM management strategies.
Funding
Department of Biotechnology (DBT) and Indian Council of Medical Research (ICMR), Government of India.
Keywords: Type 2 diabetes mellitus (T2DM), Single nucleotide polymorphism (SNP), Case–control, India, Susceptible gene
Research in context.
Evidence before this study
Before embarking on this study, our examination of articles published up to September 30, 2024 revealed a notable global surge in diabetes cases, projected to rise from 537 million in 2021 to an estimated 783 million by 2045, predominantly comprising Type 2 Diabetes Mellitus (T2DM). India, housing 17.78% of the global population and ranking second globally with 101 million T2DM patients, significantly contributes to this escalating health crisis, constituting 14% of the global burden. Existing literature underscores the intricate epidemiology of T2DM in India, marked by regional differentiations influenced by diverse factors—diet, lifestyle, genetics, geography, and environmental conditions, contributes to distinctive patterns of diabetes prevalence across the country. Technological advancements like genome-wide association studies and next-generation sequencing have identified genetic variants linked to T2DM, yet they explain only 10–20% of global incidence variability. Notably, most genetic data are European-centric, with only 22% of diabetes-related gene associations being replicable in Indian populations. Upon scrutinizing available study datasets, a prevailing trend emerges where many studies were conducted on non-representative groups, hampered by limitations such as smaller sample sizes or a focus on specific marker–trait associations. These gaps underscore the need for systematic investigations and meta-analyses to comprehensively understand the genetic landscape of T2DM in this diverse and increasingly burdened population.
Added value of this study
This study represents the first systematic review and meta-analysis focused on gene polymorphisms linked to T2DM in India, with a specific emphasis on case–control studies. It provides a comprehensive analysis of genetic variations associated with T2DM progression across diverse Indian regions, synthesizing data from 67 eligible studies. A total of 167 SNPs across 61 genes were identified, with 35 studies included in the meta-analysis. Using an Odds Ratio (OR) threshold with a significance level of P ≤ 0.05, the study revealed 89 SNPs across 46 genes associated with T2DM, uncovering significant inter-regional and intra-regional disparities. Out of the 25 eligible SNPs across 14 genes chosen for meta-analysis, 12 showed a high compatibility with the risk of T2DM with TCF7L2's variants showed strong compatibility. The study's value lies in its systematic approach to exploring the genetic landscape of T2DM in India's diverse population. By addressing regional disparities and gaps in participant representation in previous studies, it offers critical insights into the genetic underpinnings of T2DM progression, guiding future research and targeted interventions for this complex health challenge.
Implications of all the available evidence
The present study exposes a significant geographical imbalance in research under study across India, with a pronounced focus on the North and South, leaving the East, West, and Northeast regions underexplored. The nation's diverse regional divisions, marked by distinct geographical, climatic, and genetic variations, lead to studies concentrating on specific segments, providing a limited overall perspective of susceptible gene associations. Findings indicate that T2DM risk associations observed in one region may not universally apply in other regions, suggesting broader implications for the entire country. A critical finding underscores a substantial research gap, particularly in the Central Indian region, home to 8.11% of the population, yet devoid of T2DM susceptible gene studies, despite contributing significantly to diabetic and pre-diabetic cases. Our study advocates for a more inclusive T2DM research approach in India. We emphasize the need for a comprehensive roadmap that encompasses diversified, population-based studies specifically targeting genetically diverse groups. This approach is crucial in recognizing and addressing the distinct variations within each region of the country. This approach not only enhances the precision of risk prediction but also facilitates the development of targeted interventions and preventive measures, contributing to a more effective and equitable management of T2DM across India's diverse landscape.
Introduction
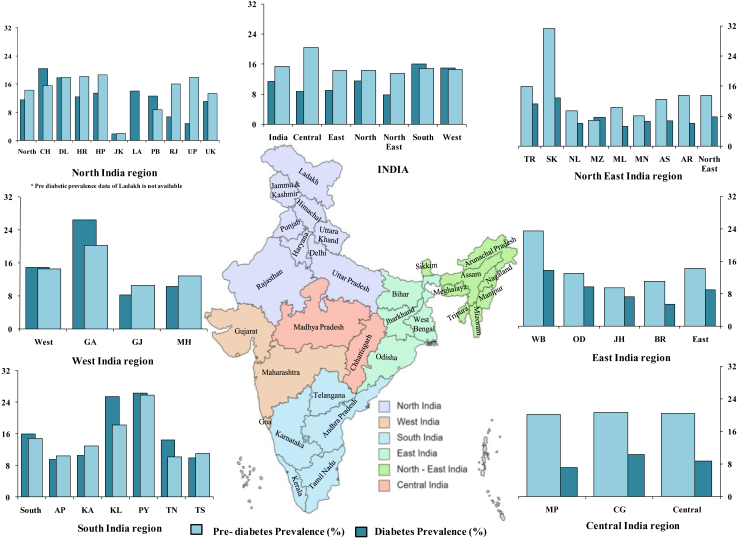
Type 2 diabetes mellitus (T2DM) is a chronic, non-communicable disease with a complex etiology influenced by lifestyle, genetics, and environmental factors. It has emerged as the top health concern of the twenty-first century and the most prevalent form of diabetes globally. The pathophysiology of T2DM is characterized by insulin resistance in somatic cells leading to persistent hyperglycemia.1,2 As of 2021, an estimated 537 million individuals aged 20–79 years had diabetes globally, with approximately 95% being T2DM cases.3,4 This number is expected to reach 783 million by 2045, reflecting a 46% increase.3,5 Globally, India has the second highest number of T2DM patients, trailing only behind China,6,7 with an estimated 101 million Indians living with the condition and an additional 136 million classified as pre-diabetic.6 Additionally, about 39.4 million Indians are estimated to have undiagnosed diabetes.3,4,6 The overall prevalence of diabetes in India has increased from 7.1% in 2009 to 11.4% in 2023, with 15.3% being pre-diabetic,4,6 with a substantial intra- and inter-regional variation (Fig. 1). Projections indicate that this burden will surge to 152 million by 2045 in India.3,8
Fig. 1.
Prevalence map of diabetes and pre-diabetes in different regions of India.
With 1.45 billion people, India is the world's most populous nation and accounts for 17.78% of the global population (https://www.worldometers.info/world-population/india-population/). The nation harbors exceptional diversity, with over 4500 anthropologically distinct communities, including castes, tribes, ethnicities, and religious groups.9 These populations distinguished by their unique characteristics such as endogamy, language, culture, physical features, geographical and climatic positioning, and genetic architecture. Notably, India displays remarkable degrees of genetic diversity, that is hardly paralleled by other nations.9,10 However, comprehensive studies to understand the genetics of T2DM in the diverse Indian population are meager.
Recent technological breakthroughs in genotyping approaches like next-generation sequencing (NGS), whole exome-sequencing, and genome-wide association studies (GWAS) have identified genetic variants linked to T2DM and quantitative glycemic markers such as glucose, insulin, and glycated haemoglobin (HbA1c) levels.11 Meta-analysis of GWAS data has revealed numerous single nucleotide polymorphism (SNPs) spread over 270 loci linked to T2DM risk across global populations.2 With an estimated heritability ranging between 20 and 80%, genetic factors are believed to play a major role in the T2DM pathogenesis.12 While there are common loci associated with T2DM susceptibility across certain population groups, ethnicity-specific variants have also been observed in India.13 Therefore, a systematic investigation is needed to understand the genetics of T2DM in India.
This study aimed to identify the important SNPs linked to the susceptibility and development of T2DM among the Indian population, through a systematic review and meta-analysis. We hope to improve our understanding of the genetic underpinnings of T2DM in this specific demography by thoroughly analyzing the existing knowledge, which will lay the foundation for developing more precise and impactful strategies.
Methods
Search strategy and selection criteria
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)14 and Strengthening the Reporting of Genetic Association Studies (STREGA),15 guidelines, for our systematic review and meta-analysis. The search strategy, based on the PECOS16 (Population, Exposure, Comparison, Outcome, Study design) approach (Table 1), targeted articles published in English and Hindi across multiple databases including, Medline (PubMed), Web of Science, Scopus, and Google Scholar, utilizing combination of specific Medical Subject Headings (MeSH) keywords and free text terms related to T2DM, genetic variations, and the Indian population. The search encompassed original articles published up to September 30, 2024. Case-control observational studies comparing T2DM patients with healthy individuals within the Indian population, irrespective of age, sex and location within the country were included. Studies including patients with T2DM and other metabolic syndromes were included only if the analysis specifically identified T2DM cases. GWAS studies, case reports, reviews, animal studies, mtDNA variants, insertion-deletion studies, and cohort studies were excluded. Two authors (LR and SK) independently screened and abstracted data based on the inclusion and exclusion criteria. The details of the search strategy and the inclusion/exclusion criteria for systematic review and meta-analysis were provided in Supplementary Table S1. The structured checklist included gene details, SNP information, allelic variations, Hardy–Weinberg Equilibrium (HWE) in controls, risk alleles, detection methods, population demographics, and study statistics. A third author (DKS) assessed the studies to resolve conflicts and duplicates. Data extraction resulted in a new tabulation, consolidating investigations of SNPs conducted by two or more groups in the Indian population. The final tabulation included gene names, SNP details, case and control allele counts, risk allele frequencies, odds ratios with 95% confidence intervals (95% CI), P-values, HWE in controls, and author names with publication years. In studies, where HWE was not mentioned, HWE was calculated from the genotype data of the respective SNPs in control population and SNPs within HWE (P > 0.5) were further considered for meta-analysis. The meta-analysis focused on studies qualified as per the inclusion and exclusion criteria mentioned in Supplementary Table S1. Risk of bias by domain and question in eligible case–control studies for meta-analysis was assessed using the Newcastle–Ottawa Scale.17 Studies were classified as good, fair and poor quality by applying Agency for Healthcare Research and Quality (AHRQ) standards using Newcastle–Ottawa scale score.18
Table 1.
Population, Exposure, Comparison, Outcome, Study design (PECOS) framework.
| Population | Indian Population of all age group, irrespective of gender from any geographical location from India |
| Exposure | Association analysis of susceptible genes with risk of T2DM |
| Comparison | Patients with T2DM verses healthy individuals, in studies which included both patients with T2DM and other metabolic syndrome, only cases with T2D were considered. |
| Outcomes | Significant association with OR (95% CI) in case and control |
| Study design | Case and Control studies |
Data analysis
The consistency of reported P-values for OR with 95% CIs were checked. When inconsistent, the same was recalculated using the method described by Altman et al.19 For the meta-analysis, we used adjusted OR values with 95% CI reported in the primary studies. However, for studies reporting only unadjusted OR, we applied the calculated multiplicative bias factor from similar type of study where adjustments for confounders (such as age, sex, and BMI) were available. The multiplicative bias factor served as the external confounding estimator. It was calculated using reported adjusted and unadjusted OR values as described by Kenneth et al.20 We used Cochran's Q, τ2, H2 and I2 statistic to assess the heterogeneity among studies included in the meta-analysis. Fixed-effect model was employed for meta-analysis if I2 was less than 25% (low heterogeneity). For I2 exceeding 25%, random effect model was used,21 and if the number of studies in meta-analysis were ≤ five, Sidik-Jonkman method was employed for random effect meta-analysis.22 Further, the potential source of heterogeneity among the studies included in the individual meta-analysis was investigated by generating a Galbraith plot.23 To assess how potential outliers affected the estimation of the overall effect size and heterogeneity, a leave-one-out meta-analysis was conducted. For meta-analysis with adequate number of studies (>10 studies), a meta-regression analysis was also performed to explore the sources of heterogeneity. We considered seven variables viz. sample size, HWE of controls, case to control ratio, study quality, publication year, study region, and genotyping method as the potential sources of heterogeneity. Initially we examined these variables in a step-wise manner independently as a source of heterogeneity under a random effect meta-regression model. In the next step, those variables found to be significant in the first step were planned to be retained (while excluding the non-significant variables) for multivariable meta-regression analysis under a random-effects model. Our random effect meta-analysis was based on the assumption that the true effects may have a normal distribution around a mean effect and vary among studies. Shannon-information value [S-value = −log2 (P)] was calculated to avoid relying solely on significance testing (P = 0.05) and improve our interpretation when assessing primary association. A higher S-value indicates stronger evidence against the null hypothesis.24 Publication bias was assessed by various methods including funnel plot analysis, Egger's and Begg's statistical test, and trim and fill method. All statistical analyses and meta-analyses executed using Stata 18.0 software (StataCorp, USA).25
Role of the funding source
LR (DBTHRDPMU/JRF/BET-20/I2020/AL/272) received a fellowship from the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, and the project was funded by the Indian Council of Medical Research (ICMR) to DKS (ICMR extramural grant no. 5/4/5-12/Diab.20-NCD-III). The funder had no involvement in the design, data collection, analysis, interpretation, report writing or any aspect pertinent to the study.
Results
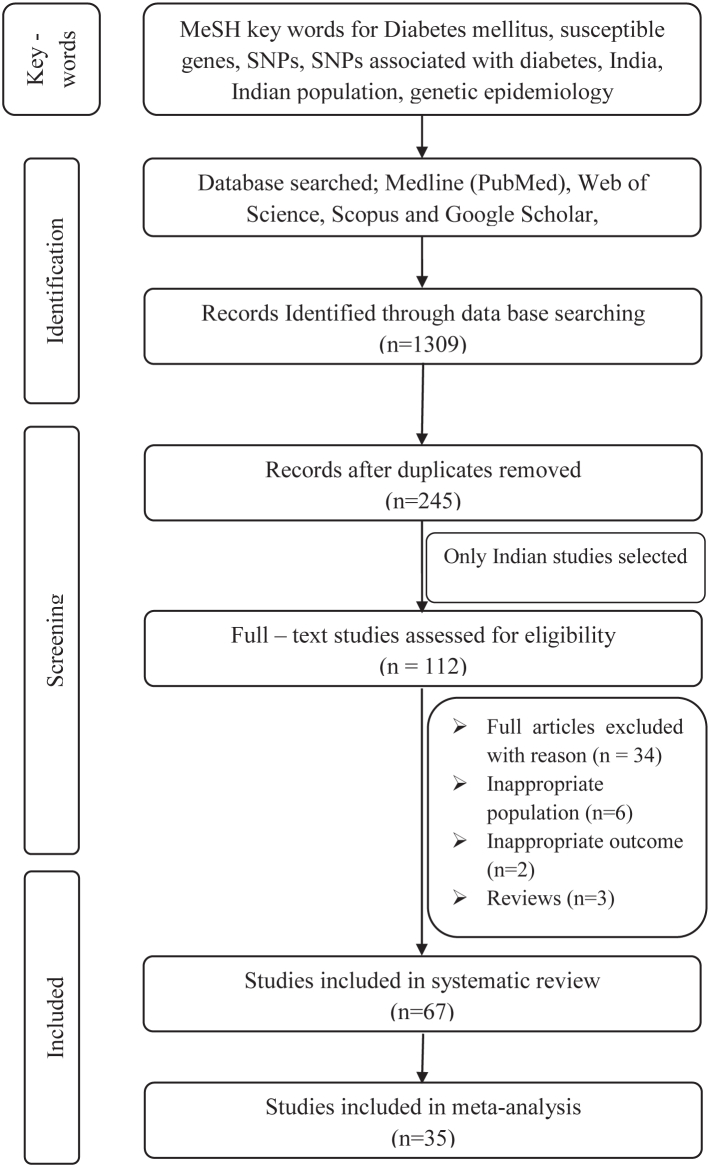
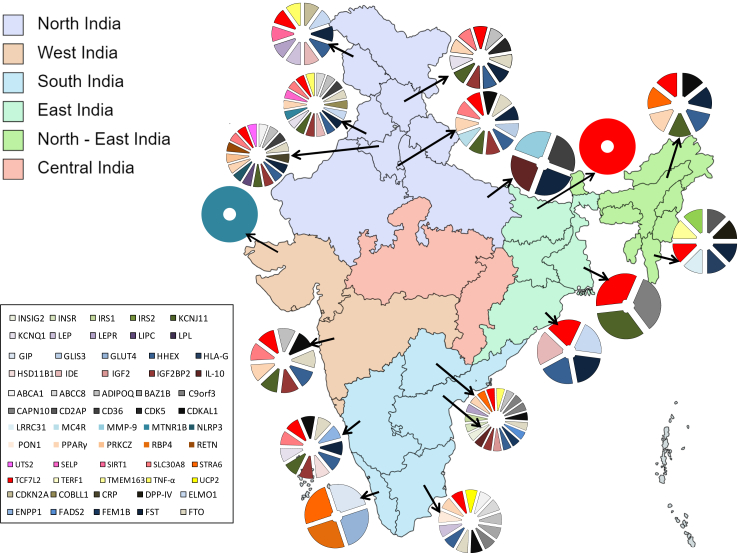
The original search resulted in 1309 articles, with 245 remaining after removing duplicates. Further screening yielded 112 publications on the Indian population, out of which 67 fulfilled the inclusion criteria for systematic review and 35 for the meta-analysis (Fig. 2, Supplementary Table S2). These 67 case–control studies collectively included 33,407 cases and 30,762 controls, and investigated 167 SNPs across 61 genes (Supplementary Table S3). The studies covered 15 Indian states and two union territories, representing five diverse Indian geographical regions (Fig. 3). The northern region accounted for highest number of studies (N = 32) across four states and two union territories, collectively investigating 91 SNPs mapped over 36 genes with 13,137 cases and 12,113 controls. The southern region of India hosted 26 studies across five states and mapping 80 SNPs on 35 genes in 15,549 cases and 14,998 controls. Four studies, conducted in three states of eastern region of India, examined 14 SNPs mapped on seven genes with 1021 cases and 1015 controls. The north-eastern region reported only three studies in two states that demonstrated 18 SNPs on 13 genes in 694 cases and 484 controls. Four studies were conducted in the western Indian region, with 3006 cases and 2566 controls, which investigated 14 SNPs on nine genes. Notably, no study was observed from the central Indian region (Fig. 3, Supplementary Table S3).
Fig. 2.
Study selection process for systematic reviews with meta-analyses. Adapted from the PRISMA flow diagram.
Fig. 3.
Geographical mapping of studied SNPs associated with T2DM in India.
Of the 67 studies included in the systematic review, 53 demonstrated statistically significant association (P < 0.05) between T2DM and 89 SNPs (mapped over 46 genes) (Supplementary Table S3). Among these, 67 SNPs were linked to an elevated risk of T2DM, while 16 exhibited a protective effect. Besides, six SNPs displayed both positive and negative association withT2DM across multiple studies, highlighting regional and study-specific disparities. The most prominent gene was Transcription Factor 7-Like 2 (TCF7L2). Majority of the examined SNPs in TCF7L2 were associated with higher risk of T2DM, with the exceptionofrs7903146 and rs12255372. Additionally, out of 15 SNPs studied for the Adiponectin (ADIPOQ) gene, eight SNPs were associated with increased risk, while one SNP demonstrated a protective effect. Further, four SNPs each in FTO Alpha-Ketoglutarate-Dependent Dioxygenase (FTO), CDK5 Regulatory Subunit Associated Protein Like 1 (CDKAL1), and Cyclin-Dependent Kinase Inhibitor 2A (CDKN2A) genes were associated with an increased risk of T2DM. An exception was observed for rs7020996 of CDKN2A, which exhibited a protective effect in one study and an increased risk in another. All three reported SNPs of potassium inwardly rectifying channel subfamily J member 1 (KCNJ1) gene were associated with an elevated risk of T2DM, while two out of three reported SNPs of HHEX exhibited a positive association with rs111875, demonstrated a protective effect in one of the reported studies. The peroxisome proliferator-activated receptor gamma (PPARG) gene was investigated in seven studies involving 15 SNPs. Among these, rs3892175 and rs11715073 showed a protective effect, while rs1801282 showed an association with both increased risk and protection. Interestingly, most of the associated SNPs were located within intronic and intergenic regions of the investigated genes (Supplementary Table S3).
Among the 67 studies included in the systematic review, 39 SNPs across 17 genes were investigated by multiple authors in 45 studies, revealing inter- and intra-regional disparities in their associations. (Supplementary Table S3). However, three specific SNPs, namely rs7020996 (CDKN2A), rs1801278 (IRS1), and rs1470579 (IGF2BP2) were excluded from further analysis due to potential overlap concerns. While there was a general concordance in terms of association with T2DM observed among most of the SNPs in these studies, discrepancies in terms of association were also noted in certain SNPs, such as rs1801216 (ABCC8), rs2241766 (ADIPOQ), rs3792267 and rs5030952 (CAPN10), rs7754840 (CDKAL1), rs10811661 (CDKN2A), rs9939609 (FTO), rs1111875, rs7923837, and rs5015480 (HHEX), rs4402960 (IGF2BP2), rs5219 (KCNJ11), rs2237892 (KCNQ1), rs1801282 (PPARG), rs13266634 (SLC30A8), rs12255372 and rs7901695 (TCF7L2), and rs1800629 (TNF-α) (Supplementary Table S3). The risk allele frequencies for individual SNPs from different studies were then combined and analyzed (Table 2). In the combined analysis, 20 SNPs mapped over 11 genes demonstrated significant risk factor for T2DM, while rs13266634 (SLC30A8) showed a protective effect. Variants like rs2241766 (ADIPOQ), rs3792267 (CAPN10), rs4402960 (IGF2BP2), and rs4506565 (TCF7L2) exhibited a highly significant association with T2DM, with OR and 95% CI of 2.51 (2.02–3.10), 3.90 (2.83–5.38), 2.04 (1.88–2.21) and 2.88 (2.39–3.47) respectively (Table 2). While, alleles of rs9940128 (FTO) and rs1800872 (IL10) were associated with T2DM in individual studies, did not exhibit susceptibility in the combined analysis. Conversely, alleles rs1421085 and rs8050136 of FTO, showed no association in individual studies, but demonstrated a significant association when considered collectively (Table 2, Supplementary Table S3). The combined analysis highlighted stronger association for several SNPs, emphasizing the importance of integrating data from multiple studies and large population for a comprehensive understanding of genetic susceptibility to T2DM.
Table 2.
Analysis of SNPs studied by two or more groups in Indian population.
| Gene name | SNP | Number of studies | Cases allele | Control allele | Risk allele frequency (%) |
OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| ABCA1 | rs1800977 | 2 | 362 | 330 | 66.6 | 31.8 | 1.12 (0.82–1.53) | 0.49 |
| rs2230806 | 2 | 457 | 304 | 37 | 38.5 | 0.96 (0.70–1.29) | 0.74 | |
| ABCC8 | rs1799854 | 2 | 837 | 863 | 34.1 | 29.9 | 1.20 (0.99–1.48) | 0.074 |
| rs1801261 | 2 | 1526 | 1469 | 0.7 | 0.3 | 2.66 (0.84–8.37) | 0.14 | |
| ADIPOQ | rs1501299 | 2 | 979 | 1039 | 17.8 | 11.9 | 1.60 (1.24–2.05) | 0.00029 |
| rs2241766 | 4 | 1154 | 1256 | 26.1 | 12.3 | 2.51 (2.02–3.10) | <0.0001 | |
| CAPN10 | rs3792267 | 3 | 1587 | 1688 | 10.8 | 3 | 3.90 (2.83–5.38) | <0.0001 |
| rs5030952 | 3 | 1607 | 1515 | 4.6 | 4 | 1.17 (0.83–1.66) | 0.42 | |
| CDKAL1 | rs7754840 | 3 | 2730 | 2369 | 22.2 | 18.7 | 1.24 (1.08–1.42) | 0.0026 |
| rs7756992 | 2 | 2314 | 2043 | 27.8 | 22.6 | 1.32 (1.15–1.52) | <0.0001 | |
| CDKN2A | rs10811661 | 5 | 5757 | 5297 | 67 | 65.1 | 1.09 (1.01–1.18) | 0.037 |
| FTO | rs1421085 | 2 | 1512 | 2250 | 38 | 34.5 | 1.16 (1.02–1.33) | 0.031 |
| rs8050136 | 3 | 2762 | 3456 | 44.2 | 40.5 | 1.16 (1.05–1.29) | 0.0036 | |
| rs9939609 | 6 | 5780 | 5478 | 36.7 | 32.5 | 1.20 (1.11–1.30) | <0.0001 | |
| rs9940128 | 2 | 1600 | 1200 | 42.7 | 39.2 | 1.15 (0.99–1.35) | 0.057 | |
| HHEX | rs1111875 | 7 | 7741 | 9361 | 44.8 | 34 | 1.57 (1.48–1.67) | <0.0001 |
| rs7923837 | 2 | 1684 | 1433 | 42.8 | 45.4 | 0.90 (0.78–1.04) | 0.15 | |
| rs5015480 | 2 | 1802 | 1501 | 61.9 | 46.2 | 1.85 (1.61–2.13) | <0.0001 | |
| IGF2BP2 | rs4402960 | 5 | 5855 | 4883 | 46.5 | 27.9 | 2.04 (1.88–2.21) | <0.0001 |
| IL-10 | rs18008 72 | 2 | 1212 | 736 | 30.7 | 30.2 | 1.02 (0.85–1.26) | 0.85 |
| KCNJ11 | rs5219 | 7 | 5364 | 5043 | 38.1 | 33.8 | 1.21 (1.11–1.31) | <0.0001 |
| KCNQ1 | rs2237892 | 3 | 4186 | 3453 | 95.9 | 94.6 | 1.39 (1.12–1.71) | 0.0023 |
| PPARG | rs1801282 | 5 | 4495 | 4510 | 75 | 65.7 | 1.57 (1.43–1.72) | <0.0001 |
| SLC30A8 | rs13266634 | 7 | 6654 | 6014 | 46.4 | 46.1 | 0.68 (0.62–0.72) | <0.0001 |
| TCF7L2 | rs10885409 | 2 | 1512 | 1474 | 49.5 | 46.1 | 1.05 (0.91–1.21) | 0.48 |
| rs11196205 | 2 | 2592 | 2264 | 47 | 40.6 | 1.16 (1.04–1.30) | 0.0091 | |
| rs12255372 | 11 | 7425 | 6064 | 29.6 | 24.8 | 1.27 (1.18–1.34) | <0.0001 | |
| rs4506565 | 2 | 1341 | 798 | 54.9 | 29.7 | 2.88 (2.39–3.47) | <0.0001 | |
| rs7901695 | 2 | 1520 | 1532 | 36 | 31.7 | 1.23 (1.07–1.44) | 0.0057 | |
| rs7903146 | 16 | 11,196 | 10,073 | 36.2 | 24.8 | 1.72 (1.62–1.83) | <0.0001 | |
| TNF-α | rs1800629 | 2 | 710 | 806 | 24.8 | 20.6 | 1.28 (0.99–1.61) | 0.051 |
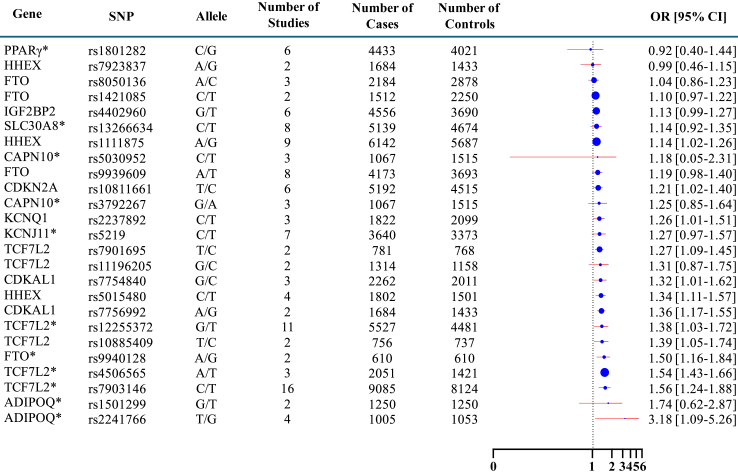
Following the predefined selection criteria, 25 SNPs from 12 distinct genes were eligible for the meta-analysis, sourced from data extracted from 35 individual studies. The TCF7L2 gene contributed six eligible SNPs (rs10885409, rs11196205, rs12255372, rs4506565, rs7901695, and rs7903146), and FTO gene featured four SNPs (rs1421085, rs8050136, rs9939609, and rs9940128). Additionally, there were three SNPs in HHEX gene (rs792837, rs1111875 and rs5015480), and two SNPs each in ADIPOQ (rs1501299 and rs2241766), CAPN10 (rs3792267 and rs5030952), and CDKAL1 (rs7754840 and rs7756992) genes respectively. Other genes, namely CDKN2A (rs10811661), IGF2BP2 (rs4402960), KCNJ11 (rs5219), KCNQ1 (rs2237892), PPARG (rs1801282), and SLC30A8 (rs13266634), contributed one eligible SNP each for inclusion in the meta-analysis (Supplementary Table S4).
Based on the 25 individual meta-analyses conducted for every shortlisted SNPs, (Supplementary Fig. S1) the consolidated representation of meta-analysis (Fig. 4) highlighted a high compatibility between the 12 examined SNPs (rs1111875 and rs5015480 of HHEX, rs11196205, rs12255372, rs10885409, rs4506565 and rs7903146 of TCF7L2, rs10811661 of CDKN2A, rs7756992 of CDKAL1, rs5030952 and rs3792267 of CAPN10, and rs9940128 of FTO) and an increased risk of developing T2DM in Indian population. Among these, TCF7L2 rs7903146 showed the highest compatibility with OR 1.56 (95% CI 1.43–1.66) and S-value 89.036. Similarly, TCF7L2 rs12255372 showed an overall OR of 1.36 (95% CI 1.17–1.35) with an S-value of 15.45. Out of the six SNPs meta-analyzed for TCF7L2, only rs7901695 showed no compatibility with T2DM (OR 1.74; 95% CI 0.62–2.87) (Supplementary Table S5).
Fig. 4.
Summary of 25 individual SNPs meta-analysis forest plot.
The I2 statistics from the 25 meta-analysis revealed significant heterogeneity in most of the studies, except for rs7923837 (HHEX), rs1421085 (FTO), rs3792267 (CAPN10), rs11196205 (TCF7L2), rs7756992 (CDKAL1), and rs1501299 (ADIPOQ) which demonstrated low heterogeneity with I2 values 9.83, 18.30, 19.41, 18.55, 0.20, and 6.87 respectively (Supplementary Table S5). Galbraith plots were generated to graphically assess the heterogeneity among studies included in the meta-analysis (Supplementary Fig. S2). Most of the studies within the individual meta-analyses were found to be within the expected range, although a few outliers were observed for certain SNPs such as rs7903146 and rs12255372 of TCF7L2 and rs9939609 of FTO. Using a leave-one-out sensitivity analysis for rs7903146 meta-analysis, minimal variation was observed, with OR ranging from 1.52 (95% CI 1.41–1.63) after excluding the data by Umajyothi et al,26 to 1.57 (95% CI 1.47–1.68) after omitting data by Ali et al.27 Similarly, the same analysis for TCF7L2 rs12255372 revealed an OR ranging from 1.33 (95% CI 1.15–1.51) after removing the data by Shitomi et al,28 to 1.42 (95% CI 1.32–1.52) after excluding the data by Shubhra et al.29 (Supplementary Fig. S3).
Further to assess different sources of heterogeneity in the meta-analysis, a meta-regression was conducted on rs7903146 and rs12255372 of TCF7L2 locus (as both SNPs had adequate numbers of studies) considering sample size, HWE of controls, case to control ratio, study quality score based on AHRQ, publication year, study region, and genotyping methods. It was observed that sample size in case of rs7903146 and genotyping method in rs12255372 contributed significantly to the observed heterogeneity (adjusted R2 values 70.59% and 36.15% respectively) (Supplementary Table S6).
Since some studies reported only crude ORs, we have adjusted these OR values using an external estimator of confounding. To further assess the impact of this adjustment on the effect size, we re-calculated the pooled estimate of OR and 95% CI by removing the externally estimated OR values in seven SNPs where externally estimated ORs were used. Minimal changes in the OR estimates were observed during this approach: from OR 1.36 (95% CI 1.17–1.55) to OR 1.40 (95% CI 1.29–1.51) for rs2255372, and from OR 1.54 (95% CI 1.43–1.66) to OR 1.55 (95% CI 1.42–1.67) for rs7903146. However, for rs3266634 of SLC30A8 locus, the OR estimates became significant after excluding the externally estimated OR values. On the contrary, the OR estimates for rs4506565 of TCF7L2 and rs2241766 of ADIPOQ became non-significant after removal of the externally estimated OR values (Supplementary Fig. S4).
We also calculated the overall gene specific effect size from the meta-analysis results of each SNP within the respective genes. All the concerned genes from our meta-analysis showed high compatibility for T2DM development. Among themTCF7L2 exhibited a strong compatibility to T2DM development with an overall OR of 1.44 (95% CI 1.36–1.52) and S-value 112.41 (Supplementary Fig. S5). These findings highlight the significant role of TCF7L2 variants in increasing the risk of T2DM in the Indian population.
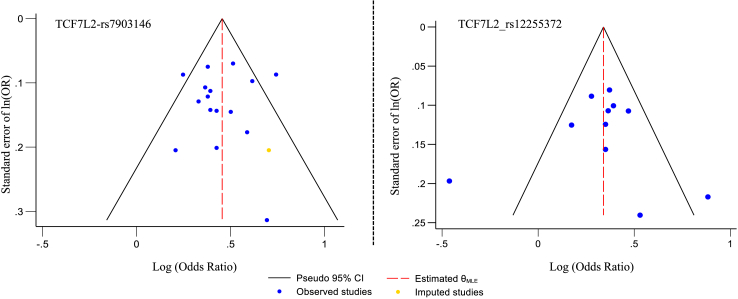
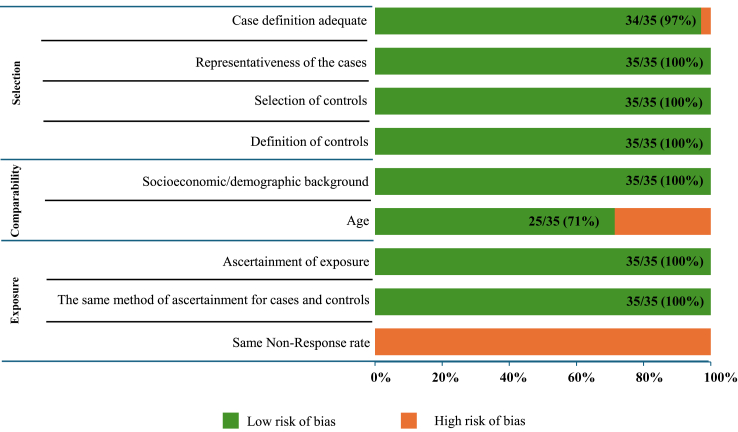
Among the 25 SNPs investigated in the meta-analysis, only TCF7L2's rs12255372 and rs7903146 met the criteria for evaluating potential publication bias, as funnel plot asymmetry tests should generally be applied only when a meta-analysis includes at least ten studies. With fewer studies, the test lacks sufficient power to differentiate between true asymmetry and random variation.30 Given that funnel plot evaluation is subjective and asymmetry may result from factors like heterogeneity or chance,18 we complemented our analysis with Begg's and Egger's tests for all the studied SNPs (Supplementary Table S5). Except for rs7901695 of TCF7L2 (Egger's P = 0.029), no significant associations were observed for the other SNPs. Funnel plot analysis revealed potential publication bias for rs12255372, where two studies fell outside the 95% CI. Using the Trim and Fill method for this SNP, no missing studies were detected. Similarly, for rs7903146, two studies were observed outside the 95% CI and one missing study was identified by the Trim and Fill method (Fig. 5). However, risk of bias by domain and question in 35 eligible case–control studies for meta-analysis using the Newcastle–Ottawa Scale revealed that 89% studies were of good quality, 11% were of fair quality and none were of poor quality, indicating no apparent publication bias in these studies (Fig. 6, Supplementary Table S7).
Fig. 5.
Funnel plot for publication bias with pseudo 95% confidence limit.
Fig. 6.
Risk of bias in 35 eligible case–control studies for meta-analysis using the Newcastle–Ottawa scale.
Discussion
The present study represents a pioneering effort in systematically investigating the genetic polymorphisms linked to T2DM within the Indian population through a comprehensive systematic review and meta-analysis of case–control studies published up to September 30, 2024. This study aimed to elucidate the genetic landscape of T2DM in India by examining 167 SNPs across 61 genes identified from 67 eligible studies, encompassing a significant number of cases (33,407) and controls (30,762) (Supplementary Table S3). The meta-analysis, focusing on 25 SNPs mapped over 14 different genes from 35 qualified studies, provided deeper insights into the genetic underpinnings of T2DM. In this systematic review, a substantial proportion of the identified SNPs (89 SNPs from 46 genes) showed significant associations with T2DM across 53 individual studies at a significance threshold of OR (P < 0.05). Among these, 73 SNPs were associated with an increased risk of T2DM, while 16 SNPs exhibited protective effects against the disease. In studies conducted by two or more authors across different regions of India, seven SNPs viz. rs7020996 (CDKN2A), rs1111875 (HHEX), rs1801282 (PPARG), rs13266634 (SLC30A8), rs1137101 (LEPR), rs7903146 and rs12255372 (TCF7L2) were found to be compatible with both increased risk and a protective effect (Supplementary Table S3).
These findings underscore the intricate genetic diversity and polygenic nature contributing to the susceptibility of T2DM within the Indian population. This relates to the previously reported statistics that indicated regional variations in diabetes prevalence in India6 which may be connected to the diverse genetic makeup of the Indian population. The present genetic understanding explains only 10–20% of T2DM etiology, and data from Europe suggests that only 22% of genes associated with T2DM are replicable in Indians.31 India, a cradle of vast diversity, embraces biological, cultural, and demographic richness shaped by its varied geography. Populations are intricately woven into tribal, non-tribal, caste, linguistic, and religious tapestries.10 The genetic mosaic echoes historic migrations and indigenous interactions.32 Studies indicate a pivotal genetic predisposition in T2DM. Genetic elements significantly contribute to the pathogenesis of diabetes, underscoring their integral role in comprehending the disease's origins and exploring potential preventive measures in the future.33,34
This analysis unveils a remarkable geographic disparity in the distribution of diabetes-related studies across different regions of India (Fig. 3, Supplementary Table S3). In the northern regions, encompassing the Himalayan mountains, foothills and plains, diabetes and pre-diabetes prevalence was previously reported as 11.59% and 14.3%, respectively6,35,36 (Fig. 1). Our systematic review found that 32 studies were reported from this region (Supplementary Table S3), with the majority from Punjab, followed by Haryana, Delhi, Jammu and Kashmir, Uttar Pradesh, and Himachal Pradesh. No study was reported from Rajasthan (Fig. 3, Supplementary Table S3). The southern region, flanked by the Western Ghats bordering the Arabian sea to the west and Eastern Ghats bordering the Bay of Bengal to the East, reported a diabetes and pre-diabetes prevalence of 16.03% and 14.8%, respectively.6 This region contributed 26 studies, mostly from Tamil Nadu, followed by Telangana, Andhra Pradesh, Karnataka, and Kerala. Limited studies were reported from the eastern (N = 4), western (N = 4), and north-eastern regions (N = 3) of the country. The eastern region, with diverse geographical features including the Eastern Himalayas and Gangetic and Mahanadi Plains, exhibited a diabetes and pre-diabetes prevalence of 9% and 14.27% respectively6 (Fig. 1). These studies were predominantly from West Bengal and Orissa followed by Bihar, however, no studies were reported from Jharkhand state. In Western India, which extends from the Western Ghats in the south to the vast Ran of Kutch in the north and bordered by the Arabian Sea to the west, displayed a diabetes prevalence of 7.78% and pre-diabetic prevalence of 13.47%.6 Studies from this region came from the states of Maharashtra and Gujarat, with none from Goa. The north-eastern region, characterized by the eastern Himalayan ranges and foothills, large forested tracts, alluvial plains of rivers Brahmaputra and Barak, and home to diverse tribal populations, reported a diabetes and pre-diabetes prevalence of 7.78% and 14.3%, respectively.6 Out of the eight states in the region, studies were reported only from the state of Assam and Mizoram. Central India, hosting approximately 8.11% of the Indian population with the highest proportion of tribal population in the country, has a diabetes prevalence of 8.7% and pre-diabetes prevalence of 20.4%.6 Surprisingly, no studies were found from this region, highlighting a significant research gap (Fig. 3, Supplementary Table S3). Studying India's diverse population across regions is crucial to comprehend the genetic, epigenetic, and environmental influences on T2DM development and progression. India's varied geography along with rich biological, cultural, and demographic landscapes necessitates a comprehensive and representative population-based research to better inform effective preventive and management strategies for T2DM.
In the present study, we found that 33 SNPs mapped across 17 genes were investigated in 39 different studies (Supplementary Table S3) from different parts of the country (Fig. 3). Among these, 16 SNPs were specifically examined in two regions, mainly in the northern and southern regions of India. Disparities in terms of association of SNPs with T2DM were evident across different regions. For instance, rs5030952 of CAPN10 exhibited an association with increased T2DM risk in the eastern region but not in the southern region, while rs3792267 of the same gene showed association in the southern region in one of the three studies but not in the eastern region. Similarly, rs111875 of HHEX demonstrated an association with increased T2DM risk in the northern region in two of four reported studies, along with western, and north-eastern part, but in the southern region its showed protective effect in one amongst the three reported studies and no association reported in the eastern part. KCNJ11's rs5219 showed association in the northern (two out of four studies), eastern, and western region, however, no association was observed in the southern and north-eastern region. rs4402960 of IGF2BP2 showed association in the northern and western part but not in the southern region. Of note, only rs7903146 of TCF7L2 was consistently associated with T2DM in all the regions. However, PPARG exhibited association with increased risk in western and in one of the three southern region studies; while in the northern part, one study suggested an increased risk, contrary to the protective effect observed in the other two studies. For rs13266634 of SLC30A8, we observed a predisposing association in one, contrary to the protective effect in other and no association in another study from the northern region (Supplementary Table S8). The GWAS studies and meta-analyses have identified numerous genes and variants associated with T2DM, but gene–environment interactions pose challenges, where phenotypic effects vary by genotype. Environmental factors such as diet, physical activity and chemical exposure can also influence T2DM risk directly by interacting with targets or indirectly through the gut microbiome.37 India's dietary sources and food practices vary across the country, which is increasingly showing a transition towards a more processed and fast food-based diet.38 This observed inter-regional and intra-regional disparity in India underscores the essential consideration of varied populations in genetic research, and the revealed associations offer promising avenues for further exploration of risk stratification and targeted management strategies.
Within this systematic review, we have identified five SNPs of four different genes which were investigated in over 10,000 individuals by two or more authors across diverse regions of India (Supplementary Fig. S6). Our investigation revealed that the minor allele frequencies (MAF%) for most SNPs included in the meta-analysis aligned with the distribution data from IndiGenome39 in the Indian population and global datasets from the 1000 Genome40 and gnomAD41 databases (Supplementary Fig. S7). However, significant differences were observed in allele frequencies for specific SNPs such as rs227892, rs1800629, rs1801282, rs1326634, and rs10811661, which displayed higher frequencies, while rs3792267, rs4121085, rs1800872, and rs5219 showed decreased trends. These variations imply differences in allele distribution among diverse ethnic groups in India, emphasizing the need for extensive genetic studies encompassing various origins and ethnicities.
A significant observation in this study was the predominant distribution of SNPs across chromosomes. Notably, 20% of the investigated SNPs (N = 33) were located on chromosome 3, whereas chromosomes 1 and 10 had 18 (11.2%) and 16 (9.9%) SNPs, respectively. No SNPs were reported on chromosomes 4, 12, 14, 21, 22, or the sex chromosomes (Supplementary Fig. S8). Predominance of SNPs on specific chromosomes highlights the need to consider these patterns in exploring genetic mechanisms and regulatory elements associated with the studied SNPs and genes. Previous studies have shown that SNPs in the protein-coding regions can influence target gene expression and are linked to numerous diseases. However, over 90% of GWAS-identified variations were found in non-coding regions, and the mechanisms by which these SNPs contribute to disease risk are unclear.42 In our analysis, 65% (N = 64) of the associated SNPs were found to be located on intronic and intergenic regions of chromosomes (Supplementary Table S3). Certain crucial regulatory elements, like super enhancers, which are clusters of transcriptional enhancers overlapping with non-coding regions, significantly influence cell-type specific gene expression.43 A recent study identified several intriguing super enhancer SNPs associated with type T2DM. Interestingly, many of these SNPs were clustered within the same or adjacent super enhancers. Several of these SNPs are involved in chromatin interaction regulation and may potentially affect the binding affinity of transcription factors.42
We performed 25 individual meta-analysis of 25 SNPs mapped on 14 different genes, and found that many of these SNPs were associated with the risk of T2DM only in individual studies (Supplementary Table S3). The gene TCF7L2 was studied extensively, and six of its variants were eligible for our meta-analysis. Except for rs7901695 (OR 1.74, 95% CI 0.62–2.86, S-value 2.64), all other variants exhibited a high compatibility with an elevated risk of T2DM. The association between the intronic SNP, rs7903146 of TCF7L2 and an elevated risk of T2DM was robust, with S-value of 89.04 and a cumulative OR of 1.54 (95% CI 1.43–1.66). Notably, this increased risk was consistently observed across various individual studies (Supplementary Table S3). Amongst the TCF7L2 SNPs the next most compatible SNP was rs12255372 with S-value 15.45 and OR 1.39 (95% CI 1.29–1.49). While doing compiled meta-analysis of TCF7L2 for all SNPs, it showed highest compatibility with the increased risk of T2DM with S-value of 112.41 and a cumulative OR 1.44 (95% CI 1.36–1.52) (Supplementary Fig. S5). TCF7L2 was reported to be associated with an increased risk of T2DM in diverse ethnic groups worldwide in various meta-analyses, implying that TCF7L2 variants may offer a potential avenue for targeted screening and preventive strategies for T2DM.44, 45, 46 In the context of glucose metabolism, TCF7L2 assumes a crucial role in governing both hepatic and pancreatic functions. Beyond its influence on glucose-dependent insulin secretion and beta cell function, TCF7L2 serves as a comprehensive signaling regulator in adipogenesis. The integrated role of TCF7L2 implies that genetic variants leading to structural alterations in this gene may contribute to adipocyte dysfunction, potentially serving as a predisposing factor for the development of T2DM.46 This multifaceted involvement underscores the significance of TCF7L2 in the complex interplay of metabolic processes implicated in T2DM.
Among the 25 SNPs studied in meta-analysis, two specific SNPs of TCF7L2, namely rs7903146 and rs12255372, met the criteria for assessing source of heterogeneity and publication bias. No publication bias was detected, as illustrated by the symmetrical funnel plot (Fig. 5). However, sample size and genotyping methods were found to be significant moderators for heterogeneity in meta-analysis of SNPs rs7903146 and rs12255372, respectively. This observed heterogeneity may be due to the less number of studies with relatively small sample size in most of the studied SNPs.47 Similarly, the genotyping errors and sensitivity of genotyping methods may also cause deviations from HWE and lead to increased heterogeneity among the studies.45
It was observed from the present study that Indians vary in genetic susceptibility, as evident from the inter- and intra-regional differences in association of SNPs with T2DM risk (Supplementary Table S8). With the world's largest population and second-highest number of diabetic patients,3 along with considerable genetic diversity and diverse phenotypes, India offers an unique opportunity to deliver innovative and globally relevant insights critical for unraveling the intricate dynamics of T2DM. Therefore, we anticipate that efforts to understand the Indian genomes39,48 will help in analyzing the genetic variations specific to Indians in relation to different health conditions. These efforts are expected to identify Indian-specific variants that may play a crucial role in the molecular pathophysiology of T2DM, thereby potentially revealing critical gene–disease relationships.
Combining risk estimates from multiple studies can enhance the strength of statistical conclusions. To the best of our knowledge, our meta-analysis is the largest of its kind that focuses specifically on the association of different SNPs with T2DM in diverse Indian populations. However, our study has certain limitations, the most significant of which was the scarcity of primary genetic epidemiological studies on T2DM from diverse regions in India. Given the country's vast geographical expanse and the presence of various ethnic groups, the limited number of studies precluded a sensitivity analysis for probing the robustness and consistency of our meta-analysis as a comprehensive representation of India. The observed heterogeneity due to the uncontrolled confounders such as limited numbers of studies with relatively small sample size, genotyping methods also influences the overall effect estimates of T2DM susceptibility. Due to the limited availability of comprehensive data on age, sex, body mass index, and other relevant covariates, our analysis relied on externally derived estimates of the odds ratio. These estimates may introduce potential biases, potentially leading to overestimations. Furthermore, the majority of SNPs were reported in fewer than ten articles, making it difficult to conduct a publication bias analysis. This highlights the importance of conducting further genetic epidemiology research in different regions of India to ensure detailed understanding of the relationship of T2DM susceptibility gene variants within the Indian population.
In conclusion, we have analyzed 167 SNPs across 61 genes from 67 case control studies, out of which 89 SNPs mapped over 46 reported genes demonstrated compatibility with T2DM, marked by inter and intra-regional disparity in association. Majority of the associated SNPs were found in intronic and intergenic regions of chromosomes. We have performed 25 individual meta-analysis of 25 SNPs mapped over 14 genes and found majority of them showing high compatibility with increased risk of T2DM with few exceptions. A key finding of our study is the disparity in the association of susceptible genes within the Indian population, emphasizing the need for highly rigorous and extensive research. Given the substantial burden of T2DM in India, future research should focus on better-controlled, inclusive epidemiological studies covering diverse population from all ethnicities to dissect the genetic architecture underlying the susceptibility of Indian patients to T2DM. It would help in identifying relevant biomarkers for enhanced risk stratification and screening for enabling effective prevention and early detection of T2DM in India. It will also facilitate the development of targeted therapies and interventions, thus contributing to a more effective and equitable management of T2DM across India's diverse landscape. This will not only enhance our understanding of the disease in the Indian context but also contribute to global knowledge and potential therapeutic strategies for T2DM.
Contributors
LR and DKS: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, software, writing—original draft; SK, SM, DD: data curation, formal analysis, methodology, writing—review & editing; KB: formal analysis; KB, SS (SwS), AT: validation, writing—review & editing, SS (SaS): writing—review & editing; MK, RRT, PKM: resources, writing—review & editing; DKS: funding acquisition, resources, supervision; LR, SK, and DKS have directly accessed and verified the underlying data. All authors critically edited, read and approved the initial and final version.
Data sharing statement
All the data used for the purpose of this review are available as supplemental material (Supplementary Table S3).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We greatly acknowledge the financial support of Department of Biotechnology (DBT) Ministry of Science and Technology, Government of India, and Indian Council of Medical Research (ICMR), Department of Health Research (DHR), Ministry of Health and Family Welfare (MoHF&W), Government of India. We are also grateful for the support, training, and mentorship received through capacity building programmes initiated by ICMR and DHR for carrying out systematic reviews and meta-analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2024.100518.
Appendix A. Supplementary data
References
- 1.World Health Organization . World Health Organization; Geneva: 2019. Classification of diabetes mellitus.https://iris.who.int/handle/10665/325182 [Google Scholar]
- 2.Barroso I. The importance of increasing population diversity in genetic studies of type 2 diabetes and related glycaemic traits. Diabetologia. 2021;64:2653–2664. doi: 10.1007/s00125-021-05575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magliano D., Boyko E.J. 10th ed. International Diabetes Federation; Brussels: 2021. IDF diabetes atlas. [Google Scholar]
- 4.Ong K.L., Stafford L.K., McLaughlin S.A., et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. 2023;402:203–234. doi: 10.1016/S0140-6736(23)01301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2016. Global report on diabetes.https://iris.who.int/handle/10665/204871 [Google Scholar]
- 6.Anjana R.M., Unnikrishnan R., Deepa M., et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17) Lancet Diabetes Endocrinol. 2023;11:474–489. doi: 10.1016/S2213-8587(23)00119-5. [DOI] [PubMed] [Google Scholar]
- 7.Barman P., Das M., Verma M. Epidemiology of type 2 diabetes mellitus and treatment utilization patterns among the elderly from the first wave of longitudinal aging study in India (2017-18)using a Heckman selection model. BMC Public Health. 2023;23:699. doi: 10.1186/s12889-023-15661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pradeepa R., Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932. doi: 10.4103/ijo.IJO_1627_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastana S.S. Unity in diversity: an overview of the genomic anthropology of India. Ann Hum Biol. 2014;41:287–299. doi: 10.3109/03014460.2014.922615. [DOI] [PubMed] [Google Scholar]
- 10.Holliday E.G. Hints of unique genetic effects for type 2 diabetes in India. Diabetes. 2013;62:1369–1370. doi: 10.2337/db12-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal S., Rani J., Bhat M.A., Vanita V. Genetics of diabetes. World J Diabetes. 2023;14:656–679. doi: 10.4239/wjd.v14.i6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meigs J.B., Cupples L.A., Wilson P.W. Parental transmission of type 2 diabetes: the framingham offspring study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 13.Lalrohlui F., Zohmingthanga J., Hruaii V., Vanlallawma A., Senthil Kumar N. Whole exome sequencing identifies the novel putative gene variants related with type 2 diabetes in Mizo population, northeast India. Gene. 2021;769 doi: 10.1016/j.gene.2020.145229. [DOI] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little J., Higgins J.P.T., Ioannidis J.P.A., et al. STrengthening the REporting of genetic association studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009;6:e22. doi: 10.1371/journal.pmed.1000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan R.L., Whaley P., Thayer K.A., Schünemann H.J. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margulis A.V., Pladevall M., Riera-Guardia N., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epdimiol. 2014;6:359. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezaeizadeh G., Mansournia M.A., Keshtkar A., et al. Maternal education and its influence on child growth and nutritional status during the first two years of life: a systematic review and meta-analysis. eClinicalMedicine. 2024;71 doi: 10.1016/j.eclinm.2024.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman D.G., Bland J.M. How to obtain the p value from a confidence interval. BMJ. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 20.Kenneth J.R., Sander G., Timothy L.L. Modern epidemiology. 2008. [Google Scholar]
- 21.Huedo-Medina T.B., Sánchez-Meca J., Marín-Martínez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Röver C., Knapp G., Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. doi: 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbraith R.F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 24.Mansournia M.A., Nazemipour M., Etminan M. P-value, compatibility, and S-value. Glob Epidemiol. 2022;4 doi: 10.1016/j.gloepi.2022.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stata statistical software. 2023. [Google Scholar]
- 26.Uma Jyothi K., Jayaraj M., Subburaj K.S., et al. Association of TCF7L2 gene polymorphisms with T2DM in the population of hyderabad, India. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali S., Chopra R., Manvati S., et al. Replication of type 2 diabetes candidate genes variations in three geographically unrelated Indian population groups. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shitomi-Jones L.M., Akam L., Hunter D., Singh P., Mastana S. Genetic risk scores for the determination of type 2 diabetes mellitus (T2DM) in north India. Int J Environ Res Public Health. 2023;20:3729. doi: 10.3390/ijerph20043729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhry S., Sonkar Kumar G., Alam R., Sonkar Kumar S., Khan M., Khan S. TCF7L2 rs12255372 (G/T) emergence as a prospective gene for type 2 diabetes mellitus predisposition. Int J Biochem Sci. 2023;23:61–68. [Google Scholar]
- 30.Duval S., Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 31.Narayan K.M.V., Varghese J.S., Beyh Y.S., et al. A strategic research framework for defeating diabetes in India: a 21st-century agenda. J Indian Inst Sci. 2023;103:33–54. doi: 10.1007/s41745-022-00354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorjani P., Thangaraj K., Patterson N., et al. Genetic evidence for recent population mixture in India. Am J Hum Genet. 2013;93:422–438. doi: 10.1016/j.ajhg.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groop L., Pociot F. Genetics of diabetes – are we missing the genes or the disease? Mol Cell Endocrinol. 2014;382:726–739. doi: 10.1016/j.mce.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Joseph A., Thirupathamma M., Mathews E., Alagu M. Genetics of type 2 diabetes mellitus in Indian and global population: a review. Egypt J Med Hum Genet. 2022;23:135. doi: 10.1186/s43042-022-00346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamid Zargar A., Kariem Khan A., Rashid Masoodi S., et al. Prevalence of Type 2 diabetes mellitus and impaired glucose tolerance in the Kashmir Valley of the Indian subcontinent. Diabetes Res Clin Pract. 2000;47:135–146. doi: 10.1016/s0168-8227(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 36.Lin B.Y., Genden K., Shen W., et al. The prevalence of obesity and metabolic syndrome in Tibetan immigrants living in high altitude areas in Ladakh, India. Obes Res Clin Pract. 2018;12:365–371. doi: 10.1016/j.orcp.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rani J., Mittal I., Pramanik A., et al. T2DiACoD: a gene atlas of type 2 diabetes mellitus associated complex disorders. Sci Rep. 2017;7:6892. doi: 10.1038/s41598-017-07238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachdev M., Misra A. Heterogeneity of dietary practices in India: current status and implications for the prevention and control of type 2 diabetes. Eur J Clin Nutr. 2023;77:145–155. doi: 10.1038/s41430-021-01067-1. [DOI] [PubMed] [Google Scholar]
- 39.Jain A., Bhoyar R.C., Pandhare K., et al. IndiGenomes: a comprehensive resource of genetic variants from over 1000 Indian genomes. Nucleic Acids Res. 2020;49(D1):D1225–D1232. doi: 10.1093/nar/gkaa923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The 1000 Genomes Project Consortium, Auton A., Brooks L.D., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karczewski K.J., Francioli L.C., Tiao G., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun W., Yao S., Tang J., et al. Integrative analysis of super enhancer SNPs for type 2 diabetes. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulger M., Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding W., Xu L., Zhang L., et al. Meta-analysis of association between TCF7L2 polymorphism rs7903146 and type 2 diabetes mellitus. BMC Med Genet. 2018;19:38. doi: 10.1186/s12881-018-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xi X., Ma J. A meta-analysis on genetic associations between transcription factor 7 like 2 polymorphisms and type 2 diabetes mellitus. Genomics. 2020;112:1192–1196. doi: 10.1016/j.ygeno.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Abuhendi N., Qush A., Naji F., et al. Genetic polymorphisms associated with type 2 diabetes in the Arab world: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2019;151:198–208. doi: 10.1016/j.diabres.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 47.IntHout J., Ioannidis J.P.A., Borm G.F., Goeman J.J. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol. 2015;68:860–869. doi: 10.1016/j.jclinepi.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Genome India. https://genomeindia.in/insights.php
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.