Abstract
Background
Cardiac lymphoma is a rare disease that can present in various ways. Additionally, atypical clinical presentation makes the diagnosis even more challenging. The most common type of cardiac lymphoma is diffuse large B-cell lymphoma. With chemotherapy, the median survival rate can be up to 2 years. In this report, we focus on the diagnostic approach and differential diagnosis.
Case summary
A 56-year-old patient presented with complete heart block and B-symptoms. Implantation of a pacemaker (PM) was initially deferred due to a junctional rhythm (50 b.p.m.). Echocardiography showed thickening of the left heart with small pericardial effusion. For better visualization of the extent and infiltration cardiac magnetic resonance imaging and computer tomography (CT) were performed. In addition to the cardiac mass, enlarged mediastinal lymph nodes were found on staging CT scan, prompting a transbronchial biopsy. Histology revealed diffuse large B-cell non-Hodgkin lymphoma. The patient was referred to a hospital with a Hemato-oncology Department for initiation of chemotherapy.
Discussion
Cardiac lymphoma can be a rare cause of complete heart block. Prior to PM implantation, basic echocardiography is important. In rare conditions like cardiac lymphoma, multimodal imaging, and interdisciplinary decision-making are crucial for management. In the future, lead-less pacemakers could be a safe and effective option for oncology patients.
Keywords: Cardiac lymphoma, Multimodality cardiac imaging, Complete heart block, Case report
Learning points.
Cardiac lymphoma is a rare cause of complete heart block.
Basic diagnostics before pacemaker implantation include echocardiography.
Multimodal imaging for infiltrative masses, for example cardiac lymphoma, is crucial.
Introduction
Primary cardiac lymphomas without extracardiac manifestation of disease are rare and represent only ∼10% of malignant cardiac tumours or 1% of all cardiac tumours, respectively. Secondary cardiac involvement is more common and occurs in up to 25% of all disseminated lymphomas. The median age at diagnosis is 60 years. Lymphomas in immunocompromised individuals usually occur as a result of HIV or EBV infections.1 Non-Hodgkin lymphomas, specifically diffuse large B-cell lymphomas, are the most common histological form.2
The right atrium is the most common site of origin, but any chamber can be affected.
Left heart involvement is rare. Cardiac lymphomas tend to encase the coronary arteries and the aortic root, but they rarely affect the heart valves.1,3,4
Cardiac lymphomas can present as single or multiple lesions and can be infiltrative and/or intramural. The pericardium is involved in about one-third of cases. Spread can occur through lymphatic, haematogenous, and/or direct extension. Myocardial involvement can mimic the appearance of restrictive cardiomyopathy. A rare cause of constrictive pericarditis is cardiac lymphoma.5,6
Common symptoms of cardiac lymphomas include dyspnoea and B-symptoms (weight loss, night sweats, and fever). Decompensated heart failure accounts for 20% of the clinical presentation. Cardiac arrhythmias, such as AV block, syncope, and rare cases of constrictive pericarditis have also been observed. Cardiogenic shock and sepsis are the most common causes of death.3,7
In computer tomography (CT), cardiac lymphomas typically appear as hypo- or iso-attenuating masses compared to the myocardium. In cardiac magnetic resonance imaging (MRI), they appear isointense on T1-weighted images and iso- to hyperintense on T2-weighted images with a heterogeneous late gadolinium enhancement (LGE) pattern.
Diffuse large B-cell lymphomas subtypes show moderate neovascularization and no significant contrast enhancement.8–10
Despite modern chemotherapy, the prognosis for cardiac lymphomas is generally poor, partly due to late diagnosis. The average survival time in patients receiving chemotherapy with rituximab is ∼22 months.11 Predictors of a poor outcome include advanced stage with extracardiac manifestation, increased age, compromised immune status, involvement of the left heart, and arrhythmias.12
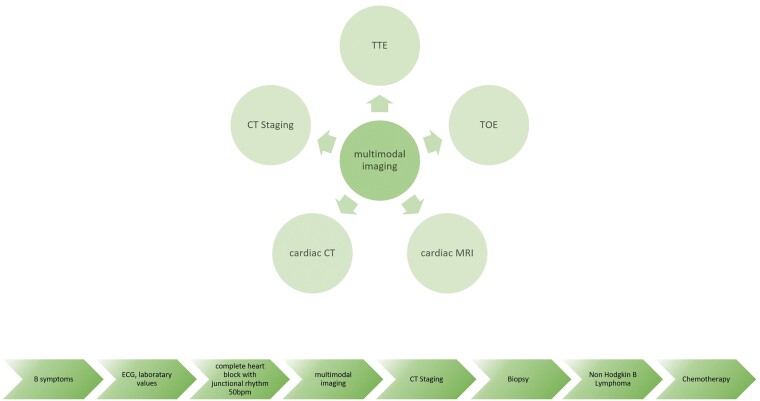
Summary figure
Case
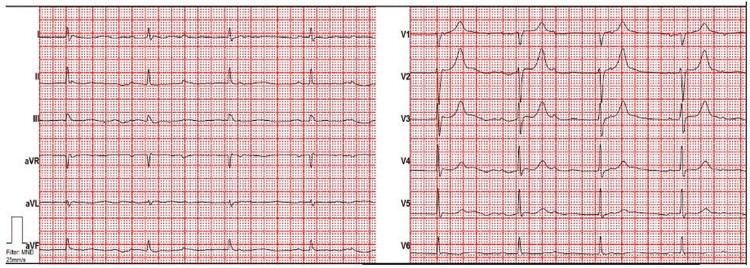
A 56-year-old male was admitted to the emergency department due to worsening of general condition. Three weeks prior, he had a respiratory infection including night sweats, chills, loss of appetite, progressive shortness of breath, chest pain, and subfebrile temperatures. Vital signs and physical examination were unremarkable. Laboratory results showed elevated inflammation markers (C-reactive protein 68 mg/L; 5 mg/L) and natriuretic peptides (>125 ng/L). Initial ECG showed complete heart block (Figure 1); however, urgent pacemaker (PM) implantation was not implanted due to a junction rhythm of 50 b.p.m., and further work-up performed.
Figure 1.
ECG demonstrating complete heart block at the time of first presentation.
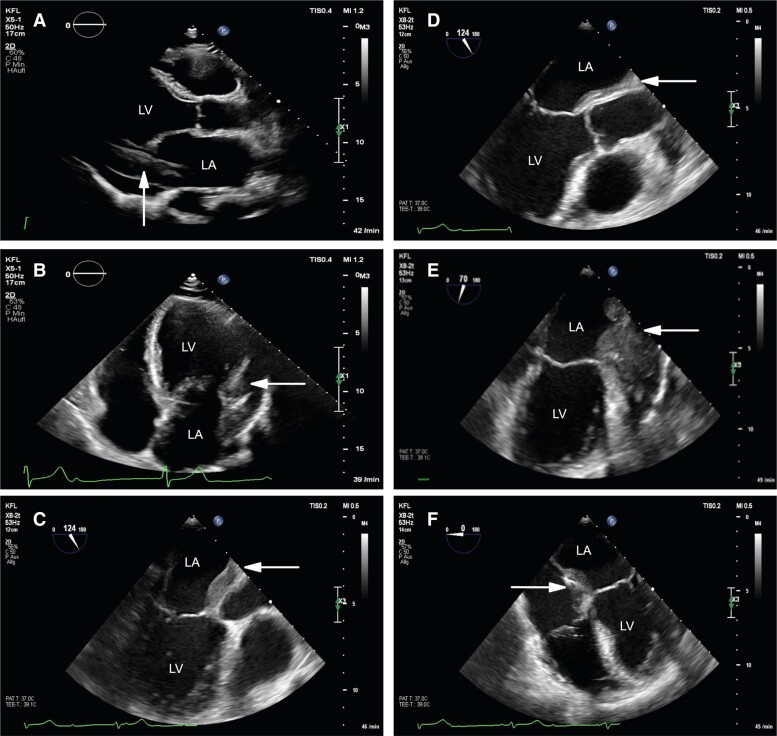
Transthoracic echocardiography (TTE) revealed abnormal thickening of the left heart with mild reduced left ventricular function and a small pericardial effusion (Figure 2A and B).
Figure 2.
Echocardiography. (A and B) Transthoracic echocardiography suspecting thickening of left heart and pericardial effusion in (A) parasternal long-axis view and in (B) apical four-chamber view (white arrow). (C–F) Transoesophageal echocardiography suggesting infiltrative character in (C) mid-oesophageal long-axis view, in (D) mid-oesophageal aortic valve long-axis view, in (E) two-chamber view and (F) four-chamber view (white arrow). LA, left atrium; LV, left ventricle.
Transoesophageal echocardiography (TOE) confirmed the presence of intramyocardial masses, which also infiltrated the region of the atrioventricular and aortic roots without valve involvement (Figure 2C–F). At this point, differential diagnosis included inflammatory processes, such as abscess or tumorous formation of unclear dignity.
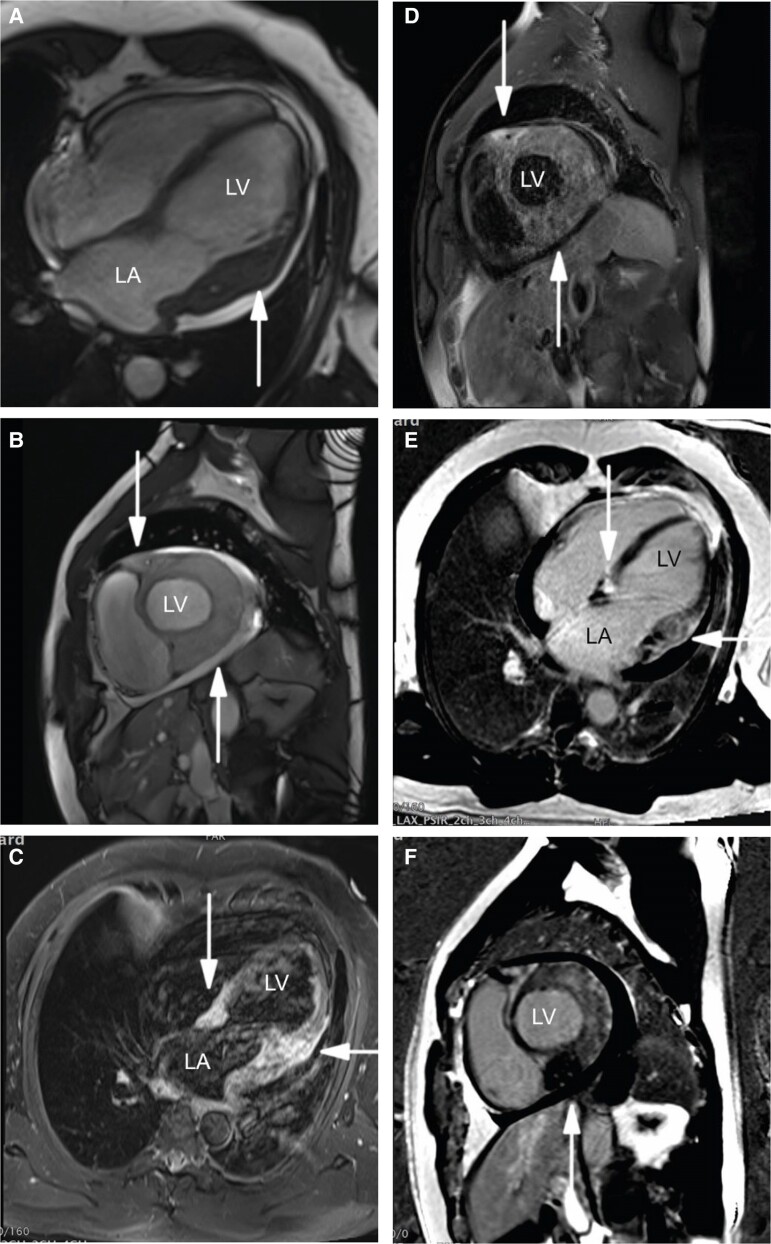
Therefore, further imaging modalities were performed. Cardiac MRI showed diffuse infiltrative thickening or mass especially of the lateral, posterolateral, inferior and anterior wall, extending into the atrial myocardium across the atrioventricular sulcus and the interventricular septum. The homogeneous process appeared isointense to the myocardium on T1- and hyperintense on T2-weighted images. The process encased the coronary arteries. Extensive enhancement of the mass was observed in the LGE sequences (Figure 3). Based on the infiltrative and space-occupying nature of the formation and the encased vessels, the suspicion of a cardiac lymphoma was raised.
Figure 3.
Magnetic resonance imaging. (A–F) Magnetic resonance imaging demonstrating the infiltrative mass in steady-state free precession in (A) four-chamber view and in (B) in short axis; hyperintensity on T2-weighted short-tau inversion recovery in (C) four-chamber view and in (D) short axis; inhomogeneous hyperenhancement in phase-sensitive inversion recovery images in (E) four-chamber view and in (F) short axis (white arrow).
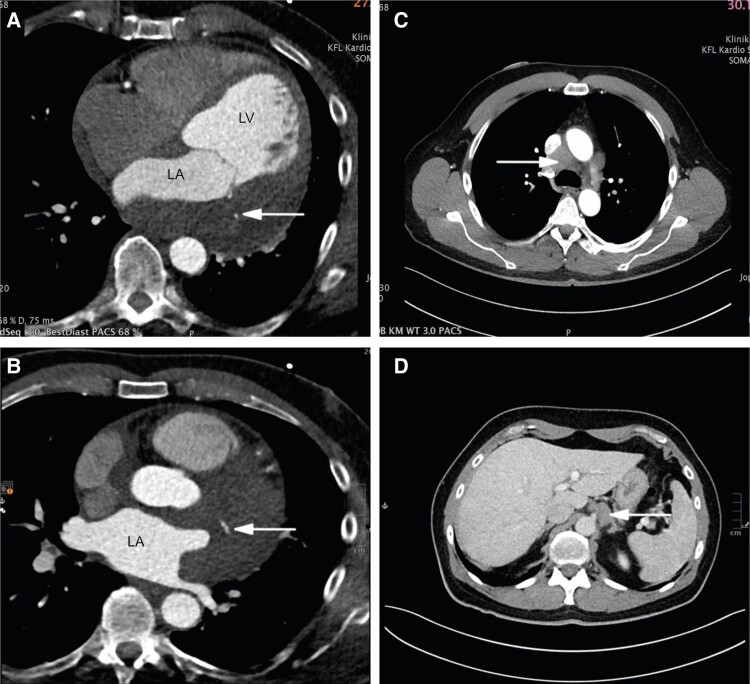
Subsequently, cardiac CT was performed. In addition to the findings on the MRI, an extension cranially into the superior pericardial recess was observed. Large vessels and coronary arteries were encased by the mass (Figure 4A and B).
Figure 4.
Computer tomography. (A and B) Coronary computer tomography revealing extent of mass and encasement of coronary arteries in (A and B) axial four-chamber views (white arrow). (C and D) Staging computer tomography detecting enlarged lymph nodes in (C) axial mediastinal window and in (D) axial subdiaphragmatic window (white arrow).
In the staging CT, a mediastinal soft tissue mass (6.1 × 2.7 × 6.2 cm) and another subdiaphragmatic soft tissue lesion (3.1 × 1.6 × 3.9 cm) were identified (Figure 4C and D).
A multidisciplinary team including pulmonologists, radiologists, and cardiologists decided to perform a transbronchial biopsy of the mediastinal lymph nodes. The final histological examination confirmed the diagnosis of an aggressive B-cell non-Hodgkin lymphoma with the features of a diffuse large B-cell lymphoma (not otherwise specified), according to Hans algorithm: germinal centre B-cell-like. Subsequently, chemotherapy consisting of liposomal doxorubicin/vincristine/cyclophosphamide plus rituximab was initiated at another hospital’s Hemato-oncology Department. We hoped the complete heart block would disappear. After 1 month, there was already partial tumour regression visible on TTE and MRI. Approximately, 3 months after finishing chemotherapy the patient kept experiencing mild shortness of breath after climbing two flights of stairs. The ECG still showed complete heart block and a junctional escape rhythm with a narrow QRS complex (heart rates 39–77/min, no pauses). Eventually, 3 months after completing treatment the patient received conduction system pacing. Immediately after implantation, he felt a significant improvement and then remained in functional Class 1.
Discussion
Cardiac lymphomas are rare. The right heart is the most common site of origin, but in our case, the left heart is affected. Clinical presentation, elevated inflammatory markers but missing elevated lactate dehydrogenase and troponin as well as the first presentation on echocardiography made the diagnosis more challenging due to a possible differential diagnosis of an infectious/inflammatory process, e.g. an abscess, which can mimic the symptoms and findings. However, no infiltrating process was observed in the valves in the TTE and TOE, and there was no evidence of, e.g. pseudoaneurysm or fistula.
The mass presented hypo-isointense on T1-weighted MRI images, which includes abscess as differential diagnosis, but no typical cavity with enhancement of the wall was found in our case. Furthermore, on T2-weighted images, the formation appeared hyperintense, suggestive of oedema or an inflammatory process. However, high-grade rapidly proliferation of lymphoma results in metabolic stress and necrosis with ‘dirty necrotic fluid’ which explains the findings like oedema on MRI.13
Cardiac lymphoma is a rare cause of complete heart block. An accurate diagnosis before invasive therapy is crucial, especially in relation to prognosis. Therefore, imaging prior to the implantation of a permanent PM is essential. In complete heart block with junctional rhythm, urgent PM implantation can be avoided.
An interdisciplinary team decides whether a PM implantation should be performed either before or during chemotherapy under CT/MRI monitoring. The benefits and risks should be carefully considered in this decision-making process, keeping in mind that pacemakers may cause artefacts in MRI/CT follow-up.
According to the 2021 ESC Guidelines for ‘cardiac pacing and cardiac resynchronization therapy’ the use of a lead-less PM may be an option for individuals with cancer to minimize the risks (e.g. infections) depending on the patient’s life expectancy.14 In future conduction, pacing system also may play a role depending on the centre’s experience.
This case highlights the importance of multimodal imaging and interdisciplinary collaboration, particularly in such rare presentations of lymphoma with cardiac involvement as recommended by the ‘cardio-oncology’ guidelines 2022. Echocardiography, MRI, and CT allow for a precise assessment of tumour extent and infiltration. If available, positron emission tomography provides additional information on metabolism and cell proliferation.15 The detection of extracardiac involvement may facilitate a targeted biopsy with less complication than a cardiac biopsy.
According to the ESC Guidelines serial echocardiography, ECG and laboratory test (troponin, natriuretic peptides) are recommended for monitoring the course of chemotherapy in order to detect complications and assess the prognosis.15
Conclusion
Cardiac lymphomas are rare and can cause complete heart block. It is important to identify the cause of a complete heart block before PM implantation, including echocardiography in the clinical work-up.
In case of cardiac lymphoma, multimodal imaging techniques and interdisciplinary collaboration play a crucial role in determining the diagnosis and best course of management.
Looking ahead, besides the conventional PM a lead-less system could be a good option for oncology patients.
Contributor Information
Ilona Grohs, Department of Internal Medicine, Klinik Oberwart, Dornburggasse 90, Oberwart 7400, Austria.
Katharina Riedl, Department of Cardiology, Klinik Floridsdorf, Brünnerstraße 68, Vienna 1210, Austria.
Christa Kliment, Department of Radiology, Klinik Floridsdorf, Brünnerstraße 68, Vienna 1210, Austria.
Georg Delle-Karth, Department of Cardiology, Klinik Floridsdorf, Brünnerstraße 68, Vienna 1210, Austria.
Lead author biography

Ilona Grohs is a senior physician and cardiologist in Austria, working in Oberwart at Klinik Oberwart as the Head of the Echolab. Her specializations include echocardiography, interventional echocardiography, and cardiac MRI, with a focus on structural heart disease.
Consent: Informed written consent was obtained from the patient for the submission and publication of this case report in accordance with COPE guidelines.
Funding: None declared.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Gowda RM, Khan IA. Clinical perspectives of primary cardiac lymphoma. Angiology 2003;54:599–604. [DOI] [PubMed] [Google Scholar]
- 2. Burke A, Tavora F, Maleszewski J, Frazier A. Hematologic tumors of the heart and pericardium. In: Tumors of the Heart and Great Vessels, Vol. 22. American Registry of Pathology; 2015, p337–352. [Google Scholar]
- 3. Zhong L, Yang S, Lei K, Jia Y. Primary cardiac lymphoma: a case report and review of the literature. Chin-Ger J Clin Oncol 2013;12:43–45. [Google Scholar]
- 4. Araoz PA, Eklund HE, Weich TJ, Breen JF. CT and MR imaging of primary cardiac malignancies. Radiographics 1999;19:1421–1434. [DOI] [PubMed] [Google Scholar]
- 5. Ho HH, Kwok HO, Chui HW, Wang E, Chau CM, Chow HW. A rare cause of constrictive pericarditis: primary cardiac lymphoma. Int J Cardiol 2008;123:208–209. [DOI] [PubMed] [Google Scholar]
- 6. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus and heart. J Thorac Oncol 2015;10:1240–1242. [DOI] [PubMed] [Google Scholar]
- 7. Carras S, Berger F, Chalabreysse L, Callet-Bauchut E, Cordier JF, Salles G, et al. Primary cardiac lymphoma; diagnosis treatment and outcome in a modern series. Hematol Oncol 2017;35:510–519. [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Li M, Zhang L, Xie M. Multimodal imaging evaluation of a primary cardiac lymphoma in an immunocompetent patient. Echocardiography 2018;35:2121–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumors. Radiographics 2005;25:1255–1276. [DOI] [PubMed] [Google Scholar]
- 10. De lucas EM, Pagola MA, Fernandez F, Lastra P, Delgado RL, Sadaba P, et al. Primary cardiac lymphoma: helical CT findings and radiopathologic correlation. Cardiovasc Intervent Radiol 2004;27:190–191. [DOI] [PubMed] [Google Scholar]
- 11. Taha AJ, Al-Kindi SG. Outcomes of cardiac diffuse large B-cell lymphoma (DLBCL) in the rituximab era. Int J Cardiol 2021;339:146–149. [DOI] [PubMed] [Google Scholar]
- 12. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma. Cancer 2011;117:581–589. [DOI] [PubMed] [Google Scholar]
- 13. Asadian S, Rezaeian N, Hosseini L, Toloueitabar Y, Komasi M. The role of cardiac CT and MRI in the diagnosis and management of primary cardiac lymphoma: a comprehensive review. Trends Cardiovasc Med 2022;32:408–420. [DOI] [PubMed] [Google Scholar]
- 14. Glikson M, Nielson JC, Kronberg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–3520. [DOI] [PubMed] [Google Scholar]
- 15. Lyon AR, Lopez-Fernandenz T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–4361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.