Abstract
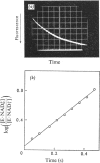
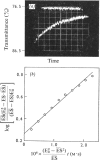
1. The binding of NAD+ and NADH to glycerol 3-phosphate dehydrogenase was studied in the pH range 6.0–9.0 at 25°C and in the temperature range 16–43°C at pH7.0. 2. The second-order velocity constants for the combination of NADH with the enzyme in the pH range 6.0–9.0 and for the combination of NAD+ with the enzyme at pH6.0 were determined. 3. The velocity constant for the dissociation of the enzyme–NAD+ complex at pH6.0 was measured.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P., Dickinson F. M. A study of the kinetics and mechanism of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1974 Oct;143(1):19–27. doi: 10.1042/bj1430019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P., Dickinson F. M., Jones I. G. Purification and properties of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1973 Dec;135(4):853–859. doi: 10.1042/bj1350853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Anderson B. M. Coenzyme binding to L-alpha-glycerophosphate dehydrogenase. J Biol Chem. 1969 Mar 25;244(6):1547–1551. [PubMed] [Google Scholar]
- VAN EYS J., NUENKE B. J., PATTERSON M. K., Jr The nonprotein component of alpha-glycerophosphate dehydrogenase. Physical and chemical properties of the crystalline rabbit muscle enzyme. J Biol Chem. 1959 Sep;234:2308–2313. [PubMed] [Google Scholar]