Abstract
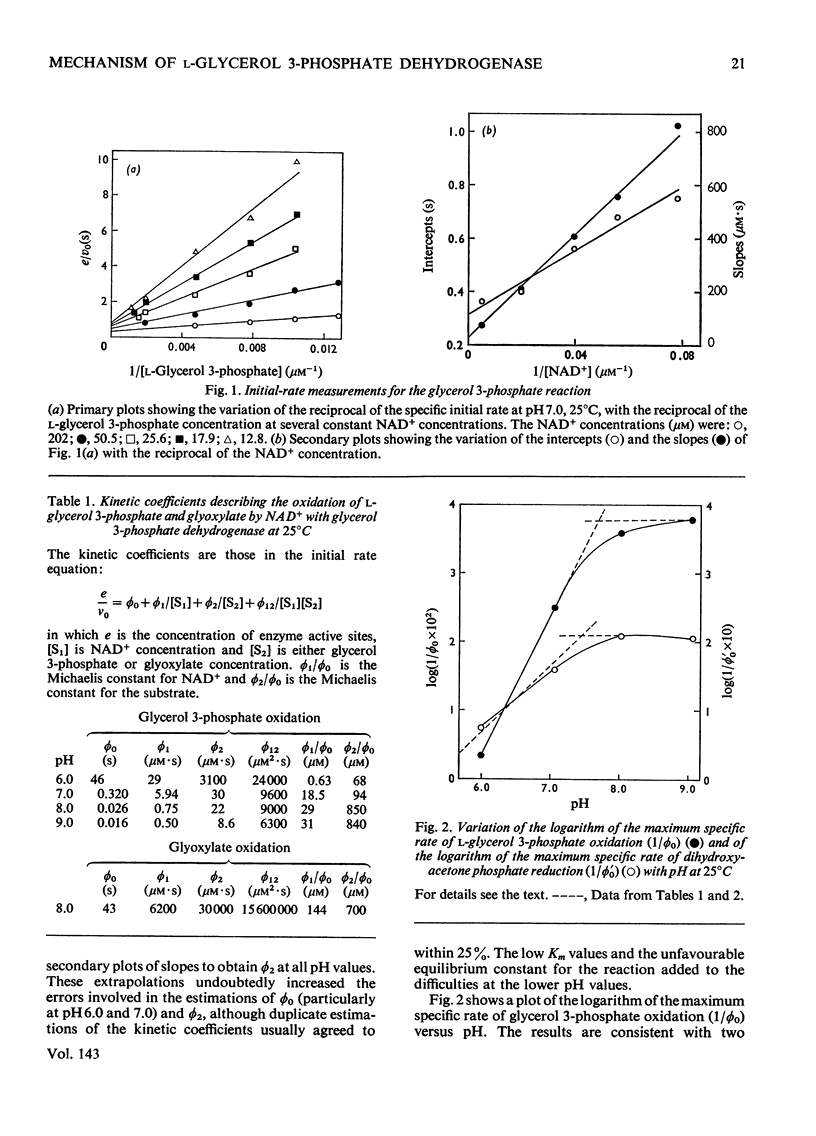
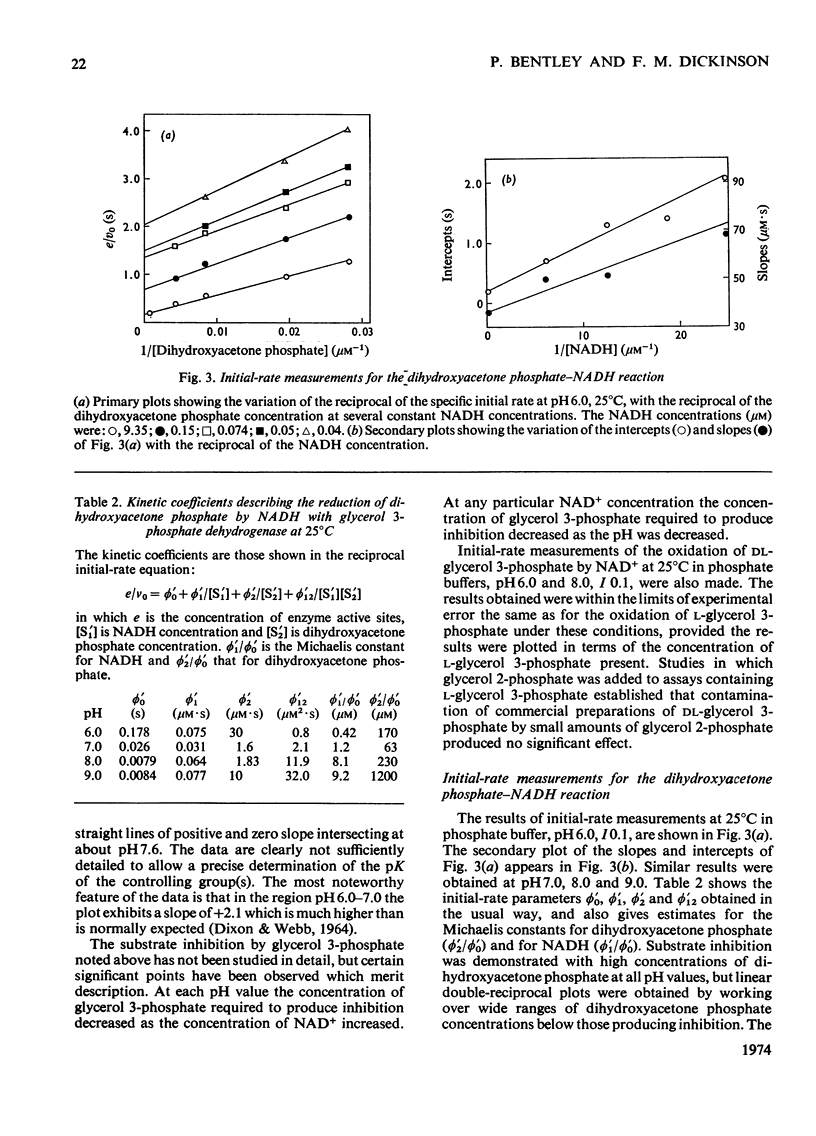
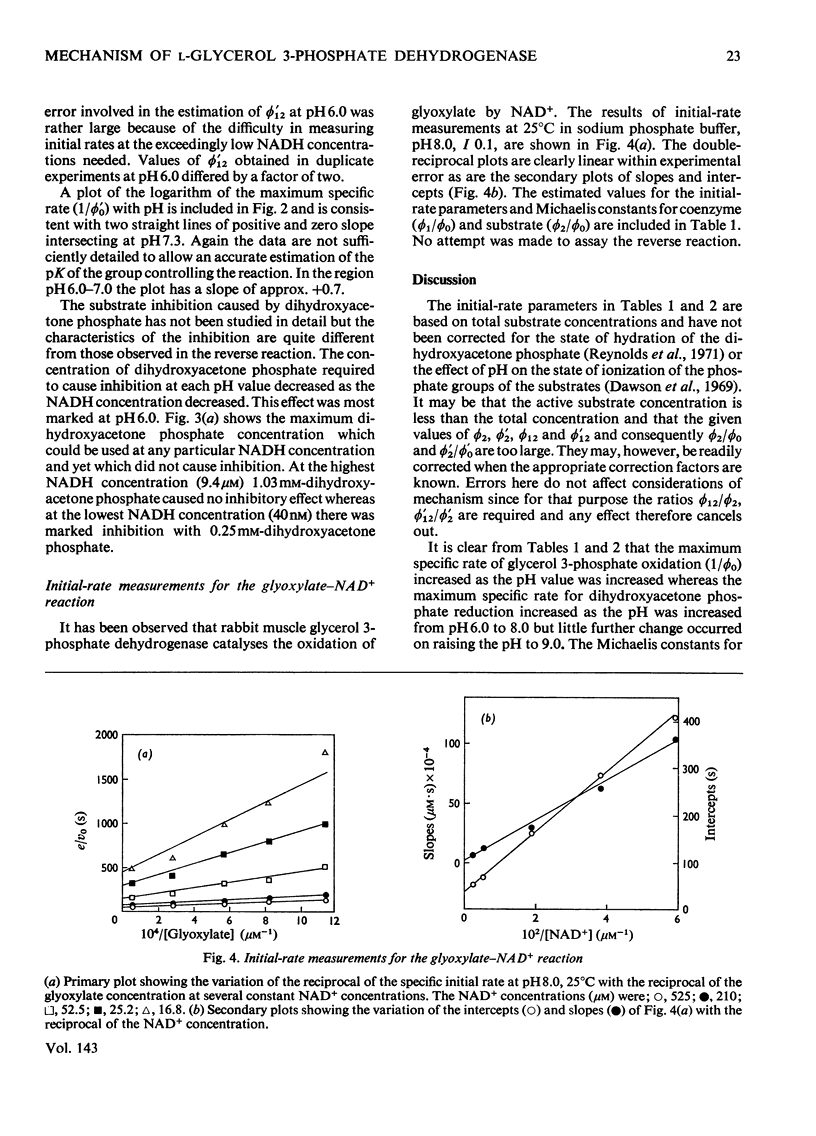
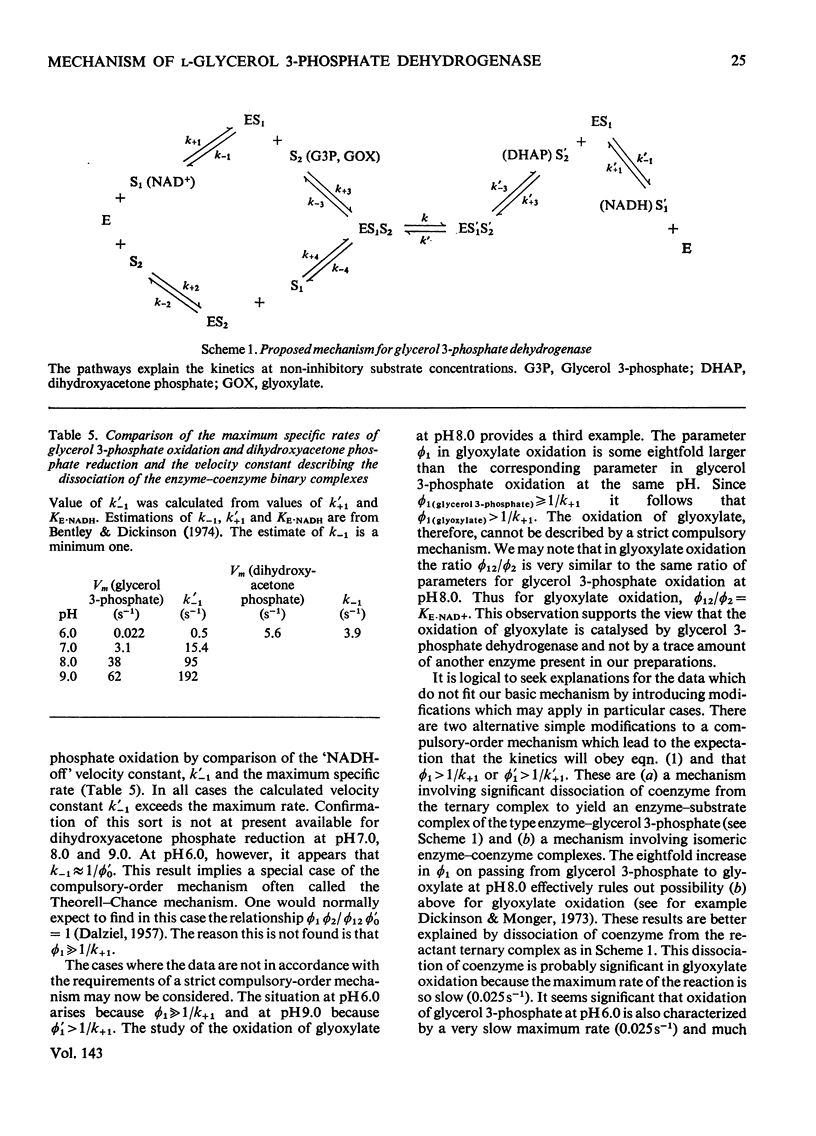
1. The kinetics of oxidation of l-glycerol 3-phosphate by NAD+ and of reduction of dihydroxyacetone phosphate by NADH catalysed by rabbit muscle glycerol 3-phosphate dehydrogenase were studied over the range pH6–9. 2. The enzyme was found to catalyse the oxidation of glyoxylate by NAD+ at pH8.0 and the kinetics of this reaction were also studied. 3. The results are consistent with a compulsory mechanism of catalysis for glycerol 3-phosphate oxidation and dihydroxyacetone phosphate reduction in the intermediate regions of pH, but modifications to the basic mechanism are required to fully explain results at the extremes of the pH range, with these substrates and for glyoxylate oxidation at pH8.0.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K., WILSON T. H. The free-energy changes for the reduction of diphosphopyridine nucleotide and the dehydrogenation of L-malate and L-glycerol 1-phosphate. Biochem J. 1953 Apr;54(1):86–94. doi: 10.1042/bj0540086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P., Dickinson F. M. A study of the coenzyme-binding characteristics of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1974 Oct;143(1):11–17. doi: 10.1042/bj1430011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P., Dickinson F. M., Jones I. G. Purification and properties of rabbit muscle L-glycerol 3-phosphate dehydrogenase. Biochem J. 1973 Dec;135(4):853–859. doi: 10.1042/bj1350853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J. Kinetic studies on the mechanism of cytoplasmic L-alpha-glycerophosphate dehydrogenase of rabbit skeletal muscle. Can J Biochem. 1966 Oct;44(10):1301–1317. doi: 10.1139/o66-150. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- Dalziel K., Dickinson F. M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem J. 1966 Jul;100(1):34–46. doi: 10.1042/bj1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy T. P., Ross C. R., Sollohub S. J. Structural studies on rabbit muscle glycerol 3-phosphate dehydrogenase and a comparison of chemical and physical determinations of its molecular weight. J Biol Chem. 1969 Mar 25;244(6):1631–1644. [PubMed] [Google Scholar]
- Holbrook J. J., Yates D. W., Reynolds S. J., Evans R. W., Greenwood C., Gore M. G. Protein fluorescence of nicotinamide nucleotide-dependent dehydrogenases. Biochem J. 1972 Jul;128(4):933–940. doi: 10.1042/bj1280933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., BAKER R. H., Jr, SHINER V. J., Jr Studies on the mechanism of enzyme-catalyzed oxidation reduction reactions. IV. A proposed mechanism for the over-all reaction catalyzed by liver alcohol dehydrogenase. Biochemistry. 1962 Jan;1:47–52. doi: 10.1021/bi00907a008. [DOI] [PubMed] [Google Scholar]
- Reynolds S. J., Yates D. W., Pogson C. I. Dihydroxyacetone phosphate. Its structure and reactivity with -glycerophosphate dehydrogenase, aldolase and triose phosphate isomerase and some possible metabolic implications. Biochem J. 1971 Apr;122(3):285–297. doi: 10.1042/bj1220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin D. L., Fondy T. P. Isolation and characterization of cytoplasmic L-glycerol-3-phosphate dehydrogenase from rabbit-renal-adipose tissue and its comparison with the skeletal-muscle enzyme. Eur J Biochem. 1973 Jul 2;36(1):97–109. doi: 10.1111/j.1432-1033.1973.tb02889.x. [DOI] [PubMed] [Google Scholar]
- YOUNG H. L., PACE N. Some physical and chemical properties of crystalline alpha-glycerophosphate dehydrogenase. Arch Biochem Biophys. 1958 May;75(1):125–141. doi: 10.1016/0003-9861(58)90403-x. [DOI] [PubMed] [Google Scholar]


