Abstract
Background
During in vitro fertilisation (IVF) procedures, human preimplantation embryos are cultured in the laboratory. While some laboratories culture in an atmospheric oxygen concentration (˜ 20%), others use a lower concentration (˜ 5%) as this is more comparable to the oxygen concentration observed in the oviduct and the uterus. Animal studies have shown that high oxygen concentration could have a negative impact on embryo quality via reactive oxygen species causing oxidative stress. In humans, it is currently unknown which oxygen concentration provides the best success rates of IVF procedures, eventually resulting in the hightest birth rate of healthy newborns.
Objectives
To determine whether embryo culture at low oxygen concentrations improves treatment outcome (better embryo development and more pregnancies and live births) in IVF and intracytoplasmic sperm injection (ICSI) as compared to embryo culture at atmospheric oxygen concentrations.
Search methods
The Menstrual Disorders and Subfertility Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO electronic databases were searched (up to 4th November 2011) for randomised controlled trials on the effect of low oxygen concentrations for human embryo culture. Furthermore, reference lists of all obtained studies were checked and conference abstracts handsearched.
Selection criteria
Only truly randomised controlled trials comparing embryo culture at low oxygen concentrations (˜ 5%) with embryo culture at atmospheric oxygen concentrations (˜ 20%) were included in this systematic review and meta‐analysis.
Data collection and analysis
Two review authors selected the trials for inclusion according to the above criteria. After that two authors independently extracted the data for subsequent analysis, and one author functioned as a referee in case of ambiguities. The statistical analysis was performed in accordance with the guidelines developed by The Cochrane Collaboration.
Main results
Seven studies with a total of 2422 participants were included in this systematic review. Meta‐analysis could be performed with the data of four included studies, with a total of 1382 participants. The methodological quality of the included trials was relatively low. Evidence of a beneficial effect of culturing in low oxygen concentration was found for live birth rate (OR 1.39; 95% CI 1.11 to 1.76; P = 0.005; I2 = 0%); this would mean that a typical clinic could improve a 30% live birth rate using atmospheric oxygen concentration to somewhere between 32% and 43% by using a low oxygen concentration. The results were very similar for ongoing and clinical pregnancy rates. There was no evidence that culturing embryos under low oxygen concentrations resulted in higher numbers of adverse events such as multiple pregnancies, miscarriages or congenital abnormalities.
Authors' conclusions
The results of this systematic review and meta‐analysis suggest that culturing embryos under conditions with low oxygen concentrations improves the success rates of IVF and ICSI, resulting in the birth of more healthy newborns.
Keywords: Female; Humans; Pregnancy; Blastocyst; Reproductive Techniques, Assisted; Embryo Culture Techniques; Embryo Culture Techniques/methods; Embryonic Development; Live Birth; Live Birth/epidemiology; Oxygen; Oxygen/administration & dosage; Pregnancy Rate; Randomized Controlled Trials as Topic
Plain language summary
Low oxygen concentrations for embryo culture in In vitro fertilisation
Couples who experience difficulty conceiving are commonly referred for assisted reproductive technologies such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) as a way to achieve pregnancy. Over 3.5 million babies have been born worldwide from IVF and ICSI procedures. One of the main focuses of research in reproductive medicine is to optimise the treatment success of IVF and ICSI procedures. One such area has focused on improving the in vitro environment to which human embryos are exposed before implantation into the uterus. An important component of this in vitro environment is the oxygen concentration. Traditionally, embryos have been cultured under atmospheric oxygen concentrations (˜ 20%), probably because culturing at lower oxygen concentration requires additional expenses. More recently there has been a shift towards the use of lower oxygen concentrations (˜ 5%) as these more closely resemble the oxygen concentration under natural conditions (2% to 8%). The results of clinical studies that have been undertaken to study the effect on the outcomes of IVF and ICSI procedures of culturing embryos under low oxygen concentrations have been conflicting. Therefore, we performed a systematic review and meta‐analysis of the literature to find the best available evidence. It has shown that culturing embryos under low oxygen concentrations does indeed improve clinical outcomes after IVF and ICSI, such as number of deliveries (live birth rate) and ongoing and clinical pregnancy rates. Furthermore, no evidence was found of an increased risk of adverse events such as multiple pregnancies, miscarriages and congenital abnormalities. We concluded that culturing embryos under low oxygen concentrations seems beneficial with an increase in the number of newborns, but more studies are needed to strongly prove this effect.
Summary of findings
Summary of findings for the main comparison. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for live birth rate.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for live birth rate | ||||||
| Patient or population: Patients with live birth rate Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: Embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Live birth rate | 309 per 1000 | 383 per 1000 (332 to 440) | OR 1.39 (1.11 to 1.76) | 1291 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In one of the trials allocation concealment did not take place and in another trial the method of allocation concealment was unclear
Summary of findings 2. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for ongoing pregnancy.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for ongoing pregnancy | ||||||
| Patient or population: Patients with ongoing pregnancy Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: Embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Ongoing pregnancy rate | 312 per 1000 | 388 per 1000 (335 to 445) | OR 1.4 (1.11 to 1.77) | 1291 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One of the trials had no allocation concealment and in another trial the method of allocation concealment was unclear
Summary of findings 3. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for clinical pregnancy rate.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for clinical pregnancy rate | ||||||
| Patient or population: Patients with clinical pregnancy rate Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: Embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Clinical pregnancy rate | 369 per 1000 | 442 per 1000 (387 to 494) | OR 1.35 (1.08 to 1.67) | 1382 (4 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One of the trials had no allocation concealment and another trial did not detail the methods of allocation concealment
Summary of findings 4. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for multiple pregnancy rate.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for multiple pregnancy rate | ||||||
| Patient or population: Patients with multiple pregnancy rate Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: Embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Multiple‐pregnancy rate | 88 per 1000 | 113 per 1000 (80 to 158) | OR 1.33 (0.91 to 1.95) | 1382 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One of the trials did not have allocation concealment and another trial did not detail the methods of allocation concealment 2 The summary effect crosses the line of no effect and substantive benefit or harm
Summary of findings 5. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for miscarriage rate.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for miscarriage rate | ||||||
| Patient or population: patients with miscarriage rate Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Miscarriage rate | 75 per 1000 | 94 per 1000 (65 to 133) | OR 1.28 (0.86 to 1.9) | 1291 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One trial had no allocation concealment and another trial did not detail the method of allocation concealment 2 The summary effect crosses the line of no effect and substantive benefit or harm
Summary of findings 6. Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for congenital abnormalities.
| Embryo culture with low oxygen concentration compared to embryo culture with atmospheric oxygen concentration for congenital abnormalities | ||||||
| Patient or population: patients with congenital abnormalities Settings: Assisted reproductive technologies Intervention: Embryo culture with low oxygen concentration Comparison: embryo culture with atmospheric oxygen concentration | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Embryo culture with atmospheric oxygen concentration | Embryo culture with low oxygen concentration | |||||
| Congenital abnormalities | 6 per 1000 | 1 per 1000 (0 to 25) | OR 0.2 (0.01 to 4.09) | 647 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was no allocation concealment 2 The summary effect crossed the line of no effect and substantive benefit or harm 3 The evidence is based on unpublished data reported by authors of a single trial
Background
Description of the condition
During in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) procedures, human preimplantation embryos are exposed to an in vitro environment for several days. Even though in vivo embryos are exposed to oxygen concentrations of 2% to 8% (Catt 2000; Fischer 1993; Yedwab 1976), historically most IVF laboratories have cultured embryos under atmospheric oxygen concentrations (˜ 20%), probably because culturing at lower oxygen concentration requires additional expenses. These costs are associated with two items, the additional costs of a specialised incubator (estimated at 2000 euros) as well as additional costs for nitrogen (N2) gas and extra maintenance costs because of the need for an O2 sensor (estimated at 200 euros per year). Atmospheric oxygen concentration can be detrimental for embryo development and gene expression as recent studies on mice and cattle embryos have shown (Feil 2006) as well as a recent study on human embryonic stem cells and embryoid bodies (Lim 2011). The evidence for an effect of low oxygen concentration on the treatment success after IVF and ICSI is confusing, with some studies reporting improved pregnancy rates while others report no differences (Kovacic 2009; Meintjes 2009).
Description of the intervention
In vitro development of human preimplantation embryos is related to, and can be affected by, a range of factors, such as the composition of the medium used (Dumoulin 2010) and the oxygen concentration in the incubator. Oxygen is relevant for early embryo development as it plays a role in cellular respiration and energy production to sustain continuous development through the various stages prior to implantation (Harvey 2007). Moreover, oxygen is important for maintaining the pH of some culture media (Kea 2007). However, oxygen can also have a potentially toxic effect on human embryos (Catt 2000; Lim 2011) via reactive oxygen species (ROS) (Catt 2000; Kovacic 2008).
How the intervention might work
Low oxygen concentrations mimic more closely the in vivo environment of human preimplantation embryos. At the same time, high levels of oxygen could cause oxidative stress via ROS, which can cause damage of DNA (resulting in abnormal protein synthesis), proteins (resulting in aberrant protein function) and lipids (affecting stability and permeability of cell membranes) (Catt 2000). This could hamper cell function of the early embryo and compromise embryo quality. Further development of these compromised embryos might lead to the birth of children with congenital abnormalities. Alternatively, the damage could influence the viability of these embryos, causing them to arrest or undergo apoptosis. Lowering the oxygen concentration during insemination, fertilisation and embryo growth could potentially reduce the harmful effects of oxygen and improve embryo viability and morphology.
Why it is important to do this review
In theory, culturing embryos under a low oxygen concentration could improve success rates of IVF. Whether this is true in clinical practice is currently unclear due to conflicting results in the available literature. These contradictory findings point out the need for a systematic review and meta‐analysis of the best available evidence, to facilitate a more robust conclusion and provide guidance for good laboratory practice. It also remains to be determined whether the effect of oxygen concentration is stage specific (that is for early cleaving embryos or blastocysts, or both) (Karagenc 2004).
Objectives
To determine whether embryo culture at low oxygen concentrations improves treatment outcome (better embryo development and more pregnancies and live births) in IVF and ICSI compared to embryo culture at atmospheric oxygen concentrations.
Methods
Criteria for considering studies for this review
Types of studies
Only truly randomised clinical trials were eligible for inclusion. Quasi and pseudo‐randomised clinical trials were excluded. Cross‐over trials could be included for completeness, but only the data from the first phase were pooled in the meta‐analysis as this design is not valid in the context of subfertility trials (Vail 2003).
Types of participants
Couples undergoing IVF and ICSI, including embryo thaw cycles.
Types of interventions
Trials comparing embryo culture at low oxygen concentrations (˜ 5%) with embryo culture at atmospheric oxygen concentrations (˜ 20%) were eligible for inclusion in the review. All embryos from a participant would have to be cultured at either low or atmospheric oxygen concentrations.
Types of outcome measures
Primary outcomes
Live birth rate, defined as the number of live births per randomised woman.
Secondary outcomes
Ongoing pregnancy rate, defined as the number of ongoing pregnancies at 12 weeks per randomised woman (demonstrated by the presence of a gestational sac with fetal heart beat on ultrasound).
Clinical pregnancy rate, defined as the number of clinical pregnancies per randomised woman (demonstrated by the presence of a gestational sac on ultrasound, normally at around six to eight weeks).
Multiple pregnancy rate, defined as the number of multiple pregnancies per randomised woman.
Miscarriage rate, defined as the number of miscarriages per randomised woman.
Congenital abnormality rate, defined as the number of congenital abnormalities per randomised woman.
Additional outcomes
Implantation rate, defined as the number of gestational sacs on ultrasound divided by the number of transferred embryos.
Embryo development, defined as the number of good quality embryos per randomised treatment cycle.
Cryopreservation rate, defined as the number of embryos that were cryopreserved divided by the total number of embryos.
It was impossible to pool the data on additional outcomes together with the primary and secondary outcomes because of the difference in the unit of analysis (randomising embryos instead of patients) (Mastenbroek 2005). However, due to the frequency at which these outcome measures were reported in the literature they were included in the review for completeness. These data were not part of the meta‐analysis but were reported as numbers under the 'Notes' sections of the 'Characteristics of included studies' table and are summarised in Table 7.
1. Additional outcome measures.
| Outcome measure |
Included trials with result |
| Implantation rate |
Kovacic 2009: low oxygen: 152/528, high oxygen 136/539 Meintjes 2009: low oxygen: 122/247, high oxygen 95/267 Sepulveda 2011: low oxygen: 38/91, high oxygen 32/87 |
| Embryo development rate |
Kovacic 2009: low oxygen 1014/1736, high oxygen 727/1742 Sepulveda 2011: low oxygen 205/370, high oxygen 161/385 |
| Cryopreservation rate |
Kovacic 2009: low oxygen 488/1736, high oxygen 420/1742 Meintjes 2009: low oxygen 99/1115, high oxygen 84/1070 Sepulveda 2011: low oxygen 114/370, high oxygen 74/385 |
The additional outcomes are not part of the meta‐analysis. However, they have been extracted for completeness.
Search methods for identification of studies
Electronic searches
All published and unpublished randomised controlled trials of embryo culture at reduced oxygen concentrations versus culture at atmospheric oxygen concentrations (from 1978 to 2011) were sought using a specific search strategy (see Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5), without language restrictions and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
The following electronic databases were searched (up to the 4th of November 2011) using the search terms reported in the appendices:
1) Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Register; 2) Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, current Issue); 3) MEDLINE, EMBASE, PsycINFO.
Other electronic sources of trials that were searched were:
CINAHL;
The Cochrane Library (www.cochrane.org/index.htm);
Handsearching of appropriate journals: lists of journals are in the MDSG Module, found in The Cochrane Library under BROWSE ‐ 'By Review Group' ‐ 'Cochrane Menstrual Disorders and Subfertility Group' ‐ then 'about this group' at the top of the page;
trial registers for ongoing and registered trials: 'Current Controlled Trials' (www.controlled‐trials.com/), 'ClinicalTrials.gov' a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home), 'The World Health Organisation International Trials Registry Platform search portal' (www.who.int/trialsearch/Default.aspx);
citation indexes (http://scientific.thomson.com/products/sci/);
conference abstracts on the ISI Web of Knowledge (http://isiwebofknowledge.com/).
Other databases that were searched were:
LILACS database, which provides a source of trials from the Portuguese and Spanish speaking world (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F);
ClinicalStudyResults, which provides clinical trial results of marketed pharmaceuticals (www.clinicalstudyresults.org/);
PubMed (www.ncbi.nlm.nih.gov/pubmed/) (filters for randomised controlled trials on PubMed can be found in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions);
OpenSIGLE database for grey literature in Europe (http://opensigle.inist.fr/).
Searching other resources
Reference lists of trials retrieved by the search were handsearched. Furthermore, the European Society of Human Reproduction and Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM) supplements were handsearched.
Data collection and analysis
Data collection and analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (SB, EM) performed a selection of the trials retrieved from the search by scanning the titles and abstracts and removing those that were clearly irrelevant. The full text was retrieved of all trials that were considered to be possibly eligible. Two review authors (SB, EM) independently examined the full text articles for compliance with the inclusion criteria and selected the eligible studies for inclusion into the review. Where required, the review authors corresponded with the study investigators to clarify study eligibility. Disagreements on eligibility were resolved by consensus or with the help of a third author (SS). Excluded articles are detailed in the table 'Characteristics of excluded studies'. Included trials were assessed against the risk of bias criteria and for methodological details. This information is presented within the table 'Characteristics of included studies' and provides a context for assessing the reliability of the results.
Time line
A search for new trials will be conducted every two years and the review will be updated as and when new trials are found for incorporation.
Data extraction and management
Data were independently extracted by two review authors (SB, EM) using a data extraction form that was designed and pilot‐tested by the authors. When disagreements could not be resolved by consensus, a third co‐author (SS) was available to resolve any discrepancies. Additional information on trial methodology or actual original trial data were requested from the authors of trials that appeared to meet eligibility criteria. This was done in order to clarify any aspects of methodology or when the data were in an unsuitable form to be included. A reminder was sent when a reply to our correspondence was not received within three weeks. When a single trial had multiple publications, the main trial report was used as the reference and additional details were supplemented from secondary papers.
Assessment of risk of bias in included studies
The included studies were assessed for risk of bias in the following domains.
1. Sequence generation
A low risk of bias was allocated when the investigators described using a random component in the sequence generation process such as:
computerised random number generator;
random numbers table.
2. Allocation concealment
A low risk of bias was allocated when the participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central computer randomisation, followed by allocation in serially numbered, sealed opaque envelopes;
third‐party allocation that was triggered by entry of a participant.
3. Blinding
A low risk of bias was allocated when blinding of participants, scientists and clinicians or nurses was ensured. The absence of blinding of participants and personnel was classified as performance bias, the absence of blinding of scientists for outcome assessment was classified as detection bias. Even though embryo viability will not be affected by the process of blinding, the outcome measures may indirectly be affected by a lack of it, for instance due to a participant's behaviour.
4. Completeness of outcome data
A low risk of bias was allocated when there were no missing data, which means live birth rate and length of follow up were stated, loss to follow up was accounted for and an intention‐to‐treat (ITT) analysis was carried out.
5. Selective outcome reporting
A low risk of bias was allocated when all of the study's primary, secondary and additional outcomes that were of interest for the review were reported in a prespecified manner.
6. Other sources of bias
A low risk of bias was allocated when the study:
reported multiple pregnancy rate in the case of an embryo transfer policy of multiple embryos per treatment cycle.
These domains were assessed by two authors (SB, EM), with any disagreements resolved by consensus or by contacting the third author (SS). All judgments are fully described. The conclusions are presented in the risk of bias figures and incorporated into the interpretation of review findings.
Measures of treatment effect
The outcomes from each study with dichotomous data (for example clinical pregnancy rate) were expressed as odds ratios (OR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis of the review was per randomised woman. Reported data that did not allow valid analysis (for example data reported per embryo transfer or per oocyte) were not pooled with the data of the primary analysis. However, when possible, these data were separately extracted from the included trials for completeness.
When possible, reported multiple live births were counted as one live birth event.
Only first‐phase data from cross‐over trials were included.
When possible, the data were analysed according to the intention‐to‐treat principle (ITT analysis). The number of randomised couples was used as the denominator.
Dealing with missing data
The data were analysed following the intention‐to‐treat principle and the original investigators were contacted regarding missing data. Patients that dropped out after randomisation were assumed not to be pregnant. When data were not presented on an intention‐to‐treat basis the study was excluded in a sensitivity analysis.
Assessment of heterogeneity
Heterogeneity was considered by the authors when the clinical and methodological characteristics of the included studies were similar enough for a meta‐analysis to give a meaningful summary. Statistical analysis was performed in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration (Higgins 2011). Heterogeneity between the results of different studies was assessed by the I2 statistic, which can be interpreted in the following broad terms:
0% to 40%, might not be important;
30% to 60%, represents moderate heterogeneity;
50% to 90%, represents substantial heterogeneity;
75% to 100%, considerable heterogeneity (Higgins 2011).
In the case of substantial or considerable heterogeneity, explanations were sought by performing a sensitivity analysis.
Assessment of reporting biases
The authors aimed to minimise the potential impact of publication and reporting biases by performing a comprehensive search for eligible studies and looking for duplication of data. Since no more than six studies were included in the analysis, a funnel plot to investigate the possibility of small study effects (a tendency for the intervention to have a bigger impact in smaller studies) was not used.
When included studies only reported interim outcomes such as clinical pregnancy and did not report the primary outcome measure of live birth, informal assessment was undertaken as to whether studies reporting the primary outcome measures reflect typical findings for the interim outcomes.
The assessment of reporting biases is addressed in the risk of bias in included studies section of the 'Results'.
Data synthesis
The data from primary studies were combined for meta‐analysis with RevMan software using a fixed‐effect model in the comparison of embryo culture at low oxygen concentrations versus embryo culture at atmospheric oxygen concentrations.
An increase in the odds of a particular outcome, either beneficial or detrimental, has been displayed graphically in the meta‐analysis to the right of the centre line. A decrease in the odds of an outcome has been displayed to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed with the live birth rate data for the timing of embryo transfer: studies performing early embryo transfer (up to and including Day 3) and those performing late embryo transfer (Days 4, 5 and 6).
Sensitivity analysis
Sensitivity analysis was performed to verify whether the conclusions about the primary outcome measures were robust to arbitrary decisions made regarding the eligibility and analysis. In this way it was checked whether conclusions differed if:
a) eligibility was restricted to studies with a low risk of bias in adequate sequence generation, allocation concealment and blinding domains of the risk of bias assessment tool;
b) studies with outlying results were excluded, as these studies might present results that were either too positive or too negative;
c) data that could not be analysed according to the intention‐to‐treat principle were excluded;
d) a random‐effects model was adopted.
When the sensitivity analysis identified particular decisions or missing data that were greatly influencing the findings of the review, the authors tried to resolve these ambiguities.
Results
Description of studies
Results of the search
A total of 127 studies were identified using the search strategies (see Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5). Thirty‐three studies appeared to meet the basic inclusion criteria. In addition, 12 studies retrieved from handsearching appeared to meet the basic inclusion criteria, resulting in a total of 45 studies that were potentially eligible for inclusion. After further in‐depth eligibility assessment, data examination and contacting the principal investigators, 38 studies were excluded and seven studies were considered eligible for inclusion. Retrospective studies, trials randomising oocytes or embryos, trials allocating alternately and double publications were excluded from the review (See Figure 1).
1.
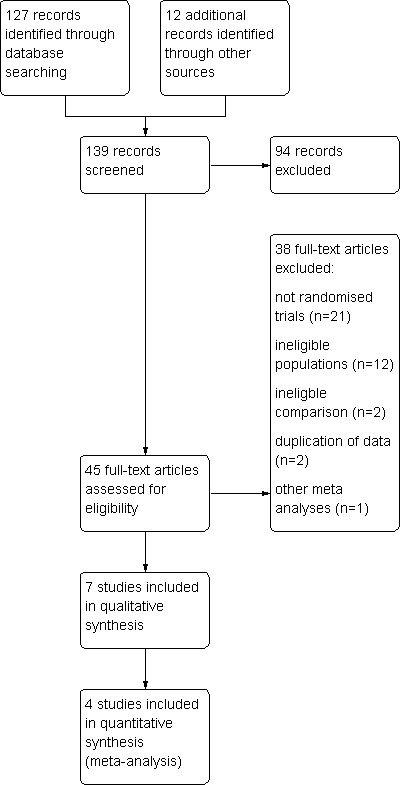
Study flow diagram.
Included studies
Seven studies with a total of 2422 analysed participants were included in this systematic review (see Characteristics of included studies). Not all published data could be used for analysis (see Appendix 6), resulting in a meta‐analysis of the data from four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009), with a total of 1382 participants. The other three included trials are to date only reported as conference abstracts and did not specify group sizes (Iacobelli 2008; Minasi 2003; Silverberg 2005). One of these studies (Minasi 2003) only reported on one of the additional outcome measures of this review and none of the primary or secondary outcome measures. Even though the data from these studies could not be extracted for meta‐analysis, they are still included in this systematic review for completeness.
Study characteristics
All included studies were randomised controlled trials comparing the results of an intervention group with low oxygen concentration (˜ 5%) and a control group with an atmospheric oxygen concentration (˜ 20%). Participant recruitment was performed in a prospective manner in all included trials. Two studies recruited participants consecutively (Kovacic 2009; Meintjes 2009). The manner of recruitment in the other studies was unclear.
Six studies were performed in a single centre (Kovacic 2009; Meintjes 2009; Minasi 2003; Sepulveda 2011; Silverberg 2005; Waldenström 2009) and one study was a multicentre trial (Iacobelli 2008). Two studies were performed in the United States of America (Meintjes 2009; Silverberg 2005), one in Italy (Minasi 2003), one both in Italy and the United States (Iacobelli 2008), one in Slovenia (Kovacic 2009), one in Peru (Sepulveda 2011) and one in Sweden (Waldenström 2009).
Five studies used strict inclusion criteria for their participant selection (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009). Four of these also used strict exclusion criteria for participant selection (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Sepulveda 2011). Examples of these selection criteria include the number of mature oocytes, the number of previous treatment failures, the participant’s age and the number of fertilised oocytes (see Characteristics of included studies). Three studies performed an a priori power calculation to determine sample size (Kovacic 2009; Meintjes 2009; Sepulveda 2011), one study did not (Waldenström 2009), and it was unclear whether a power calculation was performed in the other trials.
Three of the included trials were conference abstracts (Iacobelli 2008; Minasi 2003; Silverberg 2005) and four trials were published as full articles (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009).
Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Silverberg 2005) reported that they were free of commercial funding. The other studies did not report on funding.
Participants
Seven studies compared embryo culture at a low oxygen concentration with embryo culture at atmospheric oxygen concentration. A total of 2653 participants were randomised into either the low oxygen or the atmospheric oxygen arm of the trials, but only the data from 2422 participants have been analysed. An intention‐to‐treat principle was not adhered to in all included trials; see the incomplete outcome data sections under Characteristics of included studies for information on which trials performed an intention‐to‐treat analysis or reported whether there was any loss to follow‐up of participants, or both. Three studies allowed only a single treatment cycle per participant (Meintjes 2009; Minasi 2003; Sepulveda 2011). The number of treatment cycles per participant was unclear in the other studies.
The age of the participants was reported as a mean with standard deviation. The mean age ranged from 33.4 to 42.6 years.
One study (Kovacic 2009) reported the primary cause of subfertility of the study participants. None of the studies reported the mean duration of subfertility for participants prior to the study, or whether it concerned primary or secondary subfertility.
Three studies (Kovacic 2009; Meintjes 2009; Waldenström 2009) reported the (mean) number of previous subfertility treatments that the participants had undertaken, either as an inclusion criterion or as a study measure.
One study (Silverberg 2005) only reported on participants undergoing IVF and two studies (Iacobelli 2008; Kovacic 2009) only reported on participants undergoing ICSI. In four trials (Meintjes 2009; Minasi 2003; Sepulveda 2011; Waldenström 2009) participants were undergoing either IVF or ICSI.
Age analysis was performed in two studies (Kovacic 2009; Meintjes 2009). Kovacic 2009 compared participants of < 40 years of age with participants of > 40 years. Meintjes 2009 divided participants into subgroups of ≤ 34 years, 35 to 37 years, 38 to 40 years and > 40 years of age.
Interventions
Three studies (Iacobelli 2008; Minasi 2003; Waldenström 2009) performed randomisation of participants to either the treatment or the control arm of their trial prior to commencement of the treatment cycle. Three studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011) performed randomisation between the commencement of treatment and before the check of oocyte fertilisation one day after ovum pick‐up. The timing of randomisation was unclear in one study (Silverberg 2005).
The seven included trials did not all compare embryo culture at exactly the same oxygen concentrations. For instance, Waldenström 2009 compared culture at 5% oxygen with culture at 19% oxygen and Iacobelli 2008 compared 7% with 21%. Even though the oxygen concentration slightly varied between the included trials, they were still regarded to form similar comparison groups.
Two studies (Minasi 2003; Silverberg 2005) performed embryo transfer at an early stage of embryo development (Day 2 to 3). Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009) performed embryo transfer late in embryo development (Day 4 and later). One study (Iacobelli 2008) did not report the stage of embryo development at which the transfer was performed. The data from these trials have been analysed separately for the subgroup analysis on timing of intervention.
One study (Kovacic 2009) included both fresh and frozen‐thawed embryos in the analysis while three studies (Meintjes 2009; Sepulveda 2011; Waldenström 2009) only transferred fresh embryos. The other studies were unclear regarding inclusion of embryos after following a frozen‐thaw protocol. Of note, five studies (Kovacic 2009; Meintjes 2009; Silverberg 2005; Sepulveda 2011; Waldenström 2009) also reported the cryopreservation rate of embryos that were not used for transfer.
One study (Sepulveda 2011) only reported on oocyte recipient participants and one study (Meintjes 2009) reported on both donor and non‐donor oocytes. The policy on oocyte donation was unclear for the other studies.
One study (Iacobelli 2008) did not report the mean number of embryos per treatment cycle. All the other studies transferred multiple embryos, with a mean range of 1.23 to 2.6 embryos per treatment cycle. None of the included trials followed a policy of single embryo transfer.
One trial (Iacobelli 2008) combined the comparison of two different oxygen concentrations with a comparison of two different culture media, GIII® and Quinn’s culture media system®, resulting in four comparison groups. The data from this trial were grouped into two comparison groups (low versus high oxygen concentration) for this systematic review. One trial (Meintjes 2009) used the GIII series medium® as culture medium, two trials (Kovacic 2009; Waldenström 2009) used BlastAssist system media® as culture medium, one trial (Minasi 2003) used IVF/RS1 media® as culture medium, one trial used Global media® (Sepulveda 2011) and one trial (Silverberg 2005) used Sage cleavage medium® for embryo culture.
Pregnancy was determined by the presence of a fetal heart beat on ultrasound scan in two studies (Kovacic 2009; Waldenström 2009). Four studies (Kovacic 2009; Meintjes 2009; Minasi 2003; Waldenström 2009) used ultrasound scans to determine pregnancy by demonstrating gestational sacs. One study (Waldenström 2009) also used a biochemical pregnancy test to determine pregnancy, and one study (Sepulveda 2011) only used a biochemical pregnancy test. The method of pregnancy determination was unclear in the other studies (Iacobelli 2008; Silverberg 2005).
Outcomes
Four studies (Kovacic 2009; Meintjes 2009; Silverberg 2005; Waldenström 2009) reported on live birth rates (see Characteristics of included studies). However, the live birth rate data from Silverberg 2005 could not be extracted for meta‐analysis because live birth was reported as percentages only. The study of Kovacic 2009 was published before all participants gave birth. However, the original investigator provided us with the live birth data after making contact.
Two studies (Kovacic 2009; Silverberg 2005) reported ongoing pregnancy rate. However, the data from one of these trials (Silverberg 2005) could not be extracted because the results were reported as percentages, without giving the number of participants in each group. In addition, the live birth rate data from Meintjes 2009 and Waldenström 2009 were also pooled as ongoing pregnancy rate since these studies did not report on ongoing pregnancies.
Four studies were included in the meta‐analysis on clinical pregnancy rate. Five studies (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009) reported on this outcome measure. However, the data from one of these (Iacobelli 2008) could not be extracted because the results were reported as percentages, without giving the number of participants in each group.
Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009) reported on multiple pregnancy rate. Kovacic 2009 and Meintjes 2009 did not publish the multiple pregnancy data but reported them after contact.
Three studies (Kovacic 2009; Meintjes 2009; Waldenström 2009) reported on miscarriage rate. Meintjes 2009 reported these data after contact.
One study (Kovacic 2009) reported on the congenital abnormalities rate after contact with the principal investigator.
Five studies (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Minasi 2003; Sepulveda 2011) reported on implantation rates. Data from two studies (Iacobelli 2008; Minasi 2003) could not be extracted due to ambiguities about group sizes. Because implantation rate is reported as number of implantations per embryo transfer, it could not be part of the meta‐analysis that uses randomised women as the unit of analysis. These outcome data were extracted for completeness.
Six studies (Iacobelli 2008; Kovacic 2009; Minasi 2003; Sepulveda 2011; Silverberg 2005; Waldenström 2009) reported on embryo development rate. However, the data from three studies (Iacobelli 2008; Minasi 2003; Silverberg 2005) could not be extracted because the results were reported as percentages only. Waldenström 2009 reported embryo development as high quality blastocysts. Minasi 2003 reported embryo development as the percentage of compacted embryos. These data on embryo development were extracted for completeness and are not part of the meta‐analysis because of a difference in the unit of analysis with the primary and secondary outcome measures.
Five studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Silverberg 2005; Waldenström 2009) reported on the cryopreservation rate. However, the data from two studies (Silverberg 2005; Waldenström 2009) could not be extracted because the results were reported as percentages only. Two studies (Kovacic 2009; Meintjes 2009) reported the cryopreservation rate after contact with the principal investigator. These data on cryopreservation were extracted for completeness and are not part of the meta‐analysis because of the difference in the unit of analysis with the primary and secondary outcome measures.
Six included studies (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Minasi 2003; Sepulveda 2011; Waldenström 2009) reported on outcome measures which were not analysed in this systematic review. Iacobelli 2008 reported fertilisation rate. Kovacic 2009 reported biochemical pregnancy rate, clinically used embryos per cleaved embryos and frozen blastocysts per cleaved embryos. In addition to live birth rate, Meintjes 2009 also reported live birth implantation rate using the number of transferred embryos as the unit of analysis. Birth weights were reported as well. Minasi 2003 further reported the following outcome measures: fertilisation rate, cleavage rate and the percentage of fragments. Sepulveda 2011 reported on fertilisation rate, the blastocyst expansion rate and the MCI Grade A blastocysts rate. Waldenström 2009 also reported fertilisation rate.
The studies that reported outcome measures in such a way that they could not be incorporated in this review have been summarised in Appendix 6. The principal investigators who responded to our additional data queries and the data they provided are summarised in Appendix 7.
Excluded studies
Thirty‐eight studies were excluded (see Characteristics of excluded studies and Figure 1). Twenty‐one studies were excluded because they did not use a truly randomised design (Bahceci 2005; Curnelle 2010; Dasig 2006; Dumoulin 1995; Dumoulin 1999; Gvakharia 2008; Higdon 2008; Hoff 2008; Kasterstein 2008; Kea 2005; Kea 2007; Khabani 2008; Kim 2005; Kim 2007; Kovacic 2008; Meijers 1998; Nanassy 2010; Portmann 2008; Prados 2010; Reeka 2004; Sjoblom 2008). Twelve studies were excluded because they randomised cycles, oocytes or embryos instead of participants (Cieslak Janzen 2008; Ciray 2008; Ciray 2009; Gamiz Izquierdo 2010; Graham 2010; Loutradi 2009; Madashi 2010; Mitsoli 2011; Park 2001; Petersen 2005; Ryu 2010; Tejera 2010). Two studies (Fujiwara 2007; Mancebo 2009) were excluded because it appeared that they did not consider the comparison of interest, and one study (Meintjes 2000) was excluded because of double publication of the data. Also reported under Characteristics of excluded studies are two publications of a meta‐analysis on the effect of embryo culture under low oxygen concentrations (Sobrinho 2011), which has also been published as a conference proceeding (Oliveira 2011). See Agreements and disagreements with other studies or reviews for further details on this meta‐analysis.
Risk of bias in included studies
Based on the descriptions provided within the original publications and after contact with the original investigators, the potential risks of bias seemed moderate (Figure 2, Figure 3). See Appendix 7 for information on which ambiguities could be resolved after contact.
2.
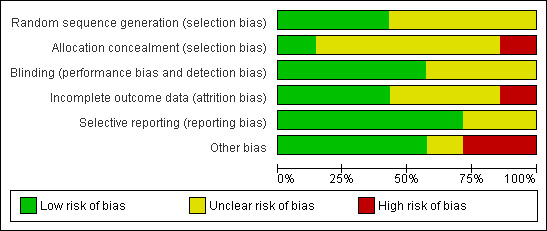
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
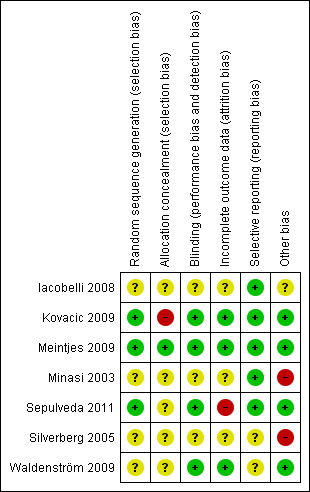
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One study (Kovacic 2009) used minimisation as the method for randomising participants, which is a method of adapted stratified sampling that can be used to make small groups closely similar with respect to several characteristics. Even though there is debate whether this is an adequate method of randomisation it has been considered to be adequate by the authors of this review, because there was a random component described in the article.
After contact with the original investigators, Meintjes 2009 reported randomisation by using stratified block randomisation where participants were allocated to the next available physician and age‐group block as they came for treatment. Sepulveda 2011 randomised their participants according to a computerised randomisation list. In the original manuscript the authors reported randomisation without explaining the actual method. Further information was supplied by the authors after contact. The remaining four studies (Iacobelli 2008; Minasi 2003; Silverberg 2005; Waldenström 2009) only reported that participants were randomly divided into a treatment and control group, without explaining the actual method of randomisation.
Allocation concealment was reported in two studies (Kovacic 2009; Meintjes 2009). In Kovacic 2009 it appeared to be inadequate. Allocation concealment could not be ensured because of the use of a computerised randomisation list. There was no mentioning of central computer randomisation or serially numbered, sealed opaque envelopes; nor did the principal investigator state that allocation concealment was ensured, after contact. Meintjes 2009 reported allocation concealment by the use of sequentially numbered identical containers, which was rated to be a proper method of allocation concealment.
The remaining studies did not report allocation concealment clearly. See Characteristics of included studies and Figure 2, Figure 3.
Blinding
Blinding to prevent performance bias was performed in four (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009) of the six studies. In Kovacic 2009 participants did not know to what arm of the study they were allocated, but no blinding was performed for clinicians and nurses or scientists. In Meintjes 2009 clinicians and nurses and participants, but not the scientists, were blinded for the participants’ allocation. Both participants and the clinicians and nurses were blinded to allocation in Sepulveda 2011. In Waldenström 2009 the clinicians or nurses, or both, treating the participants were blinded in regards to the arm of the study the participants were allocated to, but blinding of scientists or participants was unclear. Blinding to prevent detection bias (blinding of the scientists assessing the outcome measures) has not been described in any of the included trials.
Incomplete outcome data
Four studies (Kovacic 2009; Meintjes 2009; Silverberg 2005; Waldenström 2009) reported live birth rates. Kovacic 2009 did not report live birth rate in the original article. However, after contacting the original investigator the data on this primary outcome measure were obtained.
One study (Kovacic 2009) reported the length of follow up per participant after contact. In three studies (Meintjes 2009; Silverberg 2005; Waldenström 2009) the length of follow up could be determined indirectly since live births were reported. However, this was not clearly stated in the article.
Loss to follow up was described in four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009).
An intention‐to‐treat analysis was performed in one study (Kovacic 2009).
Overall, three studies (Kovacic 2009; Meintjes 2009; Waldenström 2009) have been classified as complete in reporting the outcome data, despite the fact that Meintjes 2009 and Waldenström 2009 did not perform an intention‐to‐treat analysis, nor that Kovacic 2009 did not report live birth rates in the original article. All three studies reported the loss of participants, length of follow up and the live birth rate, which is the primary outcome measure of this systematic review.
Selective reporting
Five studies (Iacobelli 2008; Kovacic 2009; Meintjes 2009; Minasi 2003; Sepulveda 2011) reported their outcome measures in a prespecified manner. Some studies reported more outcome measures than announced in the 'Introduction' or 'Material and Methods' sections of their articles, but this was not considered to be a source of bias. Two studies (Silverberg 2005; Waldenström 2009) did not specify the outcome measures beforehand and were therefore assessed as unclear.
Other potential sources of bias
See Assessment of risk of bias in included studies on how the risk of other sources of bias was assessed.
Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009) reported multiple pregnancies while transferring multiple embryos per treatment cycle. Therefore, these four studies have been regarded to be free of other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Live birth rate
Three studies (Kovacic 2009; Meintjes 2009; Waldenström 2009), with a total of 1291 participants, reported on live birth rate. The combined data showed an increased live birth rate with embryo culture under low oxygen concentrations (OR 1.39; 95% CI 1.11 to 1.76; P = 0.005; I2 = 0%) (Analysis 1.1). See Figure 4. This would mean that a typical clinic could improve from a 30% live birth rate using an atmospheric oxygen concentration to somewhere between 32% and 43% by using a low oxygen concentration.
1.1. Analysis.
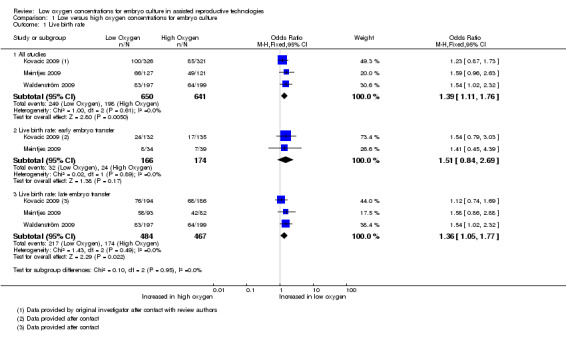
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 1 Live birth rate.
4.
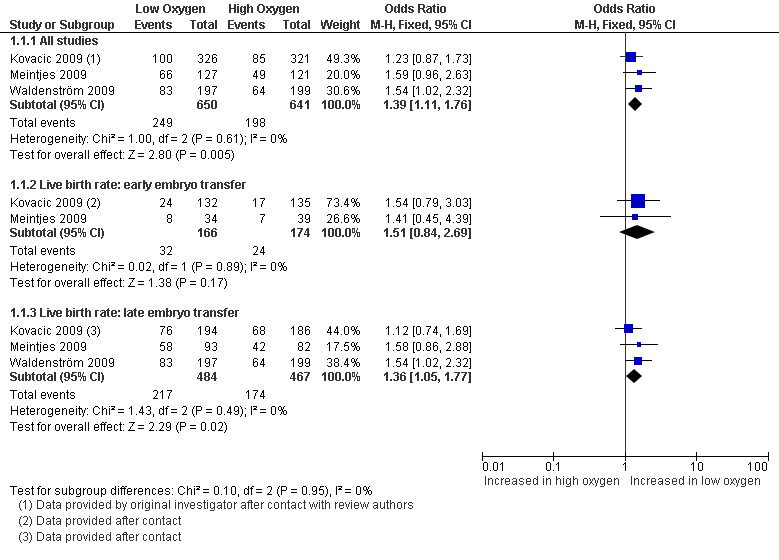
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo cultureLive birth rate, outcome: 1.1 Live birth rate.
Sensitivity analysis
Two of the planned sensitivity analyses could be performed. These showed that the evidence of a positive treatment effect of embryo culture under low oxygen concentrations on the live birth rate is based on the results of studies with a risk of bias (in one or more of the following domains: adequate sequence generation, allocation concealment and blinding).
| Sensitivity analysis | Results |
| Exclusion of trials without a low risk of bias | Kovacic 2009 and Waldenström 2009 excluded, resulting in OR 1.59 (95% CI 0.96 to 2.63; P = 0.07; N = 248) |
| Adoptation random‐effects model | OR 1.40; 95% CI 1.11 to 1.76; P = 0.005; I2 = 0%; N = 1291 |
Subgroup analysis
Live birth rate (grouped by timing of embryo transfer) (Analysis 1.1)
When pooling only the data from the two trials that performed embryo transfers at Day 3 of embryo development (Kovacic 2009; Meintjes 2009), with a total of 340 participants, there was no evidence of a difference in the live birth rate (OR 1.51; 95% CI 0.84 to 2.69; P = 0.17; I2 = 0%) between low oxygen and atmospheric oxygen concentrations.
When pooling only the data from the three trials that performed late embryo transfers (Kovacic 2009; Meintjes 2009; Waldenström 2009), with a total of 951 participants, there was evidence of a treatment effect in favour of embryo culture under low oxygen concentrations (OR 1.36; 95% CI 1.05 to 1.77; P = 0.02; I2 = 0%).
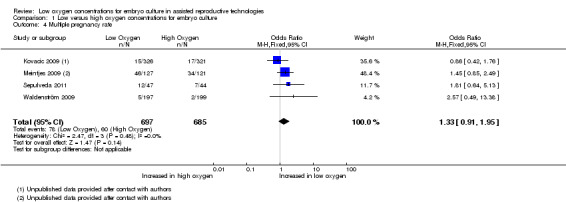
Ongoing pregnancy rate
Only one study (Kovacic 2009) reported on ongoing pregnancies, and these were pooled together with the live birth data from two other studies (Meintjes 2009; Waldenström 2009) because these trials reported live births without reporting ongoing pregnancies, resulting in three trials with a total of 1291 participants. The combined data showed an increased ongoing pregnancy rate with embryo culture under low oxygen concentrations (OR 1.40; 95% CI 1.11 to 1.77; P = 0.004; I2 = 0%) (Analysis 1.2). See Figure 5.
1.2. Analysis.
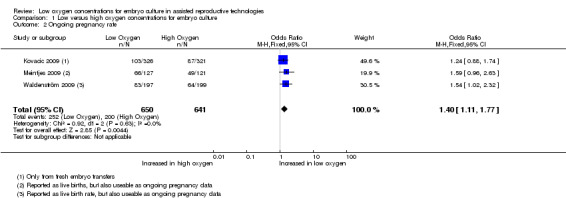
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 2 Ongoing pregnancy rate.
5.
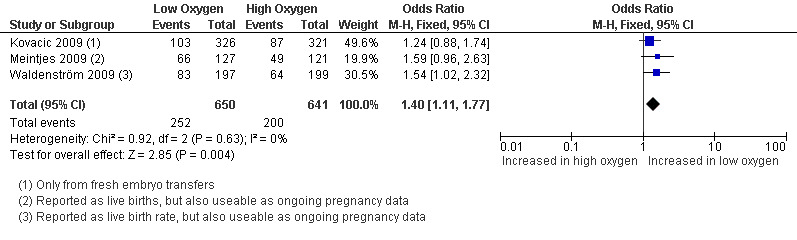
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo cultureLive birth rate, outcome: 1.2 Ongoing pregnancy rate.
Clinical pregnancy rate
Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009), with a total of 1382 participants, reported on clinical pregnancy rate. The combined data showed evidence of an increased clinical pregnancy rate with embryo culture under low oxygen concentrations (OR 1.35; 95% CI 1.08 to 1.67; P = 0.007; I2 = 0%) (Analysis 1.3). Figure 6.
1.3. Analysis.
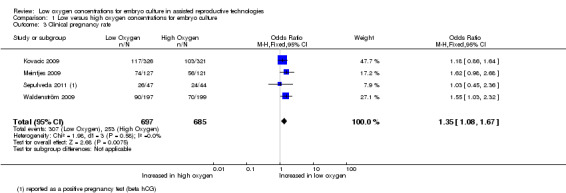
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 3 Clinical pregnancy rate.
6.
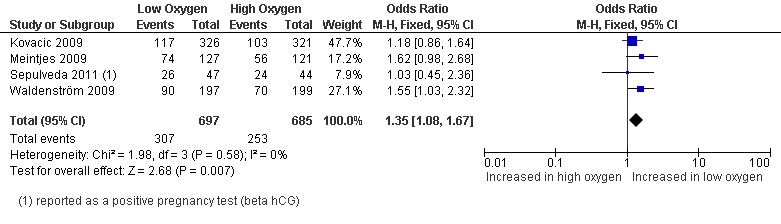
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo culture Live birth rate, outcome: 1.3 Clinical pregnancy rate.
Multiple pregnancy rate
Four studies (Kovacic 2009; Meintjes 2009; Sepulveda 2011; Waldenström 2009), with a total of 1382 participants, reported on the multiple pregnancy rate. The pooled data showed no evidence of a treatment effect from embryo culture under low oxygen concentrations on the multiple pregnancy rate (OR 1.33; 95% CI 0.91 to 1.95; P = 0.14; I2 = 0%) (Analysis 1.4). See Figure 7.
1.4. Analysis.
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 4 Multiple pregnancy rate.
7.
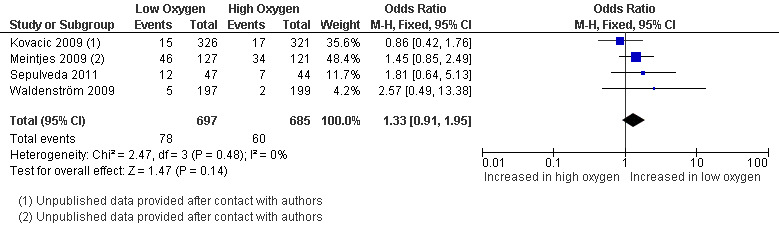
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo culture Live birth rate, outcome: 1.4 Multiple pregnancy rate.
Miscarriage rate
Three studies (Kovacic 2009; Meintjes 2009; Waldenström 2009), with a total of 1291 participants, reported on miscarriage rate. The pooled results found no evidence of a treatment effect from embryo culture under low oxygen concentrations on the miscarriage rate (OR 1.28; 95% CI 0.86 to 1.90; P = 0.22; I2 = 0%) (Analysis 1.5). See Figure 8.
1.5. Analysis.
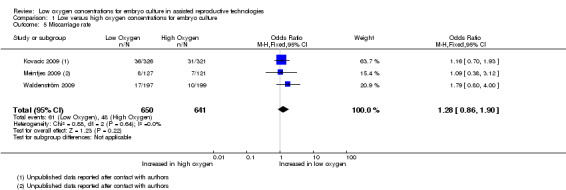
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 5 Miscarriage rate.
8.
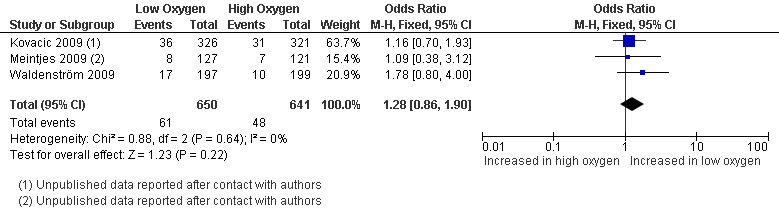
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo culture Live birth rate, outcome: 1.5 Miscarriage rate.
Congenital abnormality rate
One study (Kovacic 2009), with a total of 647 participants, reported on congenital abnormalities. There was no evidence of an increased congenital abnormality rate with embryo culture under low oxygen concentrations (OR 0.20; 95% CI 0.01 to 4.09; P = 0.29) (Analysis 1.6). See Figure 9.
1.6. Analysis.
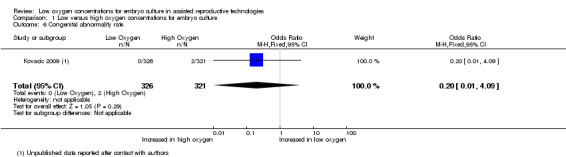
Comparison 1 Low versus high oxygen concentrations for embryo culture, Outcome 6 Congenital abnormality rate.
9.
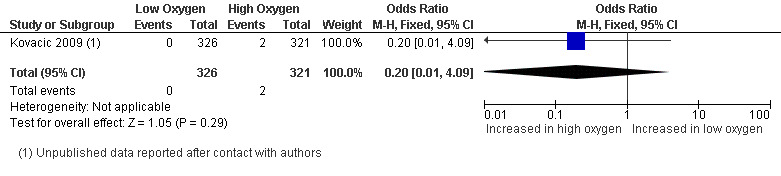
Forest plot of comparison: 1 Low versus high oxygen concentrations for embryo culture Live birth rate, outcome: 1.6 Congenital abnormality rate.
Additional outcome measures
Implantation rate, embryo development rate and cryopreservation rate could not be part of this meta‐analysis due to differences in the denominator with the other outcome measures. However, these data have been extracted from the included trials for completeness. The results are summarised in Table 7.
Discussion
Summary of main results
This meta‐analysis of the best available evidence indicates that culturing embryos under a low oxygen concentration (˜ 5%) has a clinical benefit. Historically, in vitro fertilisation (IVF) laboratories have been culturing embryos under atmospheric oxygen concentrations (˜ 20%), even though embryos are exposed to oxygen concentrations of 2% to 8% in the human oviduct and uterus. In recent years, multiple studies (45) have been performed on the effect of culturing human embryos under low oxygen concentrations, demonstrating both positive and inconclusive results. Careful consideration of these studies resulted in a total of seven studies that were eligible for inclusion in this systematic review. The data from four of these, involving a total of 1382 participants, could be incorporated in the meta‐analysis on embryo culture under low oxygen concentrations.
A beneficial treatment effect was identified for live birth rate (the primary outcome measure of this review) (see Analysis 1.1). However, this result is slightly weakened because it is based on studies with a relative risk of bias.
A similar beneficial treatment effect was identified for the secondary outcome measures of ongoing pregnancy rate and clinical pregnancy rate (Analysis 1.2, Analysis 1.3). Of note, these results are from the meta‐analysis of the same studies that reported on live birth and the same risks of bias apply.
This meta‐analysis did not find evidence of a treatment effect on multiple pregnancy rate, miscarriage rate and congenital abnormality rate (Analysis 1.4, Analysis 1.5, Analysis 1.6). Furthermore, data extraction of implantation rate, cryopreservation rate and embryo quality suggested better outcomes when embryos are cultured in low oxygen concentrations.
In addition to the meta‐analysis, a subgroup analysis was performed with the live birth rate data to determine whether the effect of oxygen concentration is stage specific (early cleaving embryos or blastocysts). Evidence of a beneficial treatment effect was only found for late embryo transfers (blastocyst) and a trend towards a beneficial treatment effect was found for early transfers. A possible explanation for this result might be that the effect is more pronounced in the blastocyst group because of increased length of exposure to the low oxygen concentration, and a smaller time of exposure may also equate to a smaller effect. However, a more likely explanation is that the difference in results is due to the difference in group sizes, with the majority of the transferred embryos being blastocysts. Based on this difference in group sizes, no robust conclusions can be drawn from these results.
Overall completeness and applicability of evidence
Even though seven studies were included in this systematic review, data could only be extracted from four of these because the other three studies only reported their outcomes as percentages without reporting the group sizes. We tried to gather these data by contacting the principal investigators but up till now we have not received an answer to our requests.
Three of the included studies reported on live birth rate, the primary outcome measure of this review. One of these studies (Kovacic 2009) did not actually publish these data, but reported them after contact with the authors of this review. Ongoing pregnancy was reported in two studies but could only be extracted from one study (Kovacic 2009); the live birth data from the other studies were therefore extrapolated as ongoing pregnancy data as well.
Also of note is the lack of reporting on congenital abnormality rate, with only one study reporting on this outcome measure. There is increasing evidence that the in vitro environment of human embryos can affect the health of offspring, and thus it is important to gather data on congenital abnormalities and the offspring's health (Dumoulin 2010). Therefore, there is a need for studies reporting on these issues and also on data on prematurity.
All original investigators of the seven included studies have been contacted regarding data queries. In total, we received responses from three study authors, which helped to resolve queries regarding the data and study characteristics. Ambiguities remain regarding the other four studies.
Quality of the evidence
Seven studies with a total of 2422 participants are included in this review. The data from only four of these studies, with a total of 1382 participants, could be extracted for meta‐analysis.
The included and pooled studies contained methodological limitations and differences in baseline characteristics of participants. Some studies allowed their participants to enrol and undertake multiple treatment cycles in the trial while others included each patient for only a single cycle. Some studies sampled participants consecutively, others did not describe their methods of sampling. Only three of the included trials performed a power calculation a priori to determine sample size. Causes and durations of subfertility largely remained unreported. The number of previous treatment cycles, which has an influence on the success rate of IVF and ICSI, was reported in three of the four analysed studies. Most studies performed randomisation prior to fertilisation check, which creates a study population that is representative for the subfertile population. All included trials adhered to a multiple embryo transfer policy, of which some resulted in multiple pregnancies. However, it was not possible to retrace which live births resulted from singleton pregnancies and which from multiple pregnancies. Therefore, it cannot be stated with 100% certainty that the number of women who had a live birth is correct, which decreases the quality of the evidence.
Regarding the methods of randomisation and allocation concealment, most studies were not clear in their published articles. Some of these ambiguities were resolved by contacting the original investigators but it remained unclear for the majority of the included studies since we received no answers from the principal investigators of those studies. Blinding was performed in all four studies that are part of the meta‐analysis, although some studies blinded only the participants and others blinded both the participants and the clinicians. Most studies reported their outcomes in a prespecified fashion. Live births and length of follow up were reported in three out of four of the analysed studies, although an intention‐to‐treat analysis was reported in only one study. Furthermore, some studies were ambiguous regarding commercial funding of their trials.
The additional outcome measures implantation rate, embryo development rate and cryopreservation rate could only be assessed per embryo cultured or transferred and were therefore not incorporated into this meta‐analysis. However, these data were extracted for completeness.
Potential biases in the review process
The authors of this systematic review decided to investigate the difference between embryo culture under high and low oxygen concentrations; 20% oxygen concentration was considered to be high concentration and 5% was considered to be low concentration. However, the oxygen concentrations varied between individual studies, for instance one study compared a 7% to 21% oxygen concentration (Iacobelli 2008) while the other compared 5% to 19% (Waldenström 2009). It was decided to group different oxygen concentrations under either a high or low oxygen concentration because under normal circumstances the oxygen concentration varies as well, for instance in the human oviduct it varies between 2% and 8%. Nevertheless, this variation potentially induces heterogeneity.
Not all of the planned sensitivity analyses could be performed with the included data, for instance we did not exclude trials with outlying results as there was only a small level of heterogeneity between the results within the meta‐analysis.
As stated in the protocol, the aim was to count multiple live births as one live birth event. However, it was not possible to do this with the data included into this review since the data on singleton and multiple live births were not reported. As stated above (Quality of the evidence), this should be taken into account regarding the conclusions based on the evidence.
Agreements and disagreements with other studies or reviews
A reasonable number of clinical trials (including randomised controlled trials) have been published on the subject, with varying results. Some trials reported a clinical benefit and others found no differences in clinical outcomes of low oxygen compared to high oxygen concentrations. To our knowledge, there is one other meta‐analysis on the effect of culturing embryos under low oxygen concentrations (Sobrinho 2011), which has also been presented as a conference abstract (Oliveira 2011). The meta‐analysis includes seven trials and reports on fertilisation rate, implantation rate and ongoing pregnancy rate. Two of the included trials (Kovacic 2009; Meintjes 2009) are also included in this systematic review. The other five trials (Bahceci 2005; Ciray 2009; Dumoulin 1999; Kea 2007; Kovacic 2008) were not included in our systematic review since they randomised oocytes instead of participants. The meta‐analysis found evidence of a positive treatment effect for the implantation rate with embryo culture under low oxygen concentrations when embryo transfer was performed on Day 5 to 6 of embryo development, which is compatible with the findings of our systematic review. However, overall they did not find evidence of a beneficial treatment effect of embryo culture under low oxygen concentrations on the implantation and ongoing pregnancy rate, which differs from the results of our meta‐analysis.
Authors' conclusions
Implications for practice.
The results of this meta‐analysis of four trials, with a total of 1382 participants, suggest that culturing embryos under low oxygen concentrations improves success rates after IVF and ICSI. A beneficial treatment effect was found for the live birth rate in favour of culturing embryos under low oxygen concentrations over atmospheric oxygen concentrations (OR 1.39; 95% CI 1.11 to 1.76). This would mean that a typical clinic could improve their IVF and ICSI success rates from a 30% live birth rate using atmospheric oxygen concentration to somewhere between 32% and 43% by using a low oxygen concentration. This beneficial treatment effect was also found for clinical pregnancy rate and ongoing pregnancy rate. There was no evidence that culturing embryos under low oxygen concentrations resulted in higher numbers of adverse events such as multiple pregnancies, miscarriages and congenital abnormalities.
Implications for research.
More and larger multicentre randomised controlled trials of good quality and also focusing on issues such as the health of the offspring are needed to get a better weighted overall view on the treatment effect of embryo culture under low oxygen concentrations for assisted reproductive technologies.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2011 | Amended | Changed subgroup analysis. Early embryo transfers are up to day 3, late transfers are from day 4 onwards. |
Acknowledgements
The authors of this systematic review would like to thank Dr B Kovacic, Dr M Meintjes, Dr D Nogueira, Dr. Ryu, Dr Priddle, Dr Sjoblom and Dr Sepulveda, authors of included and excluded studies, for aiding us by sending additional data and information on their studies.
Furthermore, we would like to thank Jane Clarke, Marian Showell and Jane Marjoribanks from the Cochrane Menstral Disorders and Subfertility Group (MDSG) for their help with this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
Searched up to 04‐11‐2011
1 exp Embryo Culture Techniques/ (33) 2 (embryo$ adj3 in vitro).tw. (331) 3 (blastocyst$ adj3 in vitro).tw. (24) 4 (embryo$ adj3 culture$).tw. (173) 5 (blastocyst$ adj3 culture$).tw. (42) 6 (embryo$ adj3 medi$).tw. (76) 7 (blastocyst$ adj3 medi$).tw. (19) 8 (incubat$ adj4 embryo$).tw. (6) 9 (incubat$ adj4 blastocyst$).tw. (0) 10 or/1‐9 (527) 11 (oxygen adj3 tension$).tw. (603) 12 (oxygen adj3 concentrat$).tw. (449) 13 (oxygen adj3 atmosphere$).tw. (38) 14 (low$ adj3 oxygen).tw. (502) 15 (reduc$ adj3 oxygen).tw. (642) 16 5% oxygen.tw. (18) 17 physiologic.tw. (2273) 18 or/11‐17 (4254) 19 10 and 18 (11) 20 limit 19 to yr="2010 ‐Current" (1)
Appendix 2. MEDLINE search strategy
Searched up to 04‐11‐2011
1 exp Embryo Culture Techniques/ (1361) 2 (embryo$ adj3 in vitro).tw. (8577) 3 (blastocyst$ adj3 in vitro).tw. (1192) 4 (embryo$ adj3 culture$).tw. (13047) 5 (blastocyst$ adj3 culture$).tw. (1101) 6 (embryo$ adj3 medi$).tw. (2130) 7 (blastocyst$ adj3 medi$).tw. (250) 8 (incubat$ adj4 embryo$).tw. (1617) 9 (incubat$ adj4 blastocyst$).tw. (104) 10 or/1‐9 (24062) 11 (oxygen adj3 tension$).tw. (10831) 12 (oxygen adj3 concentrat$).tw. (8971) 13 (oxygen adj3 atmosphere$).tw. (2082) 14 (low$ adj3 oxygen).tw. (8102) 15 (reduc$ adj3 oxygen).tw. (8742) 16 5% oxygen.tw. (351) 17 physiologic.tw. (55043) 18 or/11‐17 (88065) 19 10 and 18 (299) 20 randomised controlled trial.pt. (299815) 21 controlled clinical trial.pt. (81739) 22 randomized.ab. (216055) 23 placebo.tw. (129233) 24 clinical trials as topic.sh. (152163) 25 randomly.ab. (159585) 26 trial.ti. (92531) 27 (crossover or cross‐over or cross over).tw. (49471) 28 or/20‐27 (733494) 29 (animals not (humans and animals)).sh. (3452627) 30 28 not 29 (677972) 31 19 and 30 (11) 32 (2010$ or 2011$).ed. (1107056) 33 31 and 32 (1)
Appendix 3. EMBASE search strategy
Searched up to 04‐11‐2011
1 (oxygen adj3 tension$).tw. (11023) 2 (oxygen adj3 concentrat$).tw. (10430) 3 (oxygen adj3 atmosphere$).tw. (2167) 4 (reduc$ adj3 oxygen).tw. (9336) 5 5% oxygen.tw. (381) 6 (oxygen adj3 level$).tw. (9445) 7 (low$ adj3 oxygen).tw. (8917) 8 incubator$.tw. (3208) 9 exp embryo culture/ (4631) 10 exp embryo transfer/ (15612) 11 exp fertilization in vitro/ (31299) 12 exp intracytoplasmic sperm injection/ (8571) 13 embryo$.tw. (237879) 14 blastocyst$.tw. (14039) 15 or/1‐8 (46731) 16 or/9‐14 (262222) 17 15 and 16 (1222) 18 Clinical Trial/ (826949) 19 Randomized Controlled Trial/ (289563) 20 exp randomisation/ (53430) 21 Single Blind Procedure/ (13891) 22 Double Blind Procedure/ (101543) 23 Crossover Procedure/ (30153) 24 Placebo/ (175282) 25 Randomi?ed controlled trial$.tw. (59740) 26 Rct.tw. (6464) 27 random allocation.tw. (1020) 28 randomly allocated.tw. (15182) 29 allocated randomly.tw. (1693) 30 (allocated adj2 random).tw. (683) 31 Single blind$.tw. (10753) 32 Double blind$.tw. (116144) 33 ((treble or triple) adj blind$).tw. (234) 34 placebo$.tw. (155319) 35 prospective study/ (163667) 36 or/18‐35 (1119455) 37 case study/ (11206) 38 case report.tw. (197169) 39 abstract report/ or letter/ (770730) 40 or/37‐39 (975395) 41 36 not 40 (1087005) 42 17 and 41 (45) 43 (2010$ or 2011$).em. (1298269) 44 42 and 43 (14)
Appendix 4. PsycINFO search strategy
Searched up to 04‐11‐2011
1 embryo$.tw. (5204) 2 blastocyst$.tw. (39) 3 exp reproductive technology/ (1046) 4 (in vitro fertili?ation or intracytoplasmic sperm injection$).tw. (419) 5 (ivf or icsi).tw. (299) 6 or/1‐5 (6205) 7 (oxygen adj3 tension$).tw. (120) 8 (oxygen adj3 concentrat$).tw. (86) 9 (oxygen adj3 atmosphere$).tw. (45) 10 (reduc$ adj3 oxygen).tw. (153) 11 5% oxygen.tw. (2) 12 (oxygen adj3 level$).tw. (862) 13 (low$ adj3 oxygen).tw. (191) 14 incubator$.tw. (248) 15 or/7‐14 (1565) 16 6 and 15 (23) 17 limit 16 to yr="2010 ‐Current" (3)
Appendix 5. Menstrual Disorders and Subfertility Group Specialised Register search strategy
Searched up to 04‐11‐2011
MDSG search string for SB1283 20.05.10
Keywords CONTAINS "embryo culture techniques" or "embryo culture" or "Embryo" or "Blastocyst" or "IVF" or "ICSI" or "*Embryo Transfer" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection" or Title CONTAINS"embryo culture techniques" or "embryo culture" or "Embryo" or "Blastocyst" or "IVF" or "ICSI" or "*Embryo Transfer" or "in vitro fertilisation" or "in vitro fertilization" or "intracytoplasmic sperm injection"
AND
Keywords CONTAINS "Oxygen" or "oxygen concentration" or "oxygen concentrations" or "oxygen levels" or "oxygen partial pressure" or "oxygen tension" or "oxygen saturation" or "incubation" or "incubator"or "low oxygen" or "atmosphere" or "physiolologic condition" or Title CONTAINS "Oxygen" or "oxygen concentration" or "oxygen concentrations" or "oxygen levels" or "oxygen partial pressure" or "oxygen tension" or "oxygen saturation" or "incubation" or "incubator"or "low oxygen" or "atmosphere" or "physiolologic condition"
Appendix 6. Trials with data not incorporated in this review
| Trial | Unuseable data |
| Iacobelli 2008 | Clinical pregnancy rate, implantation rate, embryo development rate |
| Minasi 2003 | Implantation rate |
| Silverberg 2005 | Ongoing pregnancy rate, embryo development rate, cryopreservation rate |
| Waldenström 2009 | Embryo development rate, cryopreservation rate |
| Sepulveda 2011 | Fertilisation rate, blastulation rate, expanded blastocysts rate, MCI Grade A blastocysts rate |
Appendix 7. Responses to data queries
| Trial | Additional data supplied by original investigator |
| Kovacic 2009 | Live birth rate, multiple pregnancy rate, cryopreservation rate, additional information on study characteristics |
| Meintjes 2009 | Multiple pregnancy rate, miscarriage rate, cryopreservation rate, additional information on study characteristics |
| Sepulveda 2011 | Additional information on study characteristics |
Data and analyses
Comparison 1. Low versus high oxygen concentrations for embryo culture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All studies | 3 | 1291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.11, 1.76] |
| 1.2 Live birth rate: early embryo transfer | 2 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.84, 2.69] |
| 1.3 Live birth rate: late embryo transfer | 3 | 951 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.05, 1.77] |
| 2 Ongoing pregnancy rate | 3 | 1291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.11, 1.77] |
| 3 Clinical pregnancy rate | 4 | 1382 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.08, 1.67] |
| 4 Multiple pregnancy rate | 4 | 1382 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.91, 1.95] |
| 5 Miscarriage rate | 3 | 1291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.86, 1.90] |
| 6 Congenital abnormality rate | 1 | 647 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Iacobelli 2008.
| Methods | Prospective, randomised controlled trial | |
| Participants | 792 participants undergoing ICSI treatment. Inclusion criterion was a maternal age below 40 years and exclusion criterion was the complete absence of mature oocytes. Number of cycles per patient and previous treatments are unclear. | |
| Interventions | Oocytes and embryos were cultured in 6% CO2 and 21% O2 or in 6% CO2, 5% O2 and 89% N2. Timing of embryo transfer unclear. |
|
| Outcomes | Embryo development and quality, fertilisation rate, implantation rate, clinical pregnancies. Outcomes were reported as percentages. |
|
| Notes | Location: Italy Setting: European Hospital Group sizes unclear Study published as conference abstract Funding unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation has not been described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Length of follow up unclear, loss to follow up unclear and ITT analysis unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned on the method section are reported in the result section |
| Other bias | Unclear risk | Embryo transfer policy unclear |
Kovacic 2009.
| Methods | Prospective randomised controlled trial | |
| Participants | Women undergoing ICSI (n=647) with at least one matured oocyte at the time of denudation were allocated into a treatment group (n=326) or a control group (n=321). All women underwent only one treatment cycle. No donor oocytes were used. The criteria for undergoing ICSI were any male infertility, previous total fertilization failure after IVF or the woman’s age being 40 years or older. | |
| Interventions | Culturing of embryos, either at 6% CO2, 5% O2, 89% N2 or at 6% CO2 in air (˜20% O2). Embryo transfer on Day 5. |
|
| Outcomes | Live birth rate, ongoing pregnancy rates (PR), biochemical pregnancies, cumulative PRs, implantation rate and embryo quality (clinically used embryos per cleaved embryos and frozen blastocysts per cleaved embryo). | |
| Notes | Location: Slovenia Setting: University Clinical Center Maribor Additional information of the study has been obtained after contacting the authors No external funding Additional outcomes: Implantation rate: low oxygen: 152/528 high oxygen 136/539 Embryo development rate (on Day 2): low oxygen 1014/1736 high oxygen 727/1742 Cryopreservation rate: low oxygen 488/1736, high oxygen 420/1742 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation with a random component |
| Allocation concealment (selection bias) | High risk | Treatment allocation was not concealed. Allocation concealment could not be ensured because of the use of a computerised randomisation list. There was no mentioning of central computer randomisation, third party randomisation or the use of serially numbered, sealed opaque envelopes, nor did the principal investigator state that allocation concealment was ensured after contact. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the participants were blinded, clinicians and scientist not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The length of follow up, live birth rate, multiple pregnancies and congenital abnormalities were reported after contact with the primary investigator as these results were not available at the time of publication. Loss to follow up has been described and an ITT analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned on the method section are reported in the result section |
| Other bias | Low risk | Multiple pregnancy rate reported with a multiple transfer policy |
Meintjes 2009.
| Methods | Prospective, randomised controlled trial, duration one year | |
| Participants | First cycle patients (n=248) undergoing routine IVF/ICSI treatment with ejaculated sperm allocated into a treatment group (n=127) or a control group (n=121).Oocyte donations are included. Exclusion criteria were: one or more previous IVF cycles without a live birth, the need to use non‐ejaculated sperm (epididymal or testicular), no oocytes retrieved or no fertilisation, PGD or cryobanking of all embryos for any reason. 18 participants were lost to follow up (originally n=248). | |
| Interventions | Gametes and embryos were cultured in an atmosphere of 5% CO2 in air (21% O2) or in 5% CO2, 5% O2, 90% N2. Embryo transfer at Day 5. |
|
| Outcomes | Live births, implantation, clinical pregnancy, birth weights, and blastocyst cryopreservation | |
| Notes | Location: USA Setting: Frisco Institute for reproductive medicine Presbyterian Hospital ARTS Program No commercial funding Additional information over the study was obtained after contacting the authors Additional outcomes: Implantation rate: low oxygen: 122/247, high oxygen 95/267 Cryopreservation rate: low oxygen 99/1115, high oxygen 84/1070 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design where participants were allocated to next available physician/age‐group block as they came for treatment |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by using sequentially numbered, identical containers of identical drugs |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinicians or nurses and participants, but no scientists, were blinded for the allocation of the participants in the two study groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The author provided missing data after request, follow up until delivery, loss to follow up accounted for but no ITT analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned on the method section are reported in the result section |
| Other bias | Low risk | Multiple pregnancies are reported, while adhering to a multiple embryo transfer policy |
Minasi 2003.
| Methods | Prospective, randomised controlled trial | |
| Participants | 140 unselected patients undergoing IVF/ICSI treatment randomised into a treatment group (n=70) and a control group (n=70). Inclusion and exclusion criteria were not stated. Previous treatments are not reported. | |
| Interventions | Oocytes and embryos were cultured at 5% O2 or atmospheric O2 concentration. Embryo transfer at Day 2/3. |
|
| Outcomes | Embryo development until Day 3, fertilisation rate and implantation rate. | |
| Notes | Location: Italy Setting: European Hospital Study published as conference abstract Funding unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Length of follow up unclear, loss to follow up unclear and ITT analysis unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned on the method section are reported in the result section |
| Other bias | High risk | Multiple pregnancy rate is not reported, while multiple embryos are transferred |
Sepulveda 2011.
| Methods | Prospective, randomised controlled trial | |
| Participants | 100 recipients from an oocyte donation program, undergoing either IVF or ICSI were randomised into a treatment group (n=47) or a control group (n=44). 9 participants were excluded for medical reasons after randomisation. All oocytes derived from donor women. | |
| Interventions | Oocytes and embryos were cultured at 5% O2 or atmospheric (20%) O2 concentration. Embryo transfer was performed on Day 5/6 of embryo development. |
|
| Outcomes | Clinical pregnancy rate (reported as positive beta hCG pregnancy test), multiple pregnancy rate, implantation rate, embryo development rate, cryopreservation rate, fertilisation rate, blastocyst expansion rate and MCI Grade A blastocysts rate. | |
| Notes | Location: Lima, Peru Setting: Grupo PRANOR de Reproduccion Asistida Additional information regarding the study was obtained after contacting the authors No commercial funding Additional outcomes: Implantation rate: low oxygen: 38/91, high oxygen 32/87 Embryo development rate: low oxygen 205/370, high oxygen 161/385 Cryopreservation rate: low oxygen 114/370, high oxygen 74/385 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into treatment or control group according to a computerised randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment has not been specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinicians and participants were blinded for allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow up has been accounted for. No live births reported, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in a prespecified fashion |
| Other bias | Low risk | Reporting multiple pregnancies whilst adhering to a multiple embryo transfer policy |
Silverberg 2005.
| Methods | Prospective, randomised controlled trial | |
| Participants | Patients undergoing IVF (n=126). Inclusion and exclusion criteria were not stated. Information on previous treatments is not provided. | |
| Interventions | Embryos were grown in either 6% CO2, 5% O2 and 89% N2 or in 5% CO2 in air. Embryo transfer at Day 2/3. |
|
| Outcomes | Embryo development, cryopreservation rate and ongoing/delivered pregnancy rate. | |
| Notes | Location: USA Setting: Texas Fertility Center Group sizes not reported Study published as conference abstract No commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation has not been described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Length of follow up unclear, loss to follow up unclear and ITT analysis unclear |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | High risk | Multiple pregnancy rate is not reported, while multiple embryos are transferred |
Waldenström 2009.
| Methods | Prospective, randomised controlled trial | |
| Participants | 600 women undergoing IVF with at least 5 fertilised oocytes were randomised into a treatment group (n=197) and a control group (n=199) participants. 173 participants were excluded because they did not meet the inclusion criterion of at least 5 fertilised oocytes and 31 participants withdrew from the trial. Unclear whether oocyte donations were included. The patients had previous treatments but no frozen oocytes were used for this trial. | |
| Interventions | Blastocyst culture in atmospheres with either 6% CO2 in air (˜19% O2) or 6% CO2, 5% O2, 89% N2. Embryo transfer at Day 5. |
|
| Outcomes | Live birth rate, clinical pregnancy rate (reported as viable pregnancies), multiple pregnancies, miscarriages, fertilization rate, embryo development (high quality blastocysts) and cryopreservation rate. | |
| Notes | Location: Sweden Setting: In Vitro Fertilization Unit, Falun Hospital Funding is unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The clinician was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow up until delivery, loss to follow up accounted for, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Results are not described in a prespecified manner |
| Other bias | Low risk | Multiple pregnancy rate is reported with a multiple embryo transfer policy |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bahceci 2005 | Quasi‐randomised study, according to day of admission |
| Cieslak Janzen 2008 | Quasi‐randomised study, dividing oocytes |
| Ciray 2008 | Study population is oocytes |
| Ciray 2009 | Study population: oocytes |
| Curnelle 2010 | Quasi‐randomisation alternation between treatments |
| Dasig 2006 | Not a truly randomised design |
| Dumoulin 1995 | Study population: oocytes/embryos |
| Dumoulin 1999 | Study population: oocytes/embryos |
| Fujiwara 2007 | Not the comparison of interest |
| Gamiz Izquierdo 2010 | Randomisation of cycles |
| Graham 2010 | Study population: oocytes |
| Gvakharia 2008 | Study population: oocytes/embryos, retrospective study |
| Higdon 2008 | Study population: gametes, retrospective study |
| Hoff 2008 | Study population:embryos, retrospective study |
| Kasterstein 2008 | No randomised allocation |
| Kea 2005 | Quasi‐randomised study, study population: oocytes |
| Kea 2007 | Quasi‐randomised study, study population: oocytes |
| Khabani 2008 | Study population:embryos, retrospective study |
| Kim 2005 | Study population:oocytes |
| Kim 2007 | Not a truly randomised design |
| Kovacic 2008 | Quasi‐randomisation of oocytes |
| Loutradi 2009 | Randomisation of oocytes |
| Madashi 2010 | Randomisation of embryos |
| Mancebo 2009 | No relevant comparison |
| Meijers 1998 | Not a truly randomised design |
| Meintjes 2000 | Duplication of data (data also published in Meintjes 2009) |
| Mitsoli 2011 | Randomisation of oocytes, part of the data have also been published in Loutradi 2009 |
| Nanassy 2010 | Not a truly randomised design |
| Oliveira 2011 | Meta analysis of published data on embryo culture under low oxygen concentrations, conference abstract of Sobrinho 2011 |
| Park 2001 | Randomisation of oocytes instead of couples/participants |
| Petersen 2005 | Randomisation of embryos instead of couples/participants |
| Portmann 2008 | Not a truly randomised design |
| Prados 2010 | Not a randomised controlled trial |
| Reeka 2004 | Quasi‐randomisation |
| Ryu 2010 | Randomisation of cycles |
| Sjoblom 2008 | Quasi‐randomisation |
| Sobrinho 2011 | Meta‐analysis of published data on embryo culture under low oxygen concentrations |
| Tejera 2010 | Randomisation of cycles and duplication of data with Gamiz Izquierdo 2010 |
Differences between protocol and review
Change of subgroup analyses. The planned subgroup analyses were : studies where the day of embryo transfer was early stage (up to and including Day 4) compared to studies where the embryo transfer was late (Days 5 and 6). This has been changed to: early embryo transfers are up to day 3, and late transfers are from day 4 onwards.
Contributions of authors
Stephan Bontekoe (SB01) and Eleni Mantikou (EM01) wrote the protocol and the manuscript, extracted data and performed the analysis.
Srividya Seshadri (SS01) extracted data and provided feedback as an independent adviser.
Madelon van Wely (MvW01), Sjoerd Repping (SR01) and Sebastiaan Mastenbroek (SM01) provided feedback as independent advisers.
Sources of support
Internal sources
-
Academic Medical Centre University of Amsterdam, Netherlands.
Thanks to the clinical librarian.
-
Cochrane Menstral Disorders and Subfertility Group (MDSG), New Zealand.
Conducting literature search, support review process
External sources
No sources of support supplied
Declarations of interest
The authors of this systematic review have no conflict of interest to declare.
New
References
References to studies included in this review
Iacobelli 2008 {published data only}
- Iacobelli M, Nagy ZP, Minasi MG, Casciani V, Albricci L, Ferrero S, et al. The effect of reduced oxygen concentration on ICSI outcomes in two different culture media systems. Abstracts of the 24th annual meeting of the ESHRE 2008. [Google Scholar]
Kovacic 2009 {published data only}
- Kovacic B, Sajko MS, Vlaisavljevic V. A prospective, randomized trial on the effect of atmospheric versus reduced oxygen concentration on the outcome of intracytoplasmic sperm injection cycles. Fertility and Sterility 2009;94:511‐9. [DOI] [PubMed] [Google Scholar]
Meintjes 2009 {published data only}
- Meintjes M, Chantilis SJ, Douglas JD, Rodriquez AJ, Guerami AR, Bookout DM, et al. A controlled randomized trial evaluating the effect of the lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Human Reproduction 2009;24:300‐7. [DOI] [PubMed] [Google Scholar]
Minasi 2003 {published data only}
- Minasi MG, Rienzi L, Ubaldi F, Romano S, Iacobelli M, Ferrero S, et al. Effect of oxygen concentration on the culture of human embryos: a prospective, randomized study. Abstracts of the 19th annual meeting of the ESHRE 2003. [Google Scholar]
Sepulveda 2011 {published data only}
- Sepulveda S, Steurer I, Gazzo E, Escudero E, Noriega L. Effect of oxygen conditions on the results of an oocyte donation program: A prospective randomized trial [Efeito das condicoes de oxigenio no resultado de um programa de ovo‐doacao: Estudo prospectivo e randomizado]. Jornal Brasileiro de Reproducao Assistida May‐June 2011;15(3):32‐3. [Google Scholar]
Silverberg 2005 {published data only}
- Silverberg KM, Turner T, Minter T, Schuster C, Fields R, Vaughn TC. Choosing the optimal incubator environment for day 3 culture: triple gas vs. 5% CO2 in air, a prospective, randomized trial. Fertility and Sterility 2005;84 Suppl 1:88. [Google Scholar]
Waldenström 2009 {published data only}
- Waldenström U, Engström AB, Hellberg D, Nilsson S. Low‐oxygen compared with high‐oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertility and Sterility 2009;91:2461‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bahceci 2005 {published data only}
- Bahceci M, Ciray HN, Karagenc L, Ulug U, Bener F. Effect of oxygen concentration during the incubation of embryos of women undergoing ICSI and embryo transfer: A prospective randomized study. Reproductive Biomedicine Online 2005 Oct;11(4):438‐43. [DOI] [PubMed] [Google Scholar]
Cieslak Janzen 2008 {published data only}
- Cieslak Janzen JM, Graff D, Anderson S, Matt DW. Comparison of atmospheric oxygen versus low oxygen on human sibling embryo development. Fertility and Sterility 2008;90 Suppl 1:431‐2. [Google Scholar]
Ciray 2008 {published data only}
- Ciray HN, Aksoy T, Yaramanci K, Ulug U, Bahceci M. Reduced O2 concentration in first 3 days of culture yields more blastocysts with better quality: A prospective randomized study on sibling oocytes. Human Reproduction 2008;23 Suppl 1:i160. [Google Scholar]
Ciray 2009 {published data only}
- Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertility and Sterility 2009;91 Suppl(4):1459‐61. [DOI] [PubMed] [Google Scholar]
Curnelle 2010 {published data only}
- Curnelle S, Nogueira D, Guitard V, Bonald F, Guillen N, Montagut J. The effect of culture under low oxygen tension on developmental competence of post‐thawed human embryos. Abstracts of the annual meeting of the ESHRE 2010. [Google Scholar]
Dasig 2006 {published data only}
- Dasig D, Dangcil M, Telles T, Farah L, D’Amico J, Shen W. The use of low oxygen culture conditions yields similar pregnancy rates with day 2 vs day 3 embryo transfer. Fertility and Sterility 2006;86 Suppl 2:241‐2. [Google Scholar]
Dumoulin 1995 {published data only}
- Dumoulin JC, Vanvuchelen RC, Land JA, Pieters MH, Geraedts JP, Evers JL. Effect of oxygen concentration on in vitro fertilization and embryo culture in the human and the mouse. Fertility and Sterility 1995;63(1):115‐9. [DOI] [PubMed] [Google Scholar]
Dumoulin 1999 {published data only}
- Dumoulin JC, Meijers CJ, Bras M, Coonen E, Geraedts JP, Evers JL. Effect of oxygen concentration on human in‐vitro fertilization and embryo culture. Human Reproduction 1999;14(2):465‐9. [DOI] [PubMed] [Google Scholar]
Fujiwara 2007 {published data only}
- Fujiwara M, Takahashi K, Izuno M, Duan YR, Kazono M, Kimura F, Noda Y. Effect of micro‐environment maintenance on embryo culture after in‐vitro fertilization: Comparison of top‐load mini incubator and conventional front‐load incubator. Journal of Assisted Reproduction and Genetics 2007;24(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamiz Izquierdo 2010 {published data only}
- Gamiz Izquierdo P, Santos JM, Tejera A, Pellicer A, Romero JL, Galan A, et al. Culture embryos under low oxygen conditions improves clinical results. Abstract of the annual meeting of the ESHRE 2010. [Google Scholar]
Graham 2010 {published data only}
- Graham J, Richter K, Siques J, Vermilyea M, Widra E, Tucker M. Improved preimplantation development and higher pregnancy rates associated with 6% versus ambient oxygen concentration during in vitro embryo culture. Human Reproduction 2010;25 Suppl 1:i59. [Google Scholar]
Gvakharia 2008 {published data only}
- Gvakharia M, Lee SC, Palao L, Cubukcu A, Milette BJ, Adamson GD. Effects on low oxygen culture on embryo development and IVF outcome. Fertility and Sterility 2008;90 Suppl 1:434. [Google Scholar]
Higdon 2008 {published data only}
- Higdon HL, Blackhurst DW, Boone WR. Incubator management in an assisted reproductive technology laboratory. Fertility and Sterility 2008;89(3):703‐10. [DOI] [PubMed] [Google Scholar]
Hoff 2008 {published data only}
- Hoff A, Khabani A, Khabani C, Hickok L, Marshall L. Reduced oxygen tension helps increase the quality of blastocyst available on D5. Fertility and Sterility 2008;90 Suppl 1:350. [Google Scholar]
Kasterstein 2008 {published data only}
- Kasterstein E, Strassburger D, Komarovsky D, Bern O, Komsky A, Maslansky B, et al. Improved embryo development after incubation in reduced oxygen concentration. Abstracts of the 24th annual meeting of the ESHRE. 2008.
Kea 2005 {published data only}
- Kea B, Gebhardt J, Westphal L, Lathi R, Watt J, Behr B. Effects of reduced oxygen concentrations on IVF outcome. Fertility and Sterility 2005;83 Suppl 2:19. [DOI] [PubMed] [Google Scholar]
Kea 2007 {published data only}
- Kea B, Gebhardt J, Watt J, Westphal LM, Lathi RB, Milki AA, Behr B. Effect of reduced oxygen concentrations on the outcome of in vitro fertilization. Fertility and Sterility 2007;87(1):213‐6. [DOI] [PubMed] [Google Scholar]
Khabani 2008 {published data only}
- Khabani C, Marshall L, Woodford D, Khabani A, Hickok L. Improving embryo culture conditions by reducing oxygen tension may help increase the implantation potential of an embryo in the donor recipient population. Fertility and Sterility 2008;89 Suppl 2:14. [Google Scholar]
Kim 2005 {published data only}
- Kim SB, Kwon H, Kim EK, Kim JM, Choi DH, Cha KY. The comparison of embryo developments and pregnancy rates in human in vitro culture conditions at different oxygen gas phase. Fertility and Sterility 2005;84 Suppl 1:16. [Google Scholar]
Kim 2007 {published data only}
- Kim EA, Kim EK, Kang KY, Choi MA, Kwon H, Choi DH. Effect of oxygen concentrations on IVM, IVF and embryo development of immature oocytes from stimulated cycles of human IVF‐ET program. Fertility & Sterility 2007;88(Suppl 1):S319. [Google Scholar]
Kovacic 2008 {published data only}
- Kovacic B, Vlaisavljevic V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reproductive Biomedicine Online 2008;17(2):229‐36. [DOI] [PubMed] [Google Scholar]
Loutradi 2009 {published data only}
- Loutradi K, Kolibianakis EM, Mitsoli A, Venetis CA, Tzamtzoglou A, Tarlatzis BC. The effect of oxygen concentration on human embryo culture: A prospective randomized controlled trial. Fertility and Sterility 2009;92 Suppl(3):230. [Google Scholar]
Madashi 2010 {published data only}
- Madaschi C, Guilherme P, Izzo CPM, Yamakami LY, Fassolas G, Izzo CR. Evaluation the effect of lowered incubator oxygen tension on embryo quality. Fertility and Sterility. Annual Meeting of the American Society for Reproductive Medicine, ASRM 2010.
Mancebo 2009 {published data only}
- Mancebo ABA, Souza MDCB, Henriques CA, Souza MM, Cardoso FFO, Santos HCN. Prospective randomized study to compare the effects of embryo culture performed in an incubator Class 100 with High Efficiency Particulate Air filter (HEPA) plus Volatile Organic Compounds (VOC) filter on outcome measures of ICSI cycles. Jornal Brasileiro de Reproducao Assistida April‐June 2009;13 (2), 2009:9‐12. [Google Scholar]
Meijers 1998 {published data only}
- Meijers C, Dumoulin JCM, Bras M, Bergers Jansen JM, Ignoul Vanvuchelen RCM. Comparison between ambient (20%) or reduced (5%) oxygen concentration for human in‐vitro fertilization using a system of culture medium droplets under oil. Human Reproduction 1998;13:111. [Google Scholar]
Meintjes 2000 {published data only}
- Meintjes M, Hill K, Johnston S, Waugh T, Rodriguez A, Madden J. The effect of lowered incubator oxygen tension on implantation, pregnancy and cryopreservation rates in a predominantly Day‐5 embryo transfer program. Fertility and Sterility 2000;74 Suppl 1(3):256. [Google Scholar]
Mitsoli 2011 {published data only}
- Mitsoli A, Kolibianakis EM, Loutradi K, Venetis CA, Triantafilidis S, Makedos A, et al. Low oxygen embryo culture is associated with improved day 3 embryo quality: A prospective randomized controlled trial. Human Reproduction. Conference: 27th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE 2011 Stockholm Sweden 2011;26:i2. [Google Scholar]
Nanassy 2010 {published data only}
- Nanassy L, Peterson CA, Wilcox AL, Peterson CM, Hammoud A, Carrell DT. Comparison of 5% and ambient oxygen during days 3–5 of in vitro culture of human embryos. Fertility and Sterility 2010;93(2):579‐85. [DOI] [PubMed] [Google Scholar]
Oliveira 2011 {published data only}
- Oliveira JBA, Petersen CG, Mauri AL, Massoro FC, Silva LFI, Ricci J, et al. IVF/ICSI outcomes after human embryo development on low oxygen tension: A meta‐analysis. Human Reproduction. Conference: 27th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE 2011 Stockholm Sweden 2011;26:i199‐i200. [Google Scholar]
Park 2001 {published data only}
- Park SJ, Son WY, Yoon SH, Lee SW, Lim JH. Effect of low oxygen tension on human IVM/F‐ET program. http://www.liyabiotech.com/upfiles/house/1134436097.pdf 2001. [Google Scholar]
Petersen 2005 {published data only}
- Petersen A, Mikkelsen AL, Lindenberg S. The impact of oxygen tension on developmental competence of post‐thaw human embryos. Acta Obstetrica Gynecologica Scandinavica 2005;84:1181‐4. [DOI] [PubMed] [Google Scholar]
Portmann 2008 {published data only}
- Portmann MP, Morrison LS, Carney SM, Boylan CF, Feinberg RF, Kovalevsky G. Is triple gas culture effective? Practical?. Fertility and Sterility 2008;90(Suppl):S351. [Google Scholar]
Prados 2010 {published data only}
- Prados N, Ruiz M, Garca‐Ortega J, Vime P, Hernez MJ, Crespo M, et al. Improved human embryo quality in days 3 and 5 with a low oxygen closed culture system. Human Reproduction. Conference: 26th Annual Meeting of the European Society of Human Reproduction and Embryology, ESHRE Rome Italy. June 2010:25 (pp i190). [Google Scholar]
Reeka 2004 {published data only}
- Reeka N, Jelinkova L, Brucker C, Gagsteiger F. Lower oxygen concentration is beneficial for the early human embryo development. Abstracts of the 20th annual meeting of the ESHRE 2004. [Google Scholar]
Ryu 2010 {published data only}
- Ryu H, Park CY, Min SH, Choi SK, Park C, Lee SH, et al. Effect of low‐oxygen compared with high‐oxygen atmosphere on the outcomes of in vitro fertilization, a prospective randomized study. Abstracts of the annual meeting of the ESHRE 2010. [Google Scholar]
Sjoblom 2008 {published data only}
- Sjoblom C, Chamberlain S, Devlin L, Priddle H. Reduced oxygen during embryo culture increase fertilisation and subsequent pregnancy rates in IVF. Abstracts of the 24th annual meeting of the ESHRE 2008. [Google Scholar]
Sobrinho 2011 {published data only}
- Sobrinho DBG, Oliveira JBA, Petersen CG, Mauri AL, Silva LFI, Massoro FC, et al. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta‐analysis. Reproductive Biology and Endocrinology 2011;9(143). [DOI] [PMC free article] [PubMed] [Google Scholar]
Tejera 2010 {published data only}
- Tejera A, Gmiz P, Romero JL, Santos JM, Grau N, Santos MJ. Culture under low oxygen tension improves embryo quality in egg donation cycles. Molecular Human Reproduction. 25th Annual Meeting of the European Society of Human Reproduction Embryology, ESHRE Amsterdam Netherlands.
Additional references
Catt 2000
- Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Human Reproduction 2000;15 Suppl 2:199‐206. [DOI] [PubMed] [Google Scholar]
Dumoulin 2010
- Dumoulin JC, Land JA, Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Human Reproduction 2010;25(3):605‐12. [DOI] [PubMed] [Google Scholar]
Feil 2006
- Feil D, Lane M, Roberts CT, Kelley RL, Edwards LJ, Thompson JG, Kind KL. Effect of culturing mouse embryos under different oxygen concentrations on subsequent fetal and placental development. Journal of Physiology 2006;572:87‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer 1993
- Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Journal of Reproduction and Fertility 1993;99(2):673‐9. [DOI] [PubMed] [Google Scholar]
Harvey 2007
- Harvey AJ. The role of oxygen in ruminant preimplantation embryo development and metabolism. Animal Reproduction Science 2007;98(1‐2):113‐28. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Karagenc 2004
- Karagenc L, Sertkaya Z, Ciray N, Ulug U, Bahceci M. Impact of oxygen concentration on embryonic development of mouse zygotes. Reproductive Biomedicine Online 2004;9(4):409‐17. [DOI] [PubMed] [Google Scholar]
Lim 2011
- Lim HJ, Han J, Woo DH, Kim SE, Kim SK, Kang HG, Kim JH. Biochemical and morphological effects of hypoxic environment on human embryonic stem cells in long‐term culture and differentiating embryoid bodies. Molecules and Cells 2011;31:123‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastenbroek 2005
- Mastenbroek S, Bossuyt PMM, Heineman MJ, Repping S, Veen F. Comment 1 on. Design and analysis of a randomized controlled trial studying preimplantation genetic screening. Human Reproduction August 2005;20(8):2362‐3. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]
Yedwab 1976
- Yedwab GA, Paz G, Homonnai TZ, David MP, Kraicer PF. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertility and Sterility 1976;27(3):304‐9. [DOI] [PubMed] [Google Scholar]


