Abstract
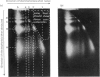
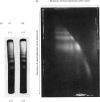
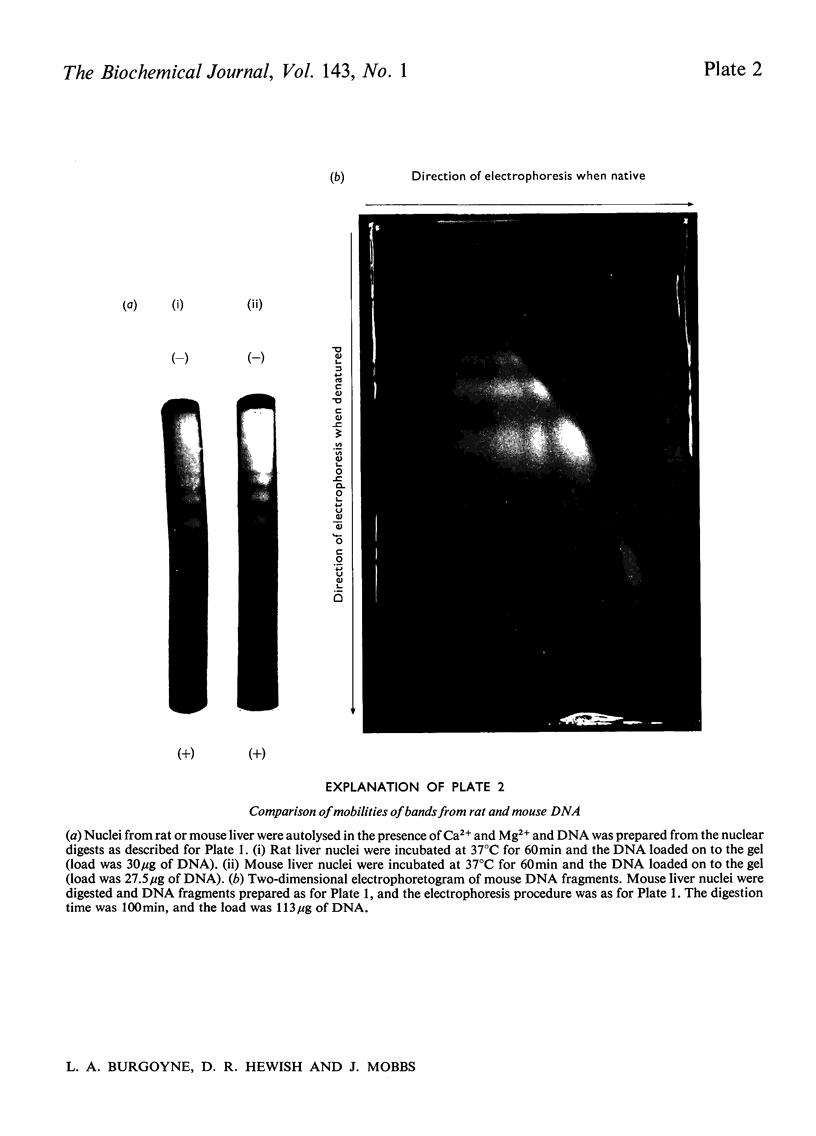
The basic regularity of chromatin substructure that has been reported in rat liver chromatin (Hewish & Burgoyne, 1973b) was also detected in mouse chromatin. The regular series of DNA fragments produced by the action of Ca–Mg endonuclease on rat chromatin were studied further. The smallest single-stranded class has a molecular weight of approx. 45000–63000 and the smallest double-stranded class has a molecular weight of approx. 120000–150000. Studies of the substructure of the DNA fragments produced by the Ca–Mg endonuclease have shown that the regular series of double-stranded fragments have regular series of single-stranded fragments within them. It was concluded that the regular series of double-stranded fragments was probably a consequence of the regular series of single-stranded fragments. Digestion time-courses are presented for mouse and rat nuclear DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONNER J., HUANG R. C. Properties of chromosomal nucleohistone. J Mol Biol. 1963 Mar;6:169–174. doi: 10.1016/s0022-2836(63)80065-0. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing R. J., Bonner J. The structure of chromatin as revealed by deoxyribonuclease digestion studies. Biochim Biophys Acta. 1972 Oct 27;281(3):453–462. doi: 10.1016/0005-2787(72)90462-5. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Wagar M. A., Atkinson M. R. Calcium-dependent priming of DNA synthesis in isolated rat liver nuclei. Biochem Biophys Res Commun. 1970 Apr 24;39(2):254–259. doi: 10.1016/0006-291x(70)90786-2. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Fisher M. P., Kakefuda T. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide gels. II. Biochemistry. 1972 Mar 28;11(7):1242–1250. doi: 10.1021/bi00757a020. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Kakefuda T., Fisher M. P. Electrophoretic properties of low molecular weight DNA fragments in agarose-acrylamide gels. Anal Biochem. 1972 Dec;50(2):519–528. doi: 10.1016/0003-2697(72)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Froholm L. O., Olsen B. R. Electron microscopy of transfer RNA. J Mol Biol. 1969 Mar 14;40(2):305–306. doi: 10.1016/0022-2836(69)90478-1. [DOI] [PubMed] [Google Scholar]
- Harley E. H., White J. S., Rees K. R. The identification of different structural classes of nucleic acids by electrophoresis in polyacrylamide gels of different concentration. Biochim Biophys Acta. 1973 Mar 19;299(2):253–263. doi: 10.1016/0005-2787(73)90348-1. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Itzhaki R. F. Structure of deoxyribonucleoprotein as revealed by its binding to polylysine. Biochem Biophys Res Commun. 1970 Oct 9;41(1):25–32. doi: 10.1016/0006-291x(70)90463-8. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky A. E., Silverman B. Blocking by histones of accessibility to DNA in chromatin. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2115–2119. doi: 10.1073/pnas.69.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky A. E., Silverman B., Panda N. C. Blocking by histones of accessibility to DNA in chromatin: addition of histones. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3243–3246. doi: 10.1073/pnas.69.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. Chromatin structure and the cell cycle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2224–2228. doi: 10.1073/pnas.69.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Silverman B., Mirsky A. E. Addition of histones to histone-depleted nuclei: effect on template activity toward DNA and RNA polymerases. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2637–2641. doi: 10.1073/pnas.70.9.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. Y. Diametric effects of histones and the non-histone proteins on DNA replication in vitro. Exp Cell Res. 1969 Oct;57(2):467–469. doi: 10.1016/0014-4827(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Williamson R. Properties of rapidly labelled deoxyribonucleic acid fragments isolated from the cytoplasm of primary cultures of embryonic mouse liver cells. J Mol Biol. 1970 Jul 14;51(1):157–168. doi: 10.1016/0022-2836(70)90277-9. [DOI] [PubMed] [Google Scholar]
- Yamada M., Nagao M., Hidaka T., Sugimura T. Effect of poly(ADP-ribose) formation on DNA synthesis and DNA fragmentation in nuclei of rat liver and rat ascites hepatoma AH-130 cells. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1567–1572. doi: 10.1016/0006-291x(73)91165-0. [DOI] [PubMed] [Google Scholar]