Abstract
Introduction
Gestational diabetes mellitus (GDM) is defined by one or more abnormal values in an oral glucose tolerance test (OGTT). The significance/importance of the number of abnormal values in relation to adverse perinatal and neonatal outcomes is unclear. We assessed the association of these outcomes with the number of abnormal glucose values in a 2‐h 75 g OGTT in a large register‐based cohort.
Material and Methods
This sub‐study of the Finnish Gestational Diabetes Study was based on the Finnish Medical Birth Register 2009 supplemented with OGTT laboratory data of 4869 pregnant women from six Finnish hospitals. The diagnostic cut‐offs in OGTT according to the Finnish guidelines for plasma samples were ≥5.3 mmol/L (fasting), ≥10.0 mmol/L 1 h or ≥8.6 mmol/L 2 h after the glucose load. As per the guidelines, women with one or several abnormal OGTT values received diet and lifestyle counseling in the primary care, self‐monitored their glucose values and received pharmacological therapy as needed. Women with GDM were categorized according to the number of abnormal glucose values. The primary outcomes, composites of adverse perinatal (pre‐eclampsia, preterm delivery, macrosomia or primary cesarean section) and neonatal outcomes (birth trauma, neonatal hypoglycemia, hyperbilirubinemia or stillbirth/perinatal mortality), were analyzed by logistic regression adjusted for maternal age, pre‐pregnancy body mass index, parity, socio‐economic status and smoking.
Results
Of all the women, 877 (18.0%) had one, 278 (5.7%) two and 79 (1.6%) three abnormal OGTT values, while 3635 (74.7%) women were normoglycemic. Women with at least two abnormal OGTT values had higher proportions of adverse perinatal composite (35.0% vs. 27.5%, adjusted odds ratio 1.36; 95% confidence interval 1.03–1.81) and neonatal composite outcomes (31.1% vs. 18.9%, adjusted odds ratio 1.88; 95% confidence interval 1.40–2.52) compared to women with one abnormal value. The risks of delivery induction and neonatal hypoglycemia were increased regardless of the number of abnormal values when compared with normoglycemic women.
Conclusions
The risk of adverse perinatal and neonatal outcomes is significantly higher in women with two or more abnormal OGTT values than in those with one abnormal value.
Keywords: composite outcomes, gestational diabetes, neonatal outcomes, oral glucose tolerance test, perinatal outcomes
This large cohort study investigated the importance of one versus several abnormal oral glucose tolerance test values and found that adverse composite perinatal and neonatal outcomes are higher in women who have two or more abnormal oral glucose tolerance values compared to women with only one abnormal value.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GDM
gestational diabetes
- GW
weeks of gestation
- IADPSG
International Association of Diabetes in Pregnancy Study Group
- ICD
International Classification of Diseases
- LGA
large for gestational age
- MBR
Medical Birth Register
- OGTT
oral glucose tolerance test
- OR
odds ratio
- SD
standard deviation
Key message.
In a study setting where all women with GDM received counseling and treatment as needed, perinatal and neonatal risks were increased in women with at least two abnormal oral glucose tolerance test values compared to those with one abnormal value.
1. INTRODUCTION
Gestational diabetes mellitus (GDM) is one of the most common medical conditions complicating pregnancies and is becoming more prevalent globally with prevalence rates of 11%–30%. 1 Currently, the most frequently used method to diagnose GDM is a 2‐h 75 g oral glucose tolerance test (OGTT), and the diagnosis is based on one or more abnormal values in the OGTT. This was endorsed by the International Association of Diabetes in Pregnancy Study Group (IADPSG) based on the Hyperglycemia and Adverse Pregnancy Outcome study.2, 3 This recommendation is widely used around the world, and organizations, such as the World Health Organization and the International Federation of Gynecology and Obstetrics, have adopted these diagnostic criteria.4, 5
Current guidelines do not share a consensus on whether a single abnormal OGTT value is of clinical significance or would it be cost‐effective to counsel and treat only women with two or three abnormal values. Studies have reported that women with one elevated OGTT value are at risk of adverse perinatal outcomes if not counseled and treated.6, 7, 8 On the contrary, it has also been reported that adverse pregnancy outcomes increase with the number of abnormal glucose values.9, 10
According to the IADPSG recommendations, GDM is diagnosed after one abnormal glucose concentration in the OGTT. 2 However, the significance of a single abnormal OGTT concentration on pregnancy outcomes remains unclear. Therefore, in the present study, our aim was to compare the significance of one vs several abnormal OGTT values on pregnancy and perinatal outcomes in a large register‐based cohort.
2. MATERIAL AND METHODS
2.1. Study population and data sources
The study has been conducted and reported applying the criteria of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 11 This study was based on data from the register‐based arm of the Finnish Gestational Diabetes Study (FinnGeDi), which was initiated in conjunction with the introduction of the new national comprehensive guidelines for GDM screening, diagnosis and treatment in Finland in 2008. 12 The study has been presented in detail previously. 13 The registry data was obtained from the Medical Birth Register (MBR), maintained by the Finnish Institute of Health and Welfare which includes data on the course and complications of pregnancy and delivery and perinatal health of newborns until the age of 7 days, as well as the 10th version of International Statistical Classification of Diseases and Related Health Problems (ICD‐10) codes for medical diagnoses of the mother and child. All live births and stillbirths from 220/7 weeks of gestation (GW) or birthweight of at least 500 grams are reported in the MBR. 14 The MBR has comprehensive coverage with high‐quality data.15, 16
The MBR also includes information on whether the OGTT was performed during pregnancy and if the result was abnormal. However, it does not include data on specific glucose concentrations. To address this, we collected numerical OGTT data from all women who delivered in 2009 and had undergone an OGTT in the laboratory in six delivery units in Finland: two tertiary level (Oulu and Tampere) and four secondary‐level (Southern Karelia, Seinäjoki, Kainuu and Satakunta) hospitals, each serving a specific geographical area. Numerical OGTT data were available from these hospitals through their laboratory data system. All OGTTs performed during pregnancy between 12 and 40 GW in the years 2008 and 2009 were linked to MBR data. The linkage was performed using unique personal identification numbers by personnel uninvolved with this study. Women with the OGTT performed before 12 GW, GDM diagnosis (ICD‐code O24.4 or O24.9) or insulin treatment during pregnancy according to the MBR but normoglycemic OGTT values and multiple pregnancies were excluded from the study. In cases where the mother had two pregnancies within the same year, only the first was included. Thereafter, the study population consisted of 4869 women who underwent OGTT between 12 and 40 GW (Figure 1).
FIGURE 1.
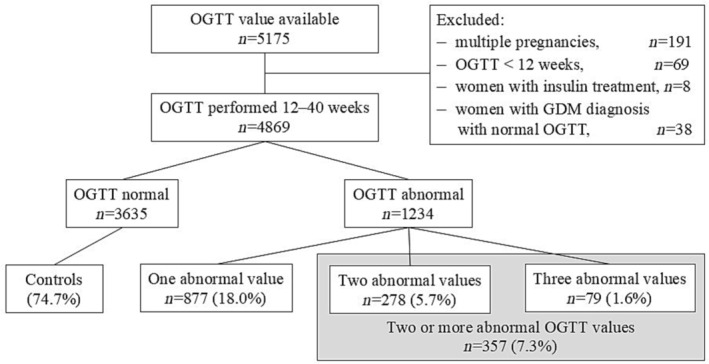
Flow chart of the study.
The Finnish national guidelines, published in 2008, introduced a comprehensive screening approach for GDM, replacing the previous risk factor‐based screening policy. 12 According to these guidelines, all women, except those with very low risk, are recommended to be screened for GDM using a two‐hour 75 g OGTT between 24 and 28 GW. The very low‐risk group comprises of <25‐year‐old primiparous women with body mass index (BMI) <25 kg/m2 and without family history of diabetes and < 40‐year‐old multiparous women with BMI <25 kg/m2 and without history of GDM or macrosomic newborn.12, 17 High‐risk women (i.e., women with prior GDM, BMI >35 kg/m2 or polycystic ovary syndrome with insulin resistance) are to undergo their first OGTT screening between 12 and 16 GW, and if the results are normal, the test is repeated between 24 and 28 GW. 12 The OGTT is performed after a 12‐h overnight fast in the laboratory nearest to the woman's residence. Blood samples are drawn from the antecubital vein into fluoride citrate tubes and analyzed within 24 h in a local laboratory using commercial enzymatic assays, with the assays used varying between laboratories. The involved laboratories in the study were accredited laboratories under ISO15189:2012 standard and had quality management systems. The laboratories performed regular internal quality control checks with controls of known concentrations and were also involved in external quality control schemes. Based on the Finnish guidelines (adapted from the American Diabetes Association guidelines 18 in 2008), the diagnostic values for plasma samples are ≥5.3 mmol/L at the baseline (fasting sample), ≥10.0 mmol/L 1 h or ≥8.6 mmol/L 2 h after the glucose load. 12 Women with one or several abnormal OGTT values, as per the guidelines, receive individualized dietary and lifestyle counseling in maternity clinics and begin glucose self‐monitoring thereafter. If, despite the dietary and lifestyle interventions, self‐monitored plasma glucose concentrations repeatedly exceed the target levels (i.e., <5.5 mmol/L fasting and <7.8 mmol/L 1 h postprandial), pharmacological therapy is considered.
2.2. Study outcomes
Maternal age was defined at the time of delivery, and parity was defined by the number of previous deliveries. BMI was calculated using self‐reported height and weight before pregnancy, both of which were recorded at the first antenatal visit. Socioeconomic status was divided into four categories using the occupation reported in the MBR: (1) upper‐level employees with administrative, managerial, professional and related occupations, (2) lower‐level employees with administrative and clerical occupations, (3) manual workers and (4) others—such as stay‐at‐home mothers, students, pensioners and self‐employed individuals. Self‐reported smoking status was categorized as nonsmokers and smokers. Neonatal morbidity was evaluated by a pediatrician as per ICD‐10 codes.
The primary study outcomes included composites of adverse perinatal and neonatal outcomes. Composite adverse perinatal outcome included pre‐eclampsia, preterm delivery (<370/7 GW), macrosomia or large for gestational age (LGA) >90% (birthweight standard deviation [SD] scores over 90%) and primary cesarean section, and composite adverse neonatal outcome included birth trauma [fracture of the clavicle (P13.4), Erb's paresis (P14.0)], neonatal hypoglycemia (P70.0–70.9), hyperbilirubinemia (P59.0–59.9), and stillbirth/perinatal mortality. The choice to use composite outcomes was based on previously published articles.19, 20, 21 The birthweight SD score is a sex‐specific parameter to estimate birthweight and length in singletons and twins born at 23–43 GW to primiparous or multiparous mothers, according to Finnish standards. 22 According to the Finnish current care guidelines, glucose concentration is mandatorily measured in all newborns of GDM mothers. 12 In addition to GDM, other potential indications for neonatal glucose screening in asymptomatic newborns included preterm birth, a birth weight of <2.5 kg or >4.5 kg or maternal use of β‐blockers. A diagnosis of hypoglycemia was recorded in the MBR if a newborn required any interventions for hypoglycemia including intravenous glucose, although there are no unified diagnostic criteria for neonatal hypoglycemia in Finland.
Secondary maternal outcomes included gestational hypertension and pre‐eclampsia (ICD‐10 codes O13 and O14 included, O10 and O11 excluded), induction of labor, Cesarean section. Secondary neonatal outcome measures included gestational age at delivery and birthweight SD scores.
2.3. Statistical analyses
Categorical variables were reported as frequencies and percentages, and continuous variables as mean and SD. Pearson's χ 2 test was used to compare the difference in proportions of demographic variables. Independent sample t‐test was used to compare the difference in the means of demographic data. Differences between each GDM group were tested using Fisher's exact test. Logistic and linear regression analyses were used to estimate odd ratios (ORs) with their 95% confidence intervals (CIs) and mean differences (with 95% CIs) of outcomes associated with GDM, respectively, according to the number of abnormal OGTT values. Logistic and linear regressions were also performed to estimate the differences between one or at least two abnormal glucose values. The results were adjusted for maternal age, pre‐pregnancy BMI, parity category (primiparity/multiparity), socio‐economic status and smoking. A two‐sided p‐value <0.05 was considered statistically significant. All statistical analyses were carried out using the SPSS 29 statistical package.
3. RESULTS
The OGTTs were performed between 12 and 40 GW (mean 26 GW, SD 4.4) on 4869 women who delivered in 2009 in the study hospitals. OGTT was abnormal and hence GDM diagnosed in 1234 (25.3%) women, and the control group consisted of 3635 (74.7%) women with normal OGTT results. Of the women with GDM, 877 (71.1%) had one, 278 (22.5%) two and 79 (6.4%) three abnormal values in the OGTT. Women with two or three abnormal OGTT values were analyzed as one group (n = 357, 7.3% of all women) (Table 1).
TABLE 1.
Maternal characteristics of normoglycemic women and women with abnormal oral glucose tolerance test values.
| Characteristics | Normoglycemic women | Women with one abnormal OGTT value | Women with two or more abnormal OGTT values | p‐value, normoglycemic vs one abnormal OGTT value | p‐value, normoglycemic vs two or more abnormal OGTT values | p‐value, one vs two or more abnormal OGTT values |
|---|---|---|---|---|---|---|
| n (%) | 3635 (74.7) | 877 (18.0) | 357 (7.3) | |||
| Age at delivery (years), mean (SD) | 29.5 (5.3) | 30.3 (5.5) | 30.8 (5.7) | <0.001 | <0.001 | 0.175 |
| Pre‐pregnancy BMI (kg/m2), mean (SD) | 25.8 (4.6) | 27.8 (5.7) | 29.6 (6.2) | <0.001 | <0.001 | < 0.001 |
| Primiparity, n (%) | 1720 (47.3) | 338 (38.5) | 134 (37.5) | <0.001 | <0.001 | 0.747 |
| Smoking, n (%) | 437 (12.5) | 137 (16.2) | 60 (17.6) | 0.005 | 0.009 | 0.605 |
| Socioeconomic status, n (%) | ||||||
| Upper‐level employee a | 630 (21.3) | 137 (18.9) | 52 (18.6) | 0.161 | 0.056 | 0.697 |
| Lower‐level employee b | 1219 (41.1) | 305 (42.2) | 112 (40.1) | |||
| Manual worker | 485 (16.3) | 139 (19.2) | 63 (22.6) | |||
| Other c | 629 (21.2) | 142 (19.6) | 52 (18.6) | |||
| Insulin treatment | ‐ (0.0) | 63 (7.2) | 57 (16.0) | <0.001 | <0.001 | < 0.001 |
Abbreviations: BMI, body mass index; OGTT, oral glucose tolerance test; SD, standard deviation.
Administrative, managerial, professional and related occupations.
Administrative and clerical occupations.
Students, pensioners, self‐employed and other.
When women with GDM were compared to normoglycemic controls, the percentage of those smoking and manual workers was higher in the GDM group. When the baseline characteristics were considered, women with at least two abnormal OGTT values had higher pre‐pregnancy BMI compared to those with one abnormal OGTT value. Of the women with one abnormal OGTT value, 7.2% received insulin treatment, while the proportion in women with at least two abnormal was 16.0% (Table 1).
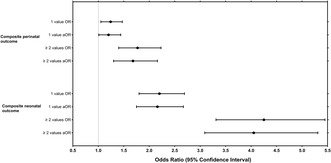
Perinatal and neonatal characteristics according to the number of abnormal OGTT values, and the unadjusted and adjusted ORs and risk estimates for the study outcomes are presented in Table 2 and in Figures 2 and 3. The proportions of composite perinatal (35.0% vs. 27.5%, adjusted OR (aOR) 1.36, 95% CI 1.03–1.81) and composite neonatal outcomes (31.1% vs. 18.9%, aOR 1.88, 95% CI 1.40–2.52) were higher in women with at least two abnormal OGTT values compared to women with one abnormal OGTT value. Furthermore, women with at least two abnormal OGTT values had higher number induction of labor (29.1% vs. 22.5%, aOR 1.36, 95% CI 1.02–1.82), preterm delivery (8.4% vs. 5.1%, aOR 1.75, 95% CI 1.05–2.93), primary cesarean section (19.3% vs. 14.4%, aOR 1.41, 95% CI 0.99–2.02) and neonatal hypoglycemia (21.0% vs. 11.9%, aOR 1.96, 95% CI 1.40–2.75) when compared to those with one abnormal value.
TABLE 2.
Perinatal characteristics of normoglycemic women and women with abnormal oral glucose tolerance test values.
| Characteristics | Normoglycemic women, n (%) | Women with one abnormal OGTT value, n (%) | Women with two or more abnormal OGTT values, n (%) | Normoglycemic vs one abnormal OGTT value, OR (95% CI), aOR (95% CI) | Normoglycemic vs two or more abnormal OGTT values, OR (95% CI), aOR (95% CI) | One vs two or more abnormal OGTT values, OR (95% CI), aOR (95% CI) |
|---|---|---|---|---|---|---|
| 3635 (74.7) | 877 (18.0) | 357 (7.3) | ||||
| Composite perinatal outcome a | 849 (23.4) | 241 (27.5) | 125 (35.0) |
1.24 (1.05–1.47) 1.20 (1.01–1.44) |
1.77 (1.40–2.23) 1.68 (1.30–2.16) |
1.42 (1.09–1.85) 1.36 (1.03–1.81) |
| Pre‐eclampsia b | 74 (2.0) | 29 (3.3) | 8 (2.2) |
1.65 (1.06–2.55) 1.77 (1.12–2.79) |
1.10 (0.53–2.31) 1.09 (0.49–2.46) |
0.66 (0.30–1.48) 0.58 (0.25–1.37) |
| Preterm delivery | 144 (3.9) | 45 (5.1) | 30 (8.4) |
1.31 (0.93–1.85) 1.35 (0.94–1.94) |
2.22 (1.48–3.35) 2.34 (1.49–3.68) |
1.70 (1.05–2.74) 1.75 (1.05–2.93) |
| Macrosomia (LGA >90%) | 343 (9.4) | 88 (10.0) | 49 (13.7) |
1.07 (0.84–1.37) 0.99 (0.76–1.28) |
1.53 (1.11–2.11) 1.38 (0.98–1.95) |
1.43 (0.99–2.08) 1.33 (0.90–1.97) |
| Primary cesarean section | 437 (12.0) | 126 (14.4) | 69 (19.3) |
1.23 (0.99–1.52) 1.23 (0.97–1.55) |
1.75 (1.32–2.32) 1.74 (1.27–2.39) |
1.43 (1.03–1.97) 1.41 (0.99–2.02) |
| Composite neonatal outcome c | 349 (9.6) | 166 (18.9) | 111 (31.1) |
2.20 (1.80–2.69) 2.16 (1.75–2.67) |
4.25 (3.31–5.45) 4.05 (3.09–5.31) |
1.93 (1.46–2.56) 1.88 (1.40–2.52) |
| Birth trauma | 46 (1.3) | 13 (1.5) | 8 (2.2) | 1.13 (0.64–2.01) 1.14 (0.63–2.06) |
1.68 (0.83–3.44) 1.84 (0.87–3.88) |
1.49 (0.64–3.43) 1.65 (0.71–3.87) |
| Neonatal hypoglycemia | 93 (2.6) | 104 (11.9) | 75 (21.0) | 5.12 (3.83–6.85) 5.06 (3.73–6.86) |
10.13 (7.30–14.05) 9.79 (6.82–14.03) |
1.98 (1.43–2.74) 1.96 (1.40–2.75) |
| Hyperbilirubinemia | 211 (5.8) | 65 (7.4) | 39 (10.1) | 1.29 (0.97–1.73) 1.27 (0.94–1.71) |
1.99 (1.39–2.85) 1.81 (1.22–2.69) |
1.53 (1.01–2.33) 1.43 (0.92–2.21) |
| Still birth/perinatal death | 8 (0.2) | 2 (0.2) | 2 (0.6) | ‐ | ‐ | ‐ |
| Secondary maternal outcomes | ||||||
| Pregnancy induced hypertension d | 234 (6.4) | 69 (7.9) | 34 (9.5) | 1.24 (0.94–1.64) 1.18 (0.88–1.57) |
1.53 (1.05–2.23) 1.24 (0.82–1.87) |
1.23 (0.80–1.90) 1.08 (0.69–1.71) |
| Induction of labor | 594 (16.3) | 197 (22.5) | 104 (29.1) | 1.48 (1.23–1.78) 1.30 (1.07–1.57) |
2.10 (1.65–2.69) 1.76 (1.35–2.28) |
1.42 (1.08–1.87) 1.36 (1.02–1.82) |
| Cesarean section (primary+secondary) | 565 (15.6) | 168 (19.0) | 94 (25.9) | 1.29 (1.06–1.56) 1.15 (0.94–1.41) |
1.94 (1.51–2.50) 1.58 (1.19–2.09) |
1.51 (1.13–2.02) 1.33 (0.97–1.83) |
| Secondary neonatal outcomes |
B coefficient (95% CI) Adjusted coefficient (95% CI) |
B coefficient (95% CI) Adjusted coefficient (95% CI) |
B coefficient (95% CI) Adjusted coefficient (95% CI) |
|||
|---|---|---|---|---|---|---|
| Gestational age at delivery, weeks mean (SD) | 39.9 (1.6) | 39.6 (1.7) | 39.2 (2.1) |
−0.08 (−0.43–‐0.20) −0.08 (−0.44–‐0.20) |
−0.13 (−0.94–‐0.58) −0.13 (−0.90–‐0.53) |
−0.11 (−0.66–‐0.22) −0.11 (−0.63–‐0.20) |
| Birthweight, SD score | −0.02 (1.02) | 0.04 (1.00) | 0.14 (1.20) | 0.08 (0.00–0.15) 0.06 (−0.02–0.14) |
0.20 (0.09–0.32) 0.16 (0.04–0.29) |
0.12 (−0.01–0.26) 0.12 (−0.02–0.26) |
Note: Adjusted for maternal age, pre‐pregnancy body mass index, parity category, socio‐economic status and smoking.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OGTT, oral glucose tolerance test; OR, odds ratios, SD, standard deviation.
Pre‐eclampsia, preterm delivery, LGA > 90% and primary cesarean section.
ICD‐10: O14 included, O10 and O11 excluded.
Birth trauma (fracture of the clavicle, Erb's paresis), neonatal hypoglycemia, hyperbilirubinemia and stillbirth/perinatal mortality.
ICD‐10: O13 included, O10, and O11 excluded.
FIGURE 2.
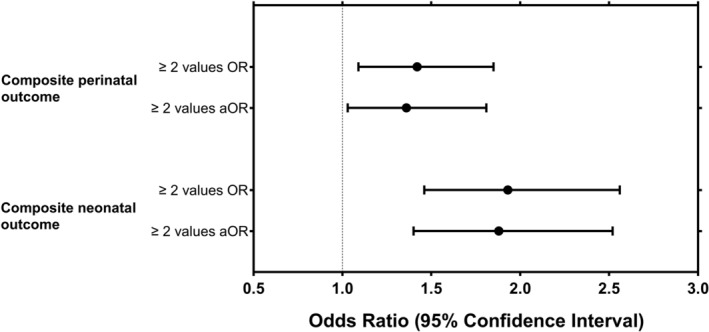
Odds ratios (ORs) and 95% confidence intervals (CIs) for the composite adverse perinatal and neonatal outcomes in women with two or more abnormal OGTT values compared to women with one abnormal OGTT value. aOR, adjusted odds ratio; OR, odds ratios. Adjusted for maternal age, pre‐pregnancy body mass index, parity category, socio‐economic status, and smoking. Composite adverse perinatal outcome included pre‐eclampsia, preterm delivery, macrosomia and primary cesarean section, and composite adverse neonatal outcome included birth trauma (fracture of the clavicle or Erb's paresis), neonatal hypoglycemia, hyperbilirubinemia, and stillbirth/perinatal mortality.
FIGURE 3.
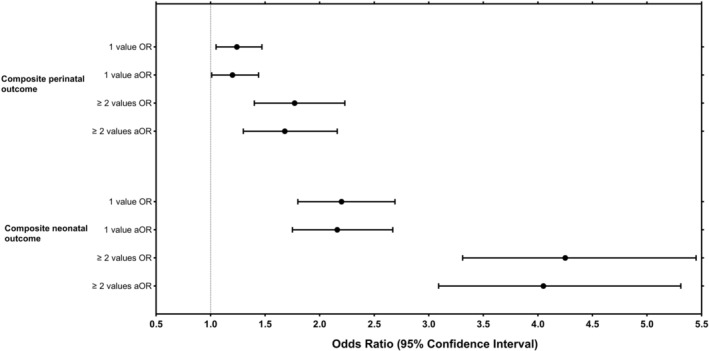
Odds ratios (ORs) and 95% confidence intervals (CIs) for the composite adverse perinatal and neonatal outcomes in women according to the number of abnormal OGTT values compared to normoglycemic controls. aOR, adjusted odds ratio; OR, odds ratios. Adjusted for maternal age, pre‐pregnancy body mass index, parity category, socio‐economic status and smoking. Composite adverse perinatal outcome included pre‐eclampsia, preterm delivery, macrosomia and primary cesarean section, and composite adverse neonatal outcome included birth trauma (fracture of the clavicle or Erb's paresis), neonatal hypoglycemia, hyperbilirubinemia, and stillbirth/perinatal mortality.
When compared to normoglycemic women, the proportions of composite perinatal (27.5% vs. 23.4%, aOR 1.20, 95% CI 1.01–1.44) and composite neonatal outcomes (18.9% vs. 9.6%, aOR 2.16, 95% CI 1.75–2.67) were higher in women with one abnormal OGTT value. Similarly, women with at least two abnormal values in the OGTT had higher proportions of composite perinatal (35.0% vs. 23.4%, aOR 1.68, 95% CI 1.30–2.16) and composite neonatal outcomes (31.1% vs. 9.6%, aOR 4.05, 95% CI 3.09–5.31) compared to normoglycemic women. Women with one abnormal OGTT value had more often pre‐eclampsia, and women with at least two abnormal OGTT values had a higher proportion of gestational hypertension, preterm delivery, primary cesarean section, macrosomia, hyperbilirubinemia, when compared to normoglycemic controls. Regardless of the number of abnormal OGTT values, women with GDM had more often induction of labor and neonatal hypoglycemia.
4. DISCUSSION
In the present study, women with two or more abnormal OGTT values had a higher risk of adverse composite perinatal and neonatal outcomes compared to women with one abnormal OGTT value, including preterm delivery, neonatal hypoglycemia and delivery induction. In addition, both GDM groups had an increased risk of delivery induction and neonatal hypoglycemia compared to the normoglycemic group. Women with two or more abnormal values also had a higher risk of preterm delivery, primary cesarean section, and hyperbilirubinemia, while women with one abnormal value had a higher risk of pre‐eclampsia. These findings indicate that women with any number of abnormal OGTT values, and hence GDM, had an increased incidence of adverse perinatal and neonatal outcomes than normoglycemic controls.
The significance of one abnormal OGTT value has been debated. Some criteria—for example, Carpenter‐Coustan and the National Diabetes Data Group criteria—set the diagnosis of GDM only after two or more abnormal values.23, 24 However, these screening policies were based on four glucose concentrations instead of three values measured in the IADPSG. According to the IADPSG recommendations—which are nowadays widely used by, for example, the World Health Organization and the International Federation of Gynecology and Obstetrics—GDM is diagnosed already after one abnormal OGTT value.4, 5
In a recent systematic review and meta‐analysis, GDM was diagnosed by a three‐hour 100 g OGTT after an abnormal one‐hour 50 g glucose challenge test, and women with only one abnormal OGTT value remained untreated. 6 A single abnormal value, hence untreated and not defined as GDM, was associated with adverse maternal and neonatal outcomes—macrosomia, cesarean delivery, pregnancy induced hypertension, neonatal intensive care unit admission, neonatal hypoglycemia and respiratory distress syndrome. Another study, wherein GDM was diagnosed by the IADPSG criteria, reported higher risks of cesarean sections and LGA in women with any one abnormal glucose value and the risk further increased in those with more than one abnormal value. 25
In the present study, two or more abnormal OGTT values led to an almost 2‐fold higher prevalence of neonatal hypoglycemia, 1.5‐fold increased prevalence of adverse composite neonatal outcomes and 2‐fold increased admission to neonatal ward compared to neonates whose mothers had only one abnormal value in the OGTT. Thus, the higher number of abnormal values in the OGTT seems to have the strongest effect on neonatal morbidity. There was a 4‐5‐fold higher prevalence of neonatal hypoglycemia and a two‐fold increased prevalence of adverse composite neonatal outcomes in women with only one abnormal OGTT value compared to normoglycemic controls. However, an increased need for care at a neonatal ward was not observed in these neonates, implying that one abnormal OGTT value could be related to an increased proportion of adverse neonatal outcomes that are relatively mild. Nevertheless, all women with GDM in this study, including those with only one abnormal OGTT value, received diet and lifestyle counseling, self‐monitored their glucose values and received pharmacological treatment when indicated, and therefore should not be considered a risk‐free group not requiring follow‐up.
Even though the significance of one abnormal OGTT value for pregnancy outcomes seems to be less significant/important compared to several abnormal values, any degree of abnormal glucose metabolism in pregnancy has been shown to independently predict an increased risk of glucose intolerance after delivery.9, 26, 27, 28 In recent Finnish studies, incidence of type 2 diabetes mellitus and metabolic syndrome increased after delivery also in women with one abnormal OGTT value during pregnancy. The probability of these disturbances was reported to increase together with the number of abnormal OGTT values obtained during follow‐up after 10 years.29, 30 Hence, regardless of the severity of glycemic disturbance during pregnancy all women with GDM should be evaluated after pregnancy for subsequent metabolic disorders.
There are several strengths in the present study. Our study included a large cohort with comprehensive data. The coverage of the Finnish national registries, especially MBR, is complete consisting of very high‐quality data.15, 16 Nonetheless, there are certain limitations. The power to estimate rare severe outcomes (such as perinatal mortality, Erb's paresis) was insufficient. There may be some uncertainty in the detection of neonatal hypoglycemia due to a lack of nationally unified diagnostic criteria in Finland. Only women with the OGTT were included and hence women with very low risk of GDM and therefore no OGTT performed, according to the national guidelines, were not included. In addition, the comparison of abnormal fasting or postprandial glucose values was not evaluated in the present study.
5. CONCLUSION
Adverse perinatal and neonatal risks are higher in women with two or more abnormal OGTT values than in women with one abnormal OGTT value. Women with any number of abnormal OGTT values are at an increased risk of induced labor, and their newborns have hypoglycemia more often than controls. However, the risk of preterm delivery, primary cesarean section, and hyperbilirubinemia is increased only if two or more values are abnormal. Of note, women with one abnormal OGTT value also received counseling and self‐monitored their glucose values and therefore should not be considered as a risk‐free group not requiring follow‐up.
AUTHOR CONTRIBUTIONS
Sanna Eteläinen contributed to the conceptualization and conduct of the study, data analysis and drafting of the manuscript. Elina Keikkala contributed to the conceptualization and critically reviewing the manuscript. Shilpa Lingaiah contributed to data analysis, visualization and reviewing and editing the manuscript. Matti Viljakainen, Tuija Männistö, Anneli Pouta, Risto Kaaja, Johan G Eriksson, Hannele Laivuori, Mika Gissler and Eero Kajantie contributed to critically reviewing the manuscript. Marja Vääräsmäki contributed to the conceptualization and conduct of the study, and critically reviewing the manuscript. All authors approved the final version of the manuscript.
FUNDING INFORMATION
The study was supported by grants from the Medical Research Centre Oulu, the Academy of Finland, the Diabetes Research Foundation, Emil Aaltonen Foundation, the Foundation for Cardiovascular Research, the Foundation of Pediatric Research, the National Graduate School of Clinical Investigation, Novo Nordisk Foundation, Pohjois‐Suomen Terveydenhuollon Tukisäätiö Foundation, Signe and Ane Gyllenberg Foundation, the Finnish Medical Foundation, Otto Malm Foundation, Yrjö Jahnsson Foundation and Sigrid Jusélius Foundation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interests.
ETHICS STATEMENT
The study was approved by the regional ethics committee of the Northern Ostrobothnia Hospital District and the Finnish Institute for Health and Welfare (Reference no. 2008/43) on June 19, 2008. According to the Finnish legislation, informed consent from study participants is not required when using anonymous register‐data.
Eteläinen S, Keikkala E, Lingaiah S, et al. Perinatal and neonatal outcomes in gestational diabetes: The importance of the number of abnormal values in an oral glucose tolerance test. Acta Obstet Gynecol Scand. 2025;104:130‐138. doi: 10.1111/aogs.14999
REFERENCES
- 1. Wang H, Li N, Chivese T, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in pregnancy study Group's criteria. Diabetes Res Clin Pract. 2022;183:4. [DOI] [PubMed] [Google Scholar]
- 2. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
- 4. Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131:S173‐S211. [DOI] [PubMed] [Google Scholar]
- 5. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization guideline. Diabetes Res Clin Pract. 2014;103:341‐363. [DOI] [PubMed] [Google Scholar]
- 6. Roeckner JT, Sanchez‐Ramos L, Jijon‐Knupp R, Kaunitz AM. Single abnormal value on 3‐hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;215:287‐297. [DOI] [PubMed] [Google Scholar]
- 7. McLaughlin GB, Cheng YW, Caughey AB. Women with one elevated 3‐hour glucose tolerance test value: are they at risk for adverse perinatal outcomes? Am J Obstet Gynecol. 2006;194:e16‐e19. [DOI] [PubMed] [Google Scholar]
- 8. Shen S, Lu J, Zhang L, et al. Single fasting plasma glucose versus 75‐g Oral glucose‐tolerance test in prediction of adverse perinatal outcomes: a cohort study. EBioMedicine. 2017;16:284‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Black MH, Sacks DA, Xiang AH, Lawrence JM. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal Oral glucose tolerance test values. Diabetes Care. 2010;33:2524‐2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ding T, Xiang J, Luo B, Luo BJ. Relationship between the IADPSG‐criteria‐defined abnormal glucose values and adverse pregnancy outcomes among women having gestational diabetes mellitus. Medicine. 2018;97:e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gestational diabetes . Current Care Guidelines. Working group set up by the Finnish Medical Society Duodecim, the Medical Advisory Board of the Finnish Diabetes Association and the Finnish Gynecological Association. 2008. Finnish Medical Society Duodecim. Accessed 15 Mar 2024. www.kaypahoito.fi
- 13. Keikkala E, Mustaniemi S, Koivunen S, et al. Cohort profile: the Finnish gestational diabetes (FinnGeDi) study. Int J Epidemiol. 2020;49:762‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finnish Institute for Health and Welfare . Medical birth register: quality description, 2018. Accessed 15 Mar 2024 https://thl.fi/en/web/thlfi‐en/statistics‐and‐data/data‐and‐services/quality‐and‐statistical‐principles/quality‐descriptions/parturients‐delivers‐and‐newborns
- 15. Gissler M, Shelley J. Quality of data on subsequent events in a routine medical birth register. Med Inform Internet Med. 2002;27:33‐38. [DOI] [PubMed] [Google Scholar]
- 16. Gissler M, Teperi J, Hemminki E, Meriläinen J. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75‐80. [DOI] [PubMed] [Google Scholar]
- 17. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30:S42‐S47. [DOI] [PubMed] [Google Scholar]
- 18. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care. 2004;27:s88‐s90. [DOI] [PubMed] [Google Scholar]
- 19. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Billionnet C, Mitanchez D, Weill A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017;60:636‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogozinska E, D'Amico MI, Khan KS, et al. Development of composite outcomes for individual patient data (IPD) meta‐analysis on the effects of diet and lifestyle in pregnancy: a Delphi survey. BJOG. 2016;123:190‐198. [DOI] [PubMed] [Google Scholar]
- 22. Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population‐based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45:446‐454. [DOI] [PubMed] [Google Scholar]
- 23. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039‐1057. [DOI] [PubMed] [Google Scholar]
- 24. O'Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278‐285. [PubMed] [Google Scholar]
- 25. Bhavadharini B, Anjana RM, Deepa M, et al. Association between number of abnormal glucose values and severity of fasting plasma glucose in IADPSG criteria and maternal outcomes in women with gestational diabetes mellitus. Acta Diabetol. 2022;59:349‐357. [DOI] [PubMed] [Google Scholar]
- 26. Corrado F, D'Anna R, Cannata ML, et al. Positive association between a single abnormal glucose tolerance test value in pregnancy and subsequent abnormal glucose tolerance. Am J Obstet Gynecol. 2007;196:339.e1‐339.e5. [DOI] [PubMed] [Google Scholar]
- 27. Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, Thangaratinam S. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta‐analysis of 95,750 women. Diabetologia. 2016;59:1403‐1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiersch L, Shah BR, Berger H, et al. The prognostic value of the oral glucose tolerance test for future type‐2 diabetes in nulliparous pregnant women testing negative for gestational diabetes. Diabetes Metab. 2022;48:101364. [DOI] [PubMed] [Google Scholar]
- 29. Hakkarainen H, Huopio H, Cederberg H, Pääkkönen M, Voutilainen R, Heinonen S. Post‐challenge glycemia during pregnancy as a marker of future risk of type 2 diabetes: a prospective cohort study. Gynecol Endocrinol. 2015;31:573‐577. [DOI] [PubMed] [Google Scholar]
- 30. Hakkarainen H, Huopio H, Cederberg H, Pääkkönen M, Voutilainen R, Heinonen S. The risk of metabolic syndrome in women with previous GDM in a long‐term follow‐up. Gynecol Endocrinol. 2016;32:920‐925. [DOI] [PubMed] [Google Scholar]


