Abstract
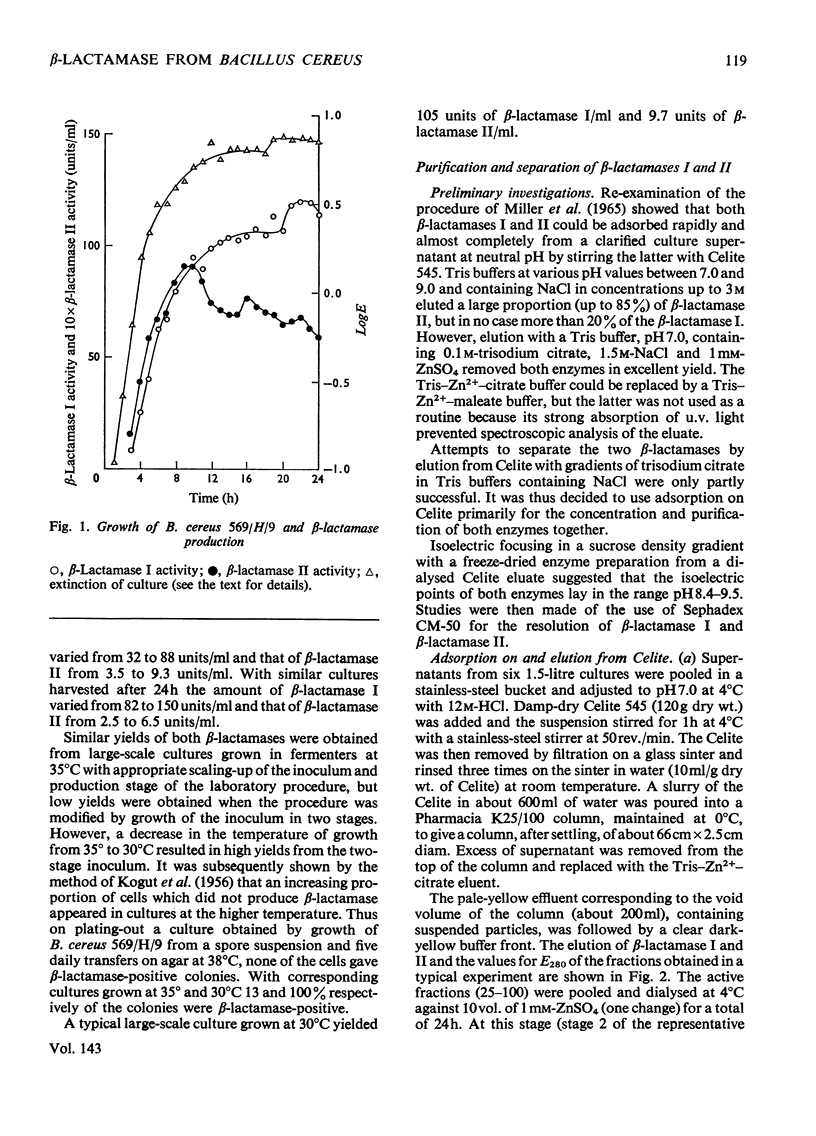
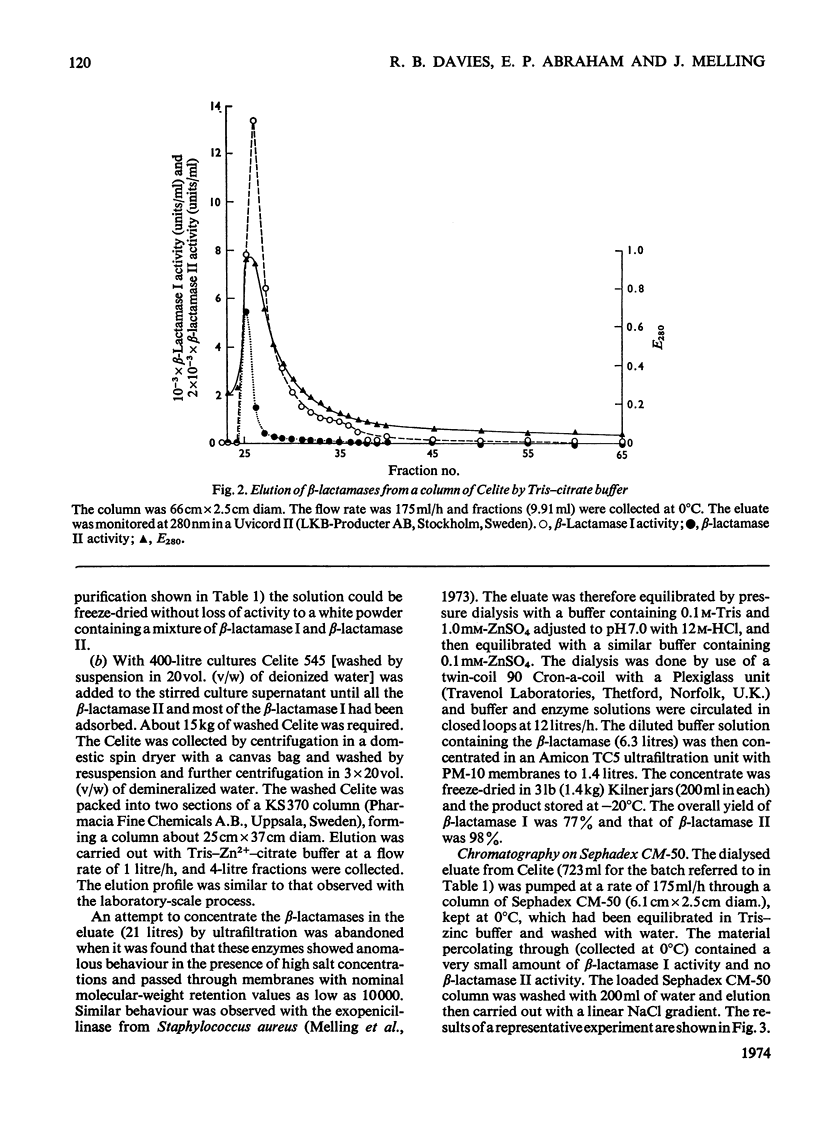
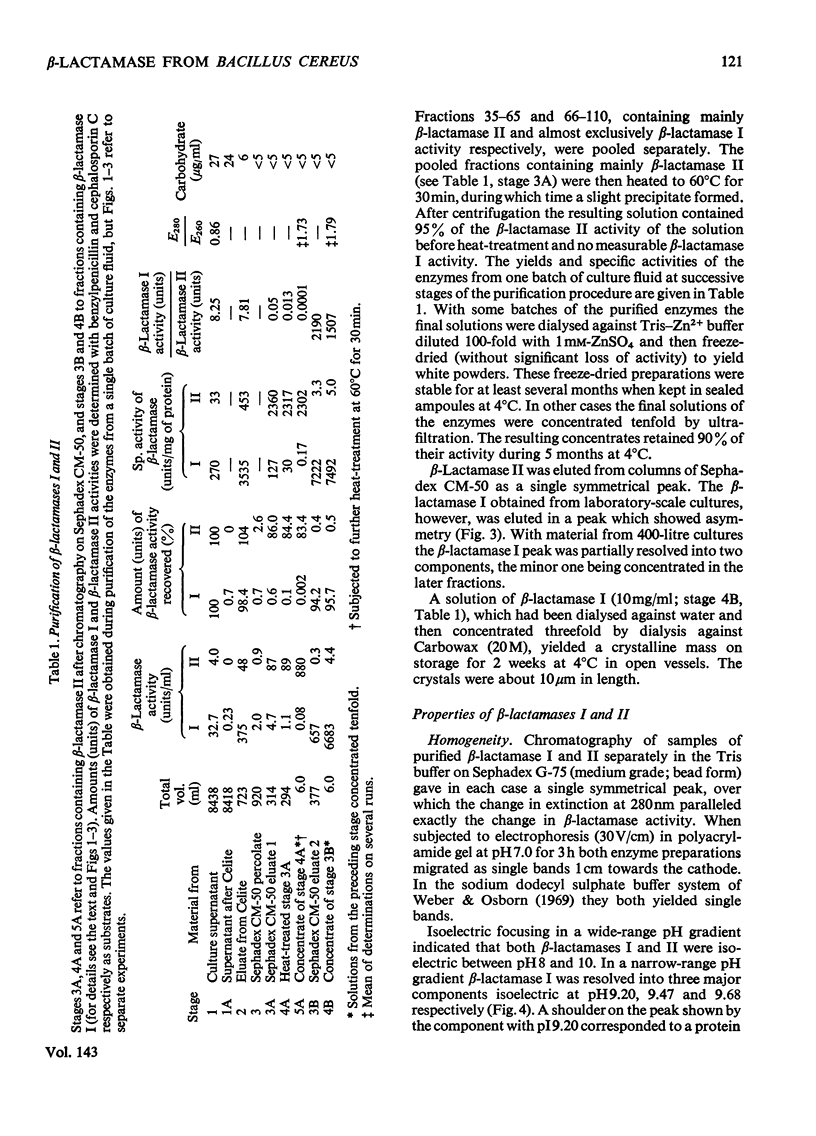
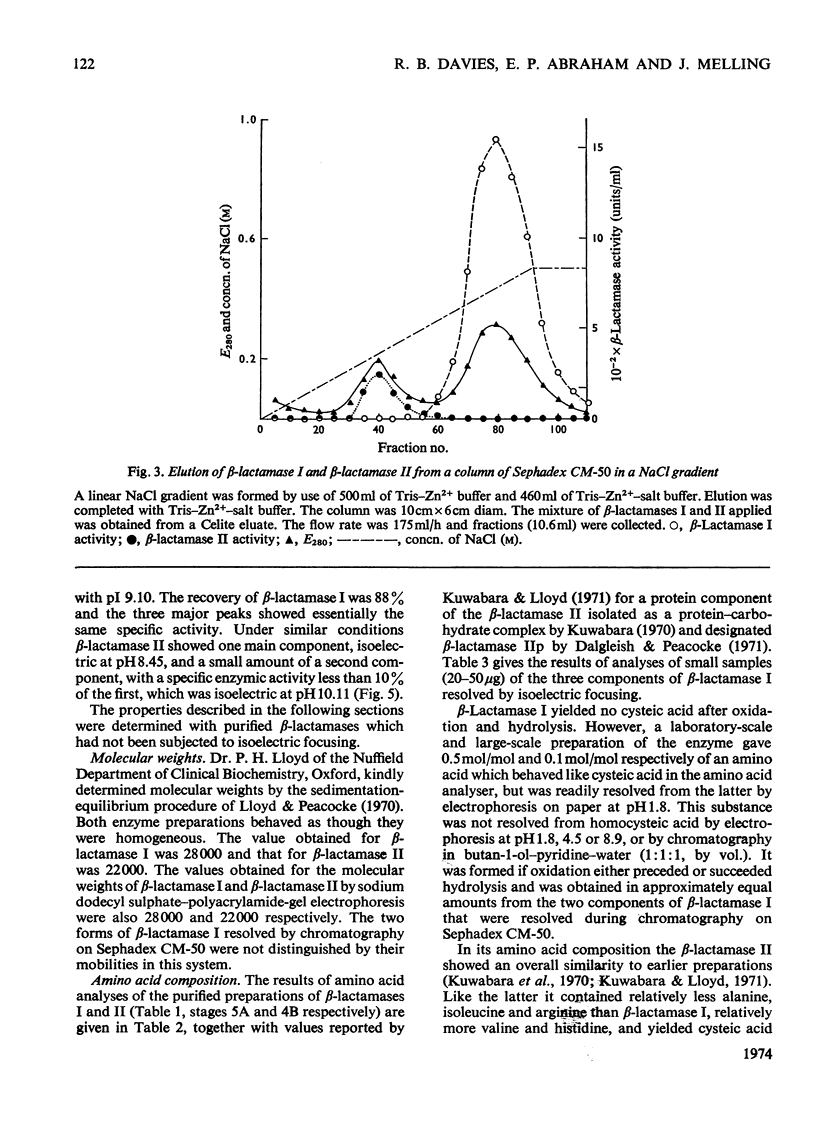
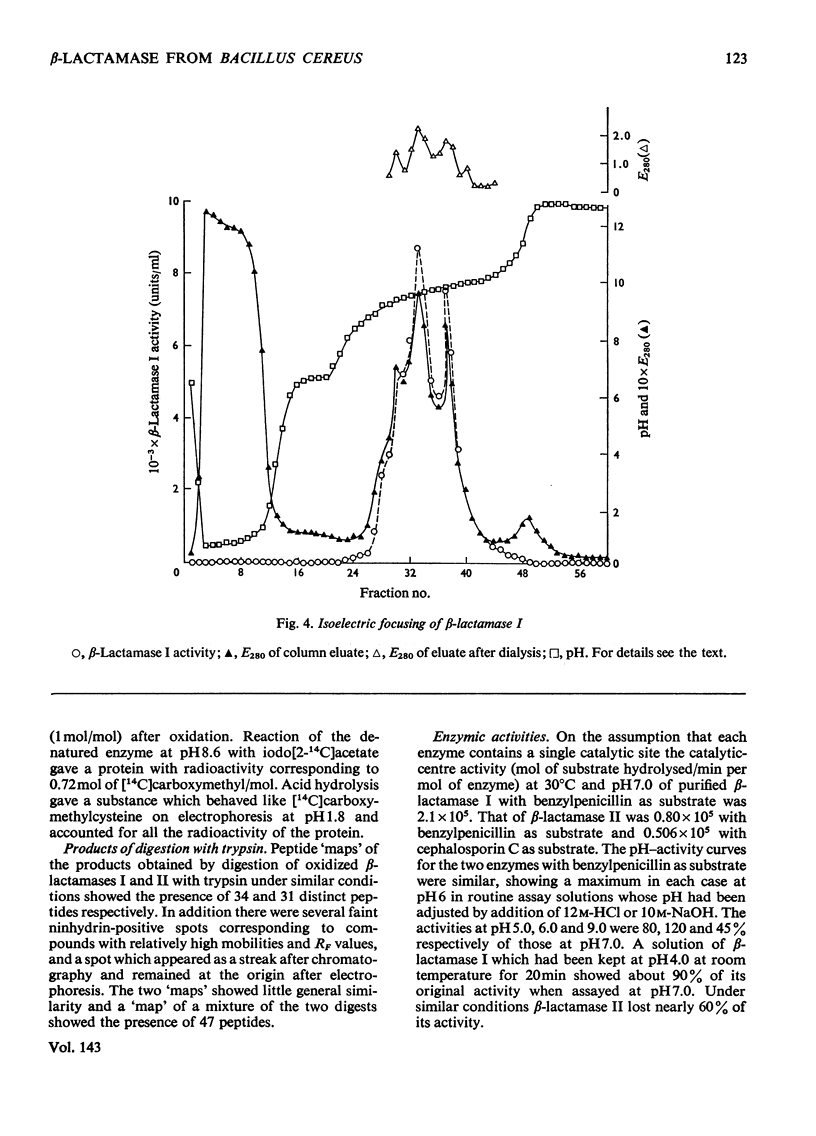
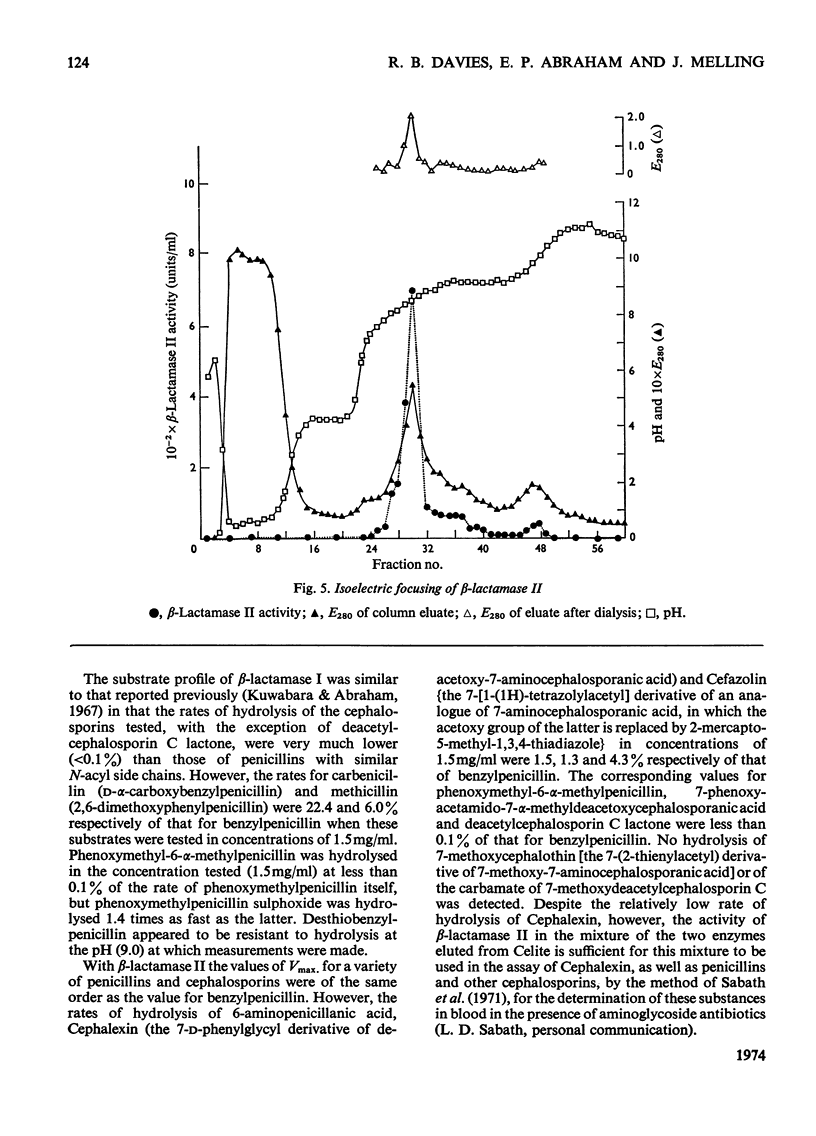
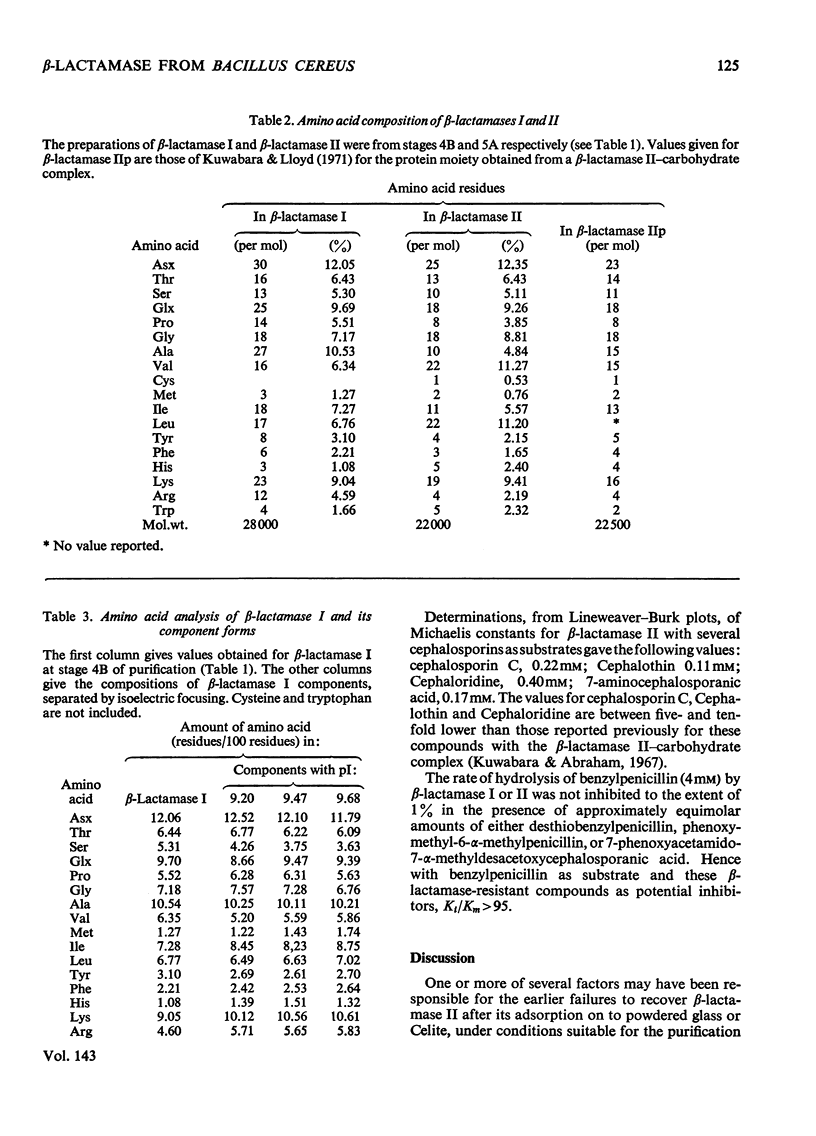
1. A procedure was devised which is suitable for the isolation of β-lactamase I and β-lactamase II from Bacillus cereus 569/H/9 on a large scale. After adsorption on to Celite both enzymes were eluted in good yield and separated by chromatography on Sephadex CM-50. 2. β-Lactamase I was separated into three main components by isoelectric focusing and into two components by chromatography. 3. The Zn2+-requiring β-lactamase II obtained by this procedure had a lower molecular weight (22000) than β-lactamase I (28000) and also differed from the latter in containing one cysteine residue. 4. The β-lactamase II contained no carbohydrate, but showed the thermostability of the enzyme isolated earlier as a protein–carbohydrate complex. 5. Amino acid analyses and tryptic-digest `maps' indicate that some degree of homology between β-lactamase I and β-lactamase II is possible, but that β-lactamase I is not composed of the entire sequence of β-lactamase II together with an additional peptide fragment. 6. A 6-methylpenicillin and a 7-methylcephalosporin showed much lower affinities for both enzymes than did penicillins and cephalosporins themselves.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcos J. M. Diferenciación de penicilinasas en Bacillus cereus. Rev Esp Fisiol. 1968 Sep;24(3):137–146. [PubMed] [Google Scholar]
- CROMPTON B., JAGO M., CRAWFORD K., NEWTON G. G., ABRAHAM E. P. Behaviour of some derivatives of 7-aminocephalosporanic acid and 6-aminopenicillanic acidas substrates, inhibitors and inducers of penicillinases. Biochem J. 1962 Apr;83:52–63. doi: 10.1042/bj0830052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri N., Pollock M. R. The biochemistry and function of beta-lactamase (penicillinase). Adv Enzymol Relat Areas Mol Biol. 1966;28:237–323. doi: 10.1002/9780470122730.ch4. [DOI] [PubMed] [Google Scholar]
- Dalgleish D. G., Peacocke A. R. Circular-dichroism studies on two -lactamases from Bacillus cereus. Biochem J. 1971 Nov;125(1):155–158. doi: 10.1042/bj1250155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOYD N. F., CAMMAROTI M. S., LAVINE T. F. THE DECOMPOSITION OF DL-METHIONINE SULFOXIDE IN 6 N HYDROCHLORIC ACID. Arch Biochem Biophys. 1963 Sep;102:343–345. doi: 10.1016/0003-9861(63)90239-x. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Newton G. G., Abraham E. P. Products of aminolysis and enzymic hydrolysis of the cephalosporins. Biochem J. 1970 Feb;116(3):371–384. doi: 10.1042/bj1160371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J., Gillin F. D., Tanis R. J., Atherly A. G. Properties of penicillinase from Bacillus cereus 569. J Biol Chem. 1970 May 10;245(9):2205–2212. [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- KOGUT M., POLLOCK M. R., TRIDGELL E. J. Purification of penicillin-induced penicillinase of Bacillus cereus NRRL 569: a comparison of its properties with those of a similarly purified penicillinase produced spontaneously by a constitutive mutant strain. Biochem J. 1956 Mar;62(3):391–401. doi: 10.1042/bj0620391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Adams E. P., Abraham E. P. The composition of beta-lactamase I and beta-lactamase II from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):475–480. doi: 10.1042/bj1180475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Lloyd P. H. Protein and carbohydrate moieties of a preparation of -lactamase II. Biochem J. 1971 Aug;124(1):215–220. doi: 10.1042/bj1240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of two extracellular beta-lactamases from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):457–465. doi: 10.1042/bj1180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd P. H., Peacocke A. R. Sedimentation-equilibrium studies on the heterogeneity of two beta-lactamases. Biochem J. 1970 Jul;118(3):467–474. doi: 10.1042/bj1180467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. A new technique for stirred aerated culture. Nature. 1949 Nov 12;164(4176):846–846. doi: 10.1038/164846a0. [DOI] [PubMed] [Google Scholar]
- Madaiah M., Day R. A. Amino acid composition and peptide maps of Bacillus cereus 569-H penicillinase. Biochim Biophys Acta. 1971 Apr 27;236(1):191–196. doi: 10.1016/0005-2795(71)90164-4. [DOI] [PubMed] [Google Scholar]
- Melling J., Scott G. K. Preparation of gram quantities of a purified R-factor-mediated penicillinase from Escherichia coli strain W3310. Biochem J. 1972 Nov;130(1):55–62. doi: 10.1042/bj1300055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECHERE J. F., ZANEN J. Possible production of several exopenicillinases by Bacillus cereus. Nature. 1962 Aug 25;195:805–806. doi: 10.1038/195805b0. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Cephalosporinase and penicillinase activity of Bacillus cereus. Antimicrob Agents Chemother (Bethesda) 1965;5:392–397. [PubMed] [Google Scholar]
- Sabath L. D., Casey J. I., Ruch P. A., Stumpf L. L., Finland M. Rapid microassay of gentamicin, kanamycin, neomycin, streptomycin, and vancomycin in serum or plasma. J Lab Clin Med. 1971 Sep;78(3):457–463. [PubMed] [Google Scholar]
- Sabath L. D., Finland M. Thiol-group binding of zinc to a beta-lactamase of Bacillus cereus: differential effects on enzyme activity with penicillin and cephalosporins as substrates. J Bacteriol. 1968 May;95(5):1513–1519. doi: 10.1128/jb.95.5.1513-1519.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Warren S. C., Newton G. G., Abraham E. P. Biosynthesis of penicillin N and cephalosporin C. Antibiotic production and other features of the metabolism of Cephalosporium sp. Biochem J. 1967 Jun;103(3):877–890. doi: 10.1042/bj1030877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



