Abstract
Underground reservoirs were used to purify water through filtration, adsorption, and biodegradation methods in coal mines. However, their effects on the composition and metabolism of microbial communities in mine water remain unknown. In this study, influent and effluent samples from underground reservoirs in seven coal mining areas were analyzed to compare their microbial community compositions and metabolites. The results indicate that the underground reservoirs can significantly reduce the chemical oxygen demand (COD) levels of mine water (values range from 7.79 to 8.50 for influent and 7.40 to 8.50 for effluent) and regulate water chemistry characteristics such as pH (values range from 7–1980 for influent and 5–20 for effluent). Since COD reflects the quantity of reducing substances in water, while total dissolved solids (TDS) indicates the total amount of dissolved solids, both parameters directly influence the physicochemical properties of water. This, in turn, affects microbial growth and metabolic pathways. Consequently, changes in these factors can lead to variations in microbial community composition as well as decreases in the Chao1 and Shannon indices. Underground reservoirs decreased the relative abundance of phyla Actinomycetota and Spirochaetota, and the decreased microbial groups were mainly belonging to phylum Bacillota. In addition, higher concentrations of metabolites, including lipids and lipid-like molecules, mixed metal/nonmetal compounds, hydrocarbon derivatives, nucleosides, nucleotides, and analogues were detected in the effluent samples, and most of them were related to taurine and hypotaurine metabolism, selenocompound metabolism, glyoxylate and dicarboxylate metabolism, riboflavin metabolism, and the citrate cycle. In summary, this study provided theoretical and experimental support for the evolution mechanism of mine water quality in coal mine underground reservoirs.
Introduction
Water scarcity has always limited human survival in arid regions of the world. However, the frequent coal mining activities, such as in the northwest arid area of China, increase the demand for water, and the produced mine water poses a serious threat to the fragile local water environment.1 Therefore, achieving coordination between coal development and water resource protection has become the core focus of ecological civilization construction in western Chinese coal mining areas.2 The mine water generated during coal mining activities mainly comes from underground sewage, coal mining working face water, and seepage from overlying rock strata. The mine water contains various components such as heavy metals, nitrogen, organic compounds, and SO42– and S2– ions that have adverse effects on the ecological environment,3 improper treatment of which may result in groundwater pollution.4
Previous studies have found that organic pollutants, total nitrogen, suspended solid particles, and total dissolved solids in mine water can be effectively removed during storage and transportation in coal gangue-encapsulated coal mine underground water reservoirs through filtration, adsorption, and biodegradation methods.3,5 Based on this, the underground reservoir has been designed in the coal mines for the recovery of mine water to effectively utilize underground waste space for protecting water resources.6,7 Its feature lies in the guidance, storage, and utilization of coal mine water.8
The technology of underground reservoirs has been used to solve the problems of mine water discharge, evaporation loss, high cost of surface water treatment plant construction and operation.9−11 It utilizes the voids between crushed rock masses in the goaf as water storage space, connects the safe coal pillars around the goaf with artificial dam bodies to form a reservoir dam body, and fully utilizes the natural purification of mine water by rock masses in goaf.12,13 Currently, a total of 35 underground reservoirs have been built in the northwest arid area of China, with a storage capacity of 26 million cubic meters. These underground reservoirs are used to meet the basic water demand of the mining areas and provide water supply for surrounding industries,14 promoting the protection and utilization of water resources in coal mines.15 For instance, the purified water from these reservoirs is redirected to coal seams for underground production reuse or to the surface for industrial and greening purposes, enhancing the conservation and utilization of water resources in coal mining. However, the effect of the underground reservoir technology on the biochemical properties of mine water has rarely been reported.
Microbial communities are the main drivers of biogeochemical cycles in various ecosystems, including underground water, and play important roles in material cycles, energy conversion, and information transfer.16 The specificity of microbial community composition can reflect and influence the physicochemical characteristics of underground water.17−19 Microbial activities are closely associated with chemical reactions of substances such as SO42–, NO3–, NO2–, NH4+, Fe, and Mn in mine water.20,21 It is also noteworthy that photons may serve as another significant electron donor driving biogeochemical processes in mining regions.22 Previous studies have shown that the activities of microbial communities were closely related to the formation of acid mine drainage and its impact on the microbial community structure and hydrochemical characteristics of surface ecosystems.23−25 For instance, during coal mining processes, some sulfides in coal are oxidized and hydrolyzed by various microorganisms, leading to the production of sulfuric acid, ferrous hydroxide, and even the formation of acid mine drainage (AMD) (pH < 6).26 Zhang et al.27 reported that when AMD flows into the Hengshui River, the bacterial community diversity along the river significantly increases, pollution levels decrease, and the relative abundance of bacteria with metal-reducing functions decreases along the AMD gradient. Therefore, understanding the implementation of underground reservoirs is crucial for the microbial community in mine water and biogeochemical cycles in groundwater ecosystems.
In this study, the microbiome and metabolome of water samples from multiple underground reservoirs were compared between the influent and effluent water. The aims were to analyze the effects of underground reservoirs on microbial communities and metabolic processes and to provide theoretical and experimental support for the evolution mechanism of mine water quality in coal mine water reservoirs.
Materials and Methods
Overview of the Study Area and Sample Collection
The Shendong Mining Area is located on the northern edge of the northern Shaanxi Plateau and the southeastern part of the Ordos Plateau, situated in the transitional zone between the northern edge of the Loess Plateau in northern Shaanxi and the eastern section of the Mu Us Desert. It falls within an arid to semiarid region. The overall terrain of the mining area is characterized by high elevation in the northwest and low elevation in the southeast, with an average altitude of 800 to 1385 m. The area is crisscrossed with valleys, well-developed crevices, and severe soil erosion. The mining area consists of two main types of landscapes: aeolian and loess landforms, with aeolian landforms accounting for 90% and reaching thicknesses of 20 to 50 m.
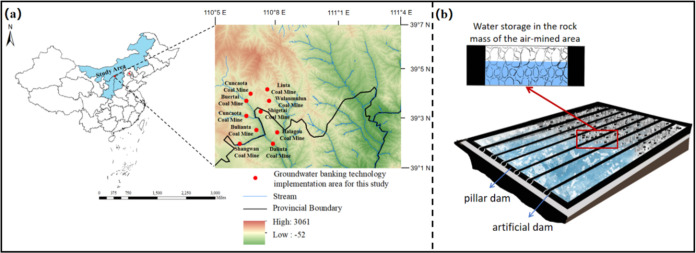
Shendong Coal Group (China) first proposed the construction of underground reservoirs in coal mines and established the theoretical framework and technical system of underground reservoirs at Buertai Coal Mine (E 109.87–110.08°, N 39.38–39.53°), Cuncaota Coal Mine (E 110.05–110.07°, N 39.48–39.49°), Wulanmulun Coal Mine (E 100.03–110.18°, N 39.42–39.55°), Bulianta Coal Mine (E 109.93–110.17°, N 39.25–39.40°), Shangwan Coal Mine (E 110.18–110.20°, N 39.28–39.30°), Daliuta Coal Mine (E 110.33–110.38°, N 39.23–39.28°), Shigetai Coal Mine (E 109.20–110.22°, N 36.27–39.28°), and other mining areas. The water sources of the underground reservoir included atmospheric precipitation entering the goaf through the fractured zone formed along the mining activities, the water content of various aquifers, and underground production wastewater injected into the goaf through drainage pipes. Various water sources gradually converge from the higher part of the goaf to the relatively low-lying area, forming a natural underground water reservoir. This research collected 13 influent samples from the injection points, central water tanks, and wastewater treatment plants and 11 effluent samples from the discharge points. Due to confidentiality agreements, the specific GPS coordinates of the sampling locations cannot be disclosed. The corresponding coal mines for the samples are illustrated in Figure 1, and the sampling for each mine is shown in Table S1.
Figure 1.
Geographical location of the implementation area of coal mine underground water reservoir technology (a) and schematic diagram of the coal mine underground water reservoir (b).
Water Chemistry Characteristics
The water chemistry characteristics were measured through on-site testing and laboratory analysis. The pH value of water samples was determined on-site using a portable pH meter (OHAUS ST20). Free HCO3– was measured in the laboratory within 48 h of sampling using an acid titration method (automatic potentiometric titrator, Metrohm, Switzerland). Anions (NO3–, F–) and cations (Ca2+, Mg2+, NH4+) were determined using ion chromatography (Dionex Integrion IC, Thermo Fisher) with a measurement accuracy of 0.1 mg/L. The total dissolved solids (TDS) were measured by gravimetry. Chemical oxygen demand (COD) was analyzed by using the potassium dichromate index method.
Total DNA Extraction and 16S rRNA Gene Sequencing
Total genomic DNA was extracted from water samples by using the AquaScreen rapid extraction method. The 16S rRNA gene V4 region fragments of the microbial community were amplified using primers 515F/806R (515F: 5′-GCC AGC MGC CGC GGT AA-3′; 806R: 5′-CCG GAC TAC HVG GGT WTC TAA T-3′) and sequenced on an Illumina high-throughput sequencing platform. The microbial community analysis and diversity assessment were performed by using the QIIME2 pipeline. Single-sample data were segregated using the DADA2 plugin, where primer sequences were removed, sequencing quality control was performed, and sequences lacking sample information, those that were too short or too long, along with sequences classified as “chloroplast”, “mitochondrial”, or “unclassified” were eliminated. Additionally, sequences were denoised, and chimeras were removed. Operational Taxonomic Units (OTUs) were classified by using the SILVA reference database (version 138.2), determining the taxonomic rank for each sequence. All detected OTUs were categorized based on a sequence similarity threshold of 97%. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, and Chinese Academy of Sciences (PRJCA031096) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
Untargeted Metabolomics Analysis
LC-QTOF/MS (Agilent 6530 Q-TOF LC/MS, America) was used in ion mode for data acquisition on various water samples. Data preprocessing is conducted using metaX,28 including imputation of missing values and removal of low-quality ions using the KNN method, and correction through Quality Control-based Robust LOESS Signal Correction (QC-RSC).29 Ions with relative standard deviation (RSD) > 30% are filtered out. Peak alignment, peak picking, normalization, deconvolution, and compound identification are performed using Progenesis QI (v 2.2), with metabolite identification based on the KEGG database.
Statistical Analysis
Normalization of sequence counts was performed through random subsampling of microorganisms. The α diversity indices, namely, Chao1 and Shannon indices, were computed using QIIME and assessed for differences through the Mann–Whitney U test. The β diversity was represented by the Bray–Curtis dissimilarity based on abundance. Nonmetric multidimensional scaling (NMDS) was conducted to illustrate the distribution patterns of microbial communities with relative abundances exceeding 1%. Principal component analysis (PCA) of the metabolite composition data from the groundwater reservoir was executed using a MetaboAnalyst. Furthermore, redundancy analysis (RDA) was conducted using the vegan package in R software to investigate the relationships between microbial communities and environmental factors, examining the impact of environmental variables on the structures of aquatic microbial communities. Statistical analysis, pathway analysis, and enrichment analysis were carried out through the official MetaboAnalyst Website (https://www.metaboanalyst.ca/).
Results
Underground Reservoirs Affected the Hydrochemical Characteristics of Mine Water
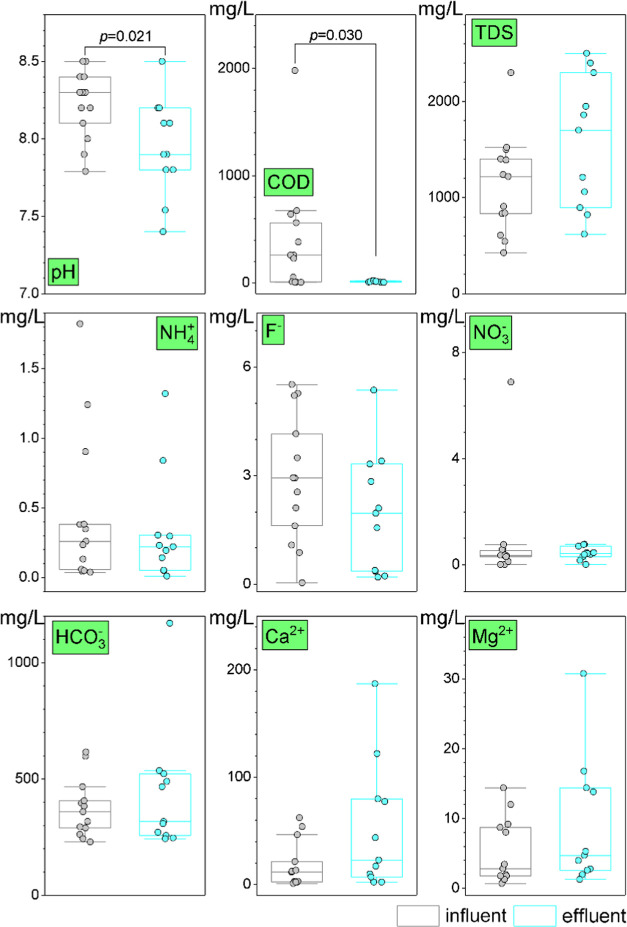
The hydrochemical characteristics of the influent and effluent water samples of the underground reservoirs showed significant fluctuations. The underground reservoirs could significantly decrease the COD content of mine water and the pH value (Figure 2). The pH value ranged from 7.79 to 8.50 in the influent water samples and from 7.40 to 8.50 in effluent water samples (p = 0.021). The COD concentration of the effluent water samples ranged from 5–20 mg/L and was significantly lower than that of the influent water samples (p = 0.030). The other hydrochemical characteristics exhibited no significant changes (Figure 2).
Figure 2.
Major hydrochemical characteristics at the influent and effluent of the underground water reservoirs in the mines. The p-values were reported for only significant differences between the influent and effluent water samples by the Mann–Whitney U test.
Microbial Diversity and Composition Differences
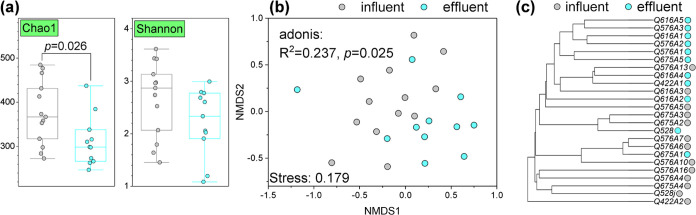
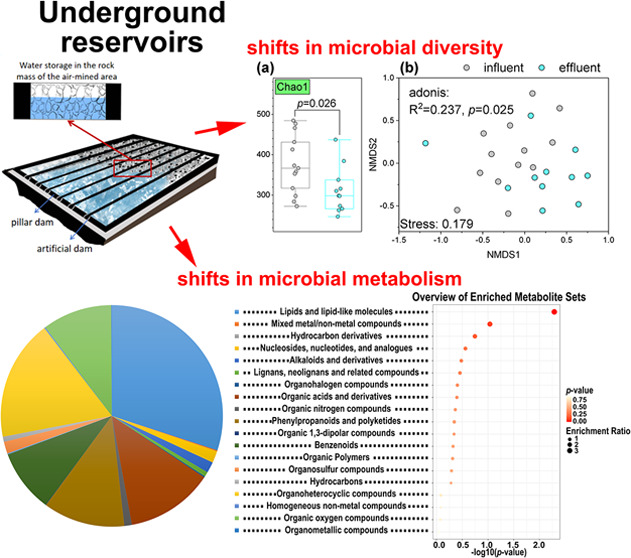
The Chao1 and Shannon indices were used to indicate the richness and diversity of microbial communities in the groundwater reservoir influent and effluent (Figure 3a). The Chao1 richness of the effluent water was significantly lower than that of the influent water. Nonmetric multidimensional scaling (NMDS) based on the Bray–Curtis similarities of a systematic composition showed a separation of microbial communities from the influent and effluent water samples (Figure 3b). The more distinct separation between the inflow and outflow is illustrated in the UPGMA tree (Figure 3c). Overall, there were significant differences in the distribution of microbial communities between the influent and effluent of the groundwater reservoirs.
Figure 3.
Changes in microbial diversities in influent and effluent of underground water reservoir. (a) Chao1 index (p < 0.05) and Shannon index. The comparison of influent and effluent water samples was conducted using the Mann–Whitney U test. (b) Sorting microbial community composition using Bray–Curtis distance and nonmetric multidimensional scaling (NMDS). (c) Hierarchical clustering of Bray–Curtis similarity matrices for influent and effluent samples (UPGMA tree).
Overall Classification and Composition Changes of Microorganisms
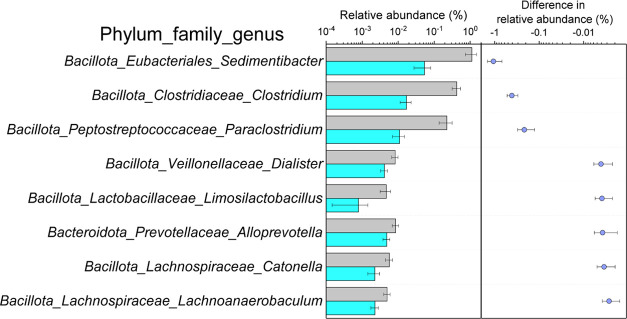
After removing low-quality and chimeric sequences, a total of 1,311,582 valid sequence reads were obtained from 24 water samples. To ensure comparability between different samples, samples were rarefied to 40,176 based on the minimum number of sequences. All sequences were assigned to 758 valid OTUs with 97% similarity. At the genus level, the nine dominant genera exhibited lower abundances in the effluent compared with the influent. This was particularly evident for the three genera—Sedimentibacter, Clostridium, and Paraclostridium—that are most abundant in the influent, as their abundances significantly decreased in the effluent (Figure 4). Additionally, we conducted analyses at the phylum and family levels to further characterize the predominant microbial taxa present in both the influent and effluent samples (Figure S1).
Figure 4.
Dominant taxa at the genus level in influent and effluent samples. The p-values were reported for only significant differences by the Mann–Whitney U test.
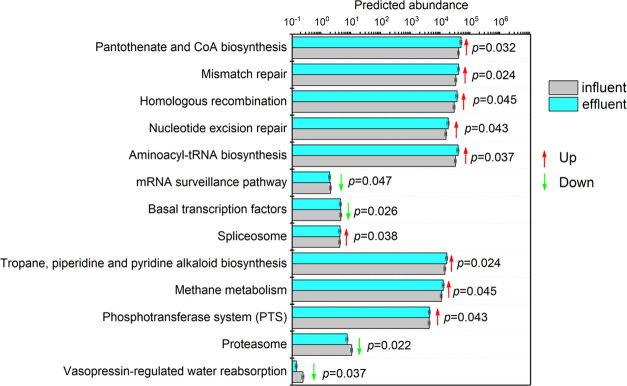
Predictive Metabolic Function Difference
The metabolic function prediction analysis of samples at the influent and effluent was performed by PICRUSt (Figures 5 and S1). Differential metabolism analysis revealed that most of the tertiary metabolic pathways in the water discharged from the coal mine underground reservoirs were upregulated compared to those in the influent water samples (Figure 5). Whereas in the influent water, four tertiary metabolic pathways, including mRNA surveillance pathway in translation, basal transcription factors in transcription, proteasome in folding, sorting, and degradation, and vasopressin-regulated water reabsorption in the excretory system, showed increased levels compared to the effluent water.
Figure 5.
Differential predictive function of the influent and effluent water at level 3. The p-values were reported for significant differences by the Mann–Whitney U text.
Difference in Metabolites between the Influent and Effluent Water Samples
The composition of metabolites in the influent and effluent water samples of the underground reservoirs exhibited a significant degree of separation, with contributions of 31.51, 23.25, and 10.57% from PC1, PC2, and PC3, respectively (Figure 6). Some samples demonstrated a degree of clustering, indicating that the composition of metabolites within the underground reservoirs experienced only a limited extent of variation. The specific composition of metabolites in the underground reservoirs is shown in Figure S3.
Figure 6.
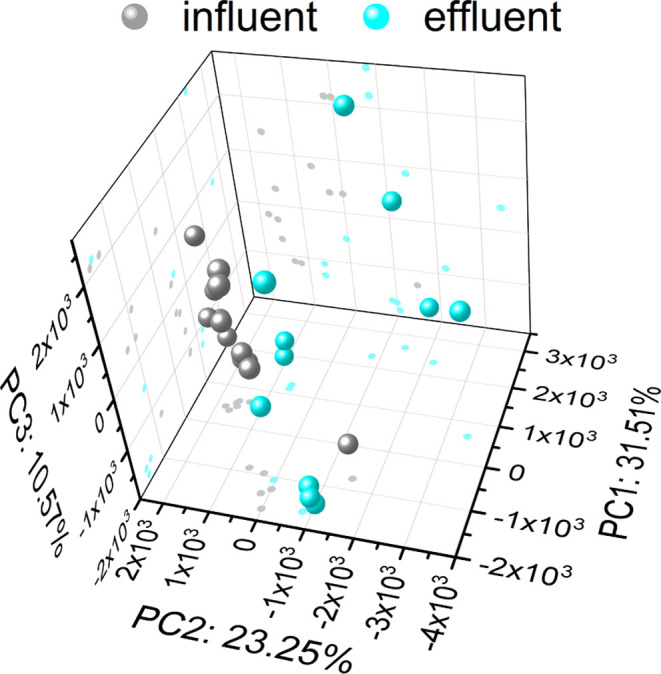
Principal component analysis (PCA) for the metabolites between the influent and effluent water samples.
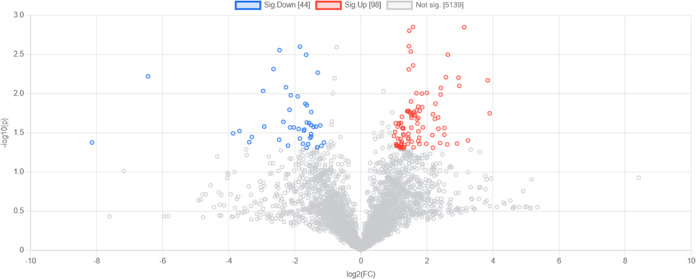
The volcano plot further displayed significant changes in metabolites between the influent and effluent of the groundwater reservoir (Figure 7). After they passed through the groundwater reservoir, a total of 98 metabolites, including γ-glutamyl-lysine, 3-iodopropanoic acid, peltatol A, and pipazethate, significantly increased. Additionally, 44 metabolites significantly decreased, including montecristin, dipivefrin, and riboflavin (Table S2).
Figure 7.
Volcano plot illustrates the fold changes in metabolites associated with the influent and effluent of the underground reservoirs, represented by p-values. Red dots indicate an increase in metabolites within the outflow samples, whereas blue dots denote a decrease in metabolites (p < 0.05, FC > 1.5). The threshold for screening differential metabolites was set at FDR < 0.05.
KEGG enrichment pathway analyses indicated that the differential metabolites derived from underground reservoir interventions in groundwater play a significant role in the metabolic processes of groundwater microorganisms (Figure 8). The metabolic pathway enrichment bubble plots, with log (p) values represented on the vertical axis and the pathway impact depicted on the horizontal axis, reflect weight calculations informed by topological analysis. This involved summing the importance measures of each matched metabolite and normalizing it by the total importance measures of all metabolites within each pathway. The primary five metabolic pathways that exhibit significant enrichment are taurine and hypotaurine metabolism, selenocompound metabolism, glyoxylate and dicarboxylate metabolism, riboflavin metabolism, and the citrate cycle (TCA cycle). The distinct impacts of groundwater banking on each of these pathways are illustrated in Figures S4–S8.
Figure 8.
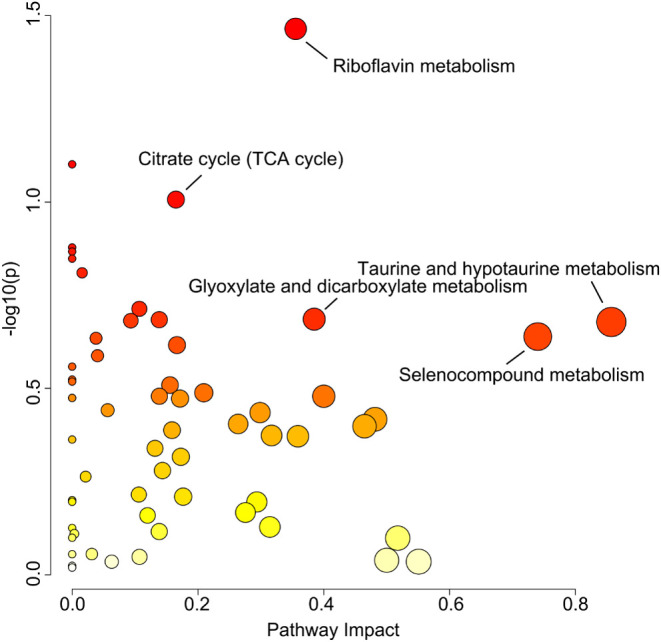
Advantageous metabolic pathways enriched in the underground water reservoir. The horizontal axis represents the impact size of the metabolic pathways. The deeper the color of the bubble, the stronger the significance of the metabolic pathway. The larger the bubble, the more metabolites are enriched in that metabolic pathway.
Factors Affecting the Components of Microbial Communities and Metabolites
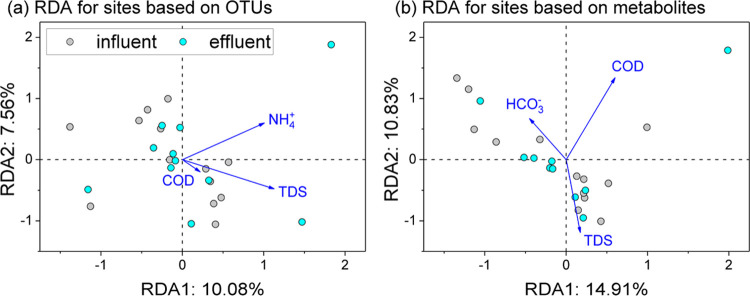
TDS, NH4+, and COD exhibited significant positive correlations with the values of the OTUs (Figure 9a). Notably, NH4+ showed a strong positive correlation with the presence of OTUs, specifically in the influent water samples, while both TDS and COD were positively correlated with the presence of OTUs across the influent and effluent water samples. Additionally, TDS, COD, and HCO3– demonstrated positive correlations with metabolites in both influent and effluent water samples (Figure 9b). It is evident that TDS and COD were primary environmental factors significantly impacting the microbial communities and their metabolic activities within underground reservoirs.
Figure 9.
Redundancy analysis (RDA) illustrates the association between the chosen environmental factors and operational taxonomic units (OTUs) (a) and metabolites (b) within the water samples. A forward selection approach for predictor variables was implemented, complemented by Monte Carlo permutation tests (999 iterations). The solid arrows denote predictor variables that exhibited significant correlations (p < 0.05) with variations in both OTU and metabolite compositions.
Discussion
In this research, we investigated the chemical properties, microbiological profiles, and metabolomic signatures of the inflow and outflow of subterranean coal mine water reservoirs. Our objective of this study was to understand the influence and mechanisms by which the deployment of these underground coal mine water reservoirs affects the dynamics of microbial community evolution in the aquatic ecosystems of subsurface environments. The results indicated that underground water reservoirs could regulate the water quality of mine water, alter the composition and diversity of microbial communities, and further affect the metabolic processes in mine water, all of which reflected the changes in the biological and chemical characteristics of underground reservoirs. Therefore, this study allowed us to deepen our understanding of the evolution mechanisms of water quality in underground reservoirs.
The underground reservoir in the study area mainly affected the pH and the COD of the mine water. The pH of the water effluent shows a noticeable decrease compared to that of the influent, and COD has been effectively removed. Previous studies have shown that weak water exchange between mine water and surrounding aquifers, along with microbial activity, can gradually improve water quality.30 The construction of underground reservoirs involved artificial dam bodies, and the materials used might adsorb and filter substances, such as organic matter, in mine water. Additionally, the ability of local microorganisms to degrade organic pollutants was limited,31 so it was essential not to overly emphasize the role of microorganisms in the purification of groundwater within underground reservoirs. We observed the influence of environmental factors on the synthesis of OTUs. Similarly to other mines, there are significant differences in COD between influent and effluent water, closely related to microorganisms and substances sensitive to oxidation–reduction reactions.30
At the same time, after the treatment of mine water through the underground reservoirs, there was a notable decline in microbial diversity, and the distribution of microbial communities underwent significant changes (Figure 3). Previous studies have indicated that in underground water heavily polluted by oil, the total number of microbial species and microbial diversity significantly decreases, leading to the enrichment of microbes capable of degrading oil pollutants.32 Therefore, it can be considered that underground reservoirs significantly reduce COD in mine water, which is an important reason for reducing microbial diversity. Like in other mining areas, aquifers, and rivers, Pseudomonadaceae dominated the underground water reservoir.33,34 A significant difference was observed with Actinomycetota and Spirochaetota, as their abundances in the effluent notably decreased. This might be related to Actinomycetota mainly being aerobic saprophytic bacteria, which were also commonly found in surface water and other oxygen-contacting environments in other mining areas.30 Given the decrease in the COD concentration in the effluent water samples, one would expect an increase in the population of the anaerobic bacteria. However, the levels of anaerobic genera such as Sedimentibacter, Clostridium, Paraclostridium, Dialister, Limosilactobacillus, Catonella, and Lachnoanaerobaculum are all declining (Figure 4). This raised the question of whether there is a deficiency of nutrients in the groundwater reservoir environment or the emergence of certain toxic substances that could be detrimental to the growth of these anaerobic bacteria.
The shifts in the microbial community further caused the change in the metabolism of mine water. The predicted functional genes indicated that a total of nine metabolic pathways, including pantothenate and CoA biosynthesis, mismatch repair, homologous recombination, nucleotide excision repair, aminoacyl-tRNA biosynthesis, spliceosome, tropane, piperidine, and pyridine alkaloid biosynthesis, methane metabolism, and the phosphotransferase system (PTS), exhibited higher abundance at the effluent water. Pantothenate and CoA biosynthesis can detect and degrade abnormal RNA;35 mismatch repair plays a crucial role in maintaining genome stability;36 homologous recombination accurately repairs DNA double-strand breaks (DSB), potential lethal damage;37 nucleotide excision repair can identify and repair extensive DNA damage caused by compounds, environmental carcinogens, and UV radiation;38 the presence of spliceosome enables transesterification to occur;39 and the phosphotransferase system is the primary mechanism through which bacteria absorb carbohydrates (especially hexoses, hexitol, and disaccharides).40 The increased abundance of these predicted functional genes indicates that the underground reservoir potential promoted the normal functioning of genes and normal life activities of microorganisms, thereby maintaining their stability. Moreover, this stability is crucial for maintaining the ecological integrity of the aquifer systems as it underlines the intricate relationships between microbial communities and their environments. The adaptability of these microorganisms, driven by the functional genes present, suggests a robust resilience to fluctuating conditions that may arise due to anthropogenic influences or natural variability. As such, understanding the genetic underpinnings of these organisms can provide valuable insights into their roles in biogeochemical cycles, ultimately contributing to better management practices for groundwater resources.
In addition, the difference in the metabolome reflects that it is not completely consistent with the predicted function genes. These changes were mainly reflected in taurine and hypotaurine metabolism, selenocompound metabolism, glyoxylate and dicarboxylate metabolism, riboflavin metabolism, and the TCA cycle. The TCA cycle was a vital aerobic pathway for the final steps of carbohydrate and fatty acid oxidation, holding significant importance in the metabolism within underground reservoirs (Figure S4). Previous studies have revealed that taurine serves as a carbon and energy source for many aerobic bacteria,41 with potentially high utilization rates in the environment. Within the enriched dominant metabolic pathway of taurine and hypotaurine metabolism, the degradation pathway of taurine was remarkably comprehensive, leading to the production of substances such as 5-glutamyl-taurine, aminoacetaldehyde, and sulfite (Figure S5). Selenium is an essential trace element in many organisms, including humans and animals.42 The underground reservoir actively engages in selenocompound metabolism (Figure S6) to maintain a balance of selenium concentrations. The metabolic systems of glyoxylate and dicarboxylate metabolism and riboflavin metabolism are extensive, with many enzymes involved in relevant processes within the underground reservoir (Figures S7 and S8). With the operation of these dominant metabolic pathways, significant separation of metabolites from inflow water occurs in mine water after it passes through the underground reservoir (Figure 7), leading to notable changes in component abundance (Figure S3). This results in a significant increase in the content of lipids and lipid-like molecules, mixed metal/nonmetal compounds, hydrocarbon derivatives, nucleosides, nucleotides, and analogues in the outflow. Further research is required to determine the specific impacts of these dominant metabolites on the underground reservoir.
Conclusions
This research highlights the impact of subterranean reservoirs on biochemical attributes, including microbial ecosystems and metabolomic profiles. Notably, these alterations were primarily associated with a significant reduction in chemical oxygen demand (COD) in the mining effluent. The capacity of underground reservoirs to modulate the biochemical characteristics of groundwater is crucial for the management and mitigation of groundwater contamination. Moreover, the study further elucidates the relationship between reservoir dynamics and the prevalence of specific microbial taxa, which appeared to thrive in altered physicochemical environments. The implications of these findings suggest that subterranean reservoirs not only serve as natural buffers against contaminants but also facilitate the bioremediation processes essential for restoring groundwater quality. Future investigations could expand on the long-term sustainability of these ecosystems, emphasizing their role in ecological resilience and the potential for integrating such systems into broader water management strategies.
Acknowledgments
This study was funded by the Open Fund of State Key Laboratory of Water Resource Protection and Utilization in Coal Mining (Grant No. WPUKFJJ2022-11).
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, and Chinese Academy of Sciences (PRJCA031096) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The other data are available from the corresponding author upon request.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c09348.
Sampling at various coal mines; the compounds in mine water visibly increase and decrease after passing through underground water reservoirs; the dominant taxa at phylum and family levels; predictive functional gene; the component of metabolites; the citrate cycle; taurine and hypotaurine metabolism; selenocompound metabolism; glyoxylate and dicarboxylate metabolism; and Riboflavin metabolism in the aquifer (PDF)
Author Contributions
∥ Y.L. and M.W. contributed equally to this work. Y.L.: Writing, methodology, formal analysis, data curation, conceptualization; M.W.: writing, methodology, formal analysis, data curation, conceptualization, and funding acquisition; H.Z.: resources and data curation; B.J.: resources and data curation; Y.B.: resources and data curation; J.L.: formal analysis; J.L.: resources and formal analysis; P.L.: resources and formal analysis; X.Y.: data curation; and T.Q.: data curation.
The authors declare no competing financial interest.
Supplementary Material
References
- Rathi B.; Siade A. J.; Donn M. J.; Helm L.; Morris R.; Davis J. A.; Berg M.; Prommer H. Multiscale Characterization and Quantification of Arsenic Mobilization and Attenuation During Injection of Treated Coal Seam Gas Coproduced Water into Deep Aquifers. Water Resour. Res. 2017, 53 (12), 10779–10801. 10.1002/2017WR021240. [DOI] [Google Scholar]
- Zhang C.; Wang F. T.; Bai Q. S. Underground space utilization of coalmines in China: A review of underground water reservoir construction. Tunnelling Underground Space Technol. 2021, 107, 103657 10.1016/j.tust.2020.103657. [DOI] [Google Scholar]
- Zhao L.; Sun C.; Yan P. X.; Zhang Q.; Wang S. D.; Luo S. H.; Mao Y. X. Dynamic changes of nitrogen and dissolved organic matter during the transport of mine water in a coal mine underground reservoir: Column experiments. J. Contam. Hydrol. 2019, 223, 103473 10.1016/j.jconhyd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Feng H. B.; Zhou J. W.; Chai B.; Zhou A. G.; Li J. Z.; Zhu H. H.; Chen H. N.; Su D. H. Groundwater environmental risk assessment of abandoned coal mine in each phase of the mine life cycle: a case study of Hongshan coal mine, North China. Environ. Sci. Pollut. Res. 2020, 27 (33), 42001–42021. 10.1007/s11356-020-10056-z. [DOI] [PubMed] [Google Scholar]
- Chen S. S.; Huang Q. X.; Xue G.; Li R. Q. Technology of underground reservoir construction and water resource utilization in Daliuta Coal Mine. Coal Sci. Technol. 2016, 44, 21–28. [Google Scholar]
- Ju J. F.; Xu J. L.; Zhu W. B. Storage capacity of underground reservoir in the Chinese western water-short coalfield. J. China Coal Soc. 2017, 42 (2), 381–387. [Google Scholar]
- Xie H.; Zhao J. W.; Zhou H. W.; Ren S. H.; Zhang R. X. Secondary utilizations and perspectives of mined underground space. Tunnelling Underground Space Technol. 2020, 96, 103129 10.1016/j.tust.2019.103129. [DOI] [Google Scholar]
- Cameira M. D. R.; Rolim J.; Valente F.; Mesquita M.; Dragosits U.; Cordovil C. M. D. S. Translating the agricultural N surplus hazard into groundwater pollution risk: Implications for effectiveness of mitigation measures in nitrate vulnerable zones. Agric., Ecosyst. Environ. 2021, 306, 107204 10.1016/j.agee.2020.107204. [DOI] [Google Scholar]
- Madlener R.; Specht J. M. An Exploratory Economic Analysis of Underground Pumped-Storage Hydro Power Plants in Abandoned Deep Coal Mines. Energies 2020, 13 (21), 5634 10.3390/en13215634. [DOI] [Google Scholar]
- Menéndez J.; Loredo J.; Galdo M.; Fernández-Oro J. M. Energy storage in underground coal mines in NW Spain: Assessment of an underground lower water reservoir and preliminary energy balance. Renewable Energy 2019, 134, 1381–1391. 10.1016/j.renene.2018.09.042. [DOI] [Google Scholar]
- Song H. Q.; Xu J. J.; Fang J.; Cao Z. G.; Yang L. Z.; Li T. X. Potential for mine water disposal in coal seam goaf: Investigation of storage coefficients in the Shendong mining area. J. Cleaner Prod. 2020, 244, 118646 10.1016/j.jclepro.2019.118646. [DOI] [Google Scholar]
- Li J. M.; Huang Y. L.; Li W.; Guo Y. C.; Ouyang S. Y.; Cao G. L. Study on dynamic adsorption characteristics of broken coal gangue to heavy metal ions under leaching condition and its cleaner mechanism to mine water. J. Cleaner Prod. 2021, 329, 129756 10.1016/j.jclepro.2021.129756. [DOI] [Google Scholar]
- Yao Q. L.; Tang C. J.; Xia Z.; Liu X. L.; Zhu L.; Chong Z. H.; Hui X. D. Mechanisms of failure in coal samples from underground water reservoir. Eng. Geol. 2020, 267, 105494 10.1016/j.enggeo.2020.105494. [DOI] [Google Scholar]
- GU D. Z. Theory framework and technological systems of coal mine underground reservoir. J. China Coal Soc. 2015, 40 (2), 239–246. 10.13225/j.cnki.jccs.2014.1661. [DOI] [Google Scholar]
- Zhu W. B.; Xu J. M.; Li Y. C. Mechanism of the dynamic pressure caused by the instability of upper chamber coal pillars in Shendong coalfield, China. Geosci. J. 2017, 21 (5), 729–741. 10.1007/s12303-017-0025-5. [DOI] [Google Scholar]
- Luís A.; Cordóba F.; Antunes C.; Loayza-Muro R.; Grande J. A.; Silva B.; Diaz-Curiel J.; da Silva E. F. Extremely Acidic Eukaryotic (Micro) Organisms: Life in Acid Mine Drainage Polluted Environments-Mini-Review. Int. J. Environ. Res. Public Health 2022, 19 (1), 376 10.3390/ijerph19010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. Y.; Ji Y.; Li C. R.; Luo P. P.; Wang W. K.; Zhang Y.; Nover D. Effects of Phytoremediation Treatment on Bacterial Community Structure and Diversity in Different Petroleum-Contaminated Soils. Int. J. Environ. Res. Public Health 2018, 15 (10), 2168 10.3390/ijerph15102168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méheust R.; Castelle C. J.; Carnevali P. B. M.; Farag I. F.; He C.; Chen L. X.; Amano Y.; Hug L. A.; Banfield J. F. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. ISME J. 2020, 14 (12), 2907–2922. 10.1038/s41396-020-0716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. S.; Lv Y. G.; Zhang T.; Zhang L.; Ma X. M.; Liu X. S.; Lian S. Characteristics of Groundwater Microbial Community Composition and Environmental Response in the Yimuquan Aquifer, North China Plain. Water 2024, 16 (3), 459 10.3390/w16030459. [DOI] [Google Scholar]
- Bomberg M.; Arnold M.; Kinnunen P. Characterization of the Bacterial and Sulphate Reducing Community in the Alkaline and Constantly Cold Water of the Closed Kotalahti Mine. Minerals 2015, 5 (3), 452–472. 10.3390/min5030452. [DOI] [Google Scholar]
- Bao Y. P.; Guo C. L.; Wang H.; Lu G. N.; Yang C. F.; Chen M. Q.; Dang Z. Fe- and S-Metabolizing Microbial Communities Dominate an AMD-Contaminated River Ecosystem and Play Important Roles in Fe and S Cycling. Geomicrobiol. J. 2017, 34 (8), 695–705. 10.1080/01490451.2016.1243596. [DOI] [Google Scholar]
- Li Y. B.; Sun X. X.; Yang R.; Guo L. F.; Li C. B.; Wang X. Y.; Li B. Q.; Liu H. Q.; Wang Q.; Soleimani M.; Ren Y. H.; Sun W. M. Phototrophic Nitrogen Fixation, a Neglected Biogeochemical Process in Mine Tailings?. Environ. Sci. Technol. 2024, 58 (14), 6192–6203. 10.1021/acs.est.3c09460. [DOI] [PubMed] [Google Scholar]
- Brantner J. S.; Senko J. M. Response of Soil-Associated Microbial Communities to Intrusion of Coal Mine-Derived Acid Mine Drainage. Environ. Sci. Technol. 2014, 48 (15), 8556–8563. 10.1021/es502261u. [DOI] [PubMed] [Google Scholar]
- de Quadros P. D.; Zhalnina K.; Davis-Richardson A. G.; Drew J. C.; Menezes F. B.; Camargo F. A. D.; Triplett E. W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. 10.1016/j.apsoil.2015.10.016. [DOI] [Google Scholar]
- Gao P.; Sun X. X.; Xiao E. Z.; Xu Z. X.; Li B. Q.; Sun W. M. Characterization of iron-metabolizing communities in soils contaminated by acid mine drainage from an abandoned coal mine in Southwest China. Environ. Sci. Pollut. Res. 2019, 26 (10), 9585–9598. 10.1007/s11356-019-04336-6. [DOI] [PubMed] [Google Scholar]
- Kefeni K. K.; Msagati T. A. M.; Mamba B. B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Cleaner Prod. 2017, 151, 475–493. 10.1016/j.jclepro.2017.03.082. [DOI] [Google Scholar]
- Zhang X. H.; Tang S.; Wang M.; Sun W. M.; Xie Y. W.; Peng H.; Zhong A. M.; Liu H. L.; Zhang X. W.; Yu H. X.; Giesy J. P.; Hecker M. Acid mine drainage affects the diversity and metal resistance gene profile of sediment bacterial community along a river. Chemosphere 2019, 217, 790–799. 10.1016/j.chemosphere.2018.10.210. [DOI] [PubMed] [Google Scholar]
- Wen B.; Mei Z.; Zeng C.; Liu S. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinf. 2017, 18 (1), 183 10.1186/s12859-017-1579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. B.; David B.; Paul B.; Eva Z.; Sue F. M.; Nadine A.; Marie B.; Knowles J. D.; Antony H.; Haselden J. N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6 (7), 1060–1083. 10.1038/nprot.2011.335. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Xu Z. M.; Sun Y. J.; Gao Y. T.; Zhu L. L. Coal Mining Activities Driving the Changes in Microbial Community and Hydrochemical Characteristics of Underground Mine Water. Int. J. Environ. Res. Public Health 2022, 19 (20), 13359 10.3390/ijerph192013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckenstock R. U.; Elsner M.; Griebler C.; Lueders T.; Stumpp C.; Aamand J.; Agathos S. N.; Albrechtsen H. J.; Bastiaens L.; Bjerg P. L.; Boon N.; Dejonghe W.; Huang W. E.; Schmidt S. I.; Smolders E.; Sorensen S. R.; Springael D.; van Breukelen B. M. Biodegradation: Updating the Concepts of Control for Microbial Cleanup in Contaminated Aquifers. Environ. Sci. Technol. 2015, 49 (12), 7073–7081. 10.1021/acs.est.5b00715. [DOI] [PubMed] [Google Scholar]
- Wang J. L.; Zhang Y. L.; Ding Y.; Song H. W.; Liu T. Analysis of microbial community resistance mechanisms in groundwater contaminated with SAs and high NH4+-Fe-Mn. Sci. Total Environ. 2022, 817, 153036 10.1016/j.scitotenv.2022.153036. [DOI] [PubMed] [Google Scholar]
- An X. L.; Baker P.; Li H.; Su J. Q.; Yu C. P.; Cai C. The patterns of bacterial community and relationships between sulfate-reducing bacteria and hydrochemistry in sulfate-polluted groundwater of Baogang rare earth tailings. Environ. Sci. Pollut. Res. 2016, 23 (21), 21766–21779. 10.1007/s11356-016-7381-y. [DOI] [PubMed] [Google Scholar]
- Hu Y. T.; Liu T.; Chen N.; Feng C. P. Changes in microbial community diversity, composition, and functions upon nitrate and Cr(VI) contaminated groundwater. Chemosphere 2022, 288, 132476 10.1016/j.chemosphere.2021.132476. [DOI] [PubMed] [Google Scholar]
- Eulalio A.; Behm-Ansmant I.; Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007, 8 (1), 9–22. 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Hsieh P. Molecular mechanisms of DNA mismatch repair. Mutat. Res. 2001, 486 (2), 71–87. 10.1016/S0921-8777(01)00088-X. [DOI] [PubMed] [Google Scholar]
- Ouyang K. J.; Woo L. L.; Ellis N. A. Homologous recombination and maintenance of genome integrity: Cancer and aging through the prism of human RecQ helicases. Mech. Ageing Dev. 2008, 129 (7–8), 425–440. 10.1016/j.mad.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H.; Patronas N. J.; Schiffmann R.; Brooks B. P.; Tamura D.; Digiovanna J. J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience 2007, 145 (4), 1388–1396. 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. B.; Schellenberg M. J.; MacMillan A. M. Spliceosome structure: Piece by piece. Biochim. Biophys. Acta, Gene Regul. Mech. 2009, 1789 (9–10), 624–633. 10.1016/j.bbagrm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Mukherjee A.; Mammel M. K.; LeClerc J. E.; Cebula T. A. Altered utilization of N-acetyl-D-galactosamine by Escherichia coli O157:H7 from the 2006 spinach outbreak. J. Bacteriol. 2008, 190 (5), 1710–1717. 10.1128/JB.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denger K.; Ruff J.; Schleheck D.; Cook A. M. Rhodococcus opacus expresses the xsc gene to utilize taurine as a carbon source or as a nitrogen source but not as a sulfur source. Microbiology 2004, 150 (6), 1859–1867. 10.1099/mic.0.27077-0. [DOI] [PubMed] [Google Scholar]
- Zhang C. H.; Xu B. Y.; Zhao C. R.; Sun J. W.; Lai Q. X.; Yu C. L. Comparative de novo transcriptomics and untargeted metabolomic analyses elucidate complicated mechanisms regulating celery (Apium graveolens L.) responses to selenium stimuli. PLoS One 2019, 14 (12), e0226752 10.1371/journal.pone.0226752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, and Chinese Academy of Sciences (PRJCA031096) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa. The other data are available from the corresponding author upon request.