Abstract
A series of coumarin-3-carboxylic acid derivatives containing a thioether quinoline moiety were designed and synthesized. The structures of these compounds were determined using 1H NMR, 13C NMR, and HRMS. The antibacterial activity of the compounds was evaluated against Xanthomonas oryzae pv oryzae (Xoo), Ralstonia solanacearum (Rs), and Acidovorax citrulli (Aac). The results showed that most of the compounds exhibited significant antibacterial activity against these pathogens. Particularly, compound A9 demonstrated potent activity against Xoo and Aac, with EC50 values of 11.05 and 8.05 μg/mL respectively. In addition, A9 indicated strong protective and curative effects against Aac in vivo, with efficacy rates of 61.50 and 54.86%, respectively, surpassing those of the positive control thiodiazole copper. The scanning electron microscopy observations revealed that treatment of Aac cells with A9 at a concentration of 2EC50 resulted in a curved and sunken cell morphology, along with destroyed cell membrane integrity. Additionally, the motility and exopolysaccharide production of Aac were inhibited, and biofilm formation was prevented. Consequently, these newly developed derivatives of coumarin-3-carboxylic acid, incorporating the thioether quinoline moiety, hold promise as potential templates for the development of innovative antibacterial agents.
1. Introduction
Crop bacterial diseases caused by phytopathogenic bacteria pose significant challenges in terms of control due to their rapid spread, extensive range, and high resistance to pesticides.1 Examples of such diseases include rice bacterial leaf blight caused by Xanthomonas oryzae pv oryzae (Xoo),2,3 tomato bacterial wilt caused by Ralstonia solanacearum (Rs)4, and melon bacterial fruit blotch caused by Acidovorax citrulli (Aac).5 These diseases have a detrimental impact on crop growth, jeopardizing both the yield and quality. Currently, chemical control serves as the primary approach to managing bacterial diseases, predominantly relying on copper preparations and antibiotics. However, copper preparations exhibit limited efficacy and are susceptible to produce drug damage to crops,6 while antibiotics are prone to induce antibiotic resistance and pollute the environment.7 Consequently, there is a pressing need to develop a novel, ecofriendly bactericide that is both sustainable and environmentally benign for effective control of bacterial diseases.
Coumarin-3-carboxylic acid (3-CCA) is a naturally occurring derivative of coumarin that has been shown to possess antitumor properties,8 as well as anticancer effects9−11 and antimicrobial activities.12,13 Previous investigations have demonstrated the broad-spectrum antibacterial activity of 3-CCA;14 however, the precise structure–activity relationship (SARs) remains incompletely understood. Prior research has indicated that structural modifications of 3-CCA can yield compounds with potent fungicidal,15 antibacterial,16 and antagonist properties.17 It is anticipated that the enhancement of bactericidal activity can be achieved through the incorporation of diverse agricultural bactericidal active groups at the 3 sites.
Quinoline, a significant bioactive natural alkaloid, has garnered considerable interest in recent years due to its remarkable antifungal,18,19 antimalaria,20 antitumor,21−23 antinucleation,24,25 and other biological activities.26 Consequently, it has emerged as a prominent lead compound for the development of novel pesticides. Notably, several quinoline-based compounds, including Quinoxyfen, Quinclorac, and Tebufloquin have been successfully developed and introduced to the market.
In our previous work, the incorporation of thioether quinoline into the molecular framework of myricetin was achieved using active splicing methodology, resulting in the synthesis of myricetin derivative B6 featuring containing thioether quinoline moieties that exhibit a pronounced affinity toward TMV-P.27 This finding suggests that thioether quinoline, when utilized as a pharmacophore, can augment the antiviral efficacy of the parent compound. Nevertheless, the structural characterization of 3-CCA compounds incorporating thioether quinoline groups remains unexplored in the literature.
In this study, the thioether quinoline was introduced into the structure of 3-CCA through active splicing. A total of thirty-eight derivatives of 3-CCA containing thioether quinoline were synthesized and evaluated for their antibacterial activities against Xoo, Rs, and Aac in vitro. The EC50 values and in vivo efficacy of the most effective compound were determined. The effects of potent compounds on physiological and biochemical indicators of sensitive bacteria, such as bacterial morphology, motility, and cell membrane integrity, were also assessed. The results of this study may offer valuable insights into the development of novel antibacterial agents derived from coumarin compounds.
2. Materials and Methods
2.1. Instruments and Chemicals
2.1.1. Instruments
The chemical reaction processes were monitored through thin-layer chromatography (TLC) under an ultraviolet lamp. The melting points of the compounds were determined by using a melting point apparatus (Shanghai INESA Optical Instrument Co., Ltd., China) without temperature calibration. The 1H and 13C NMR spectra were obtained using a Bruker DKX500 NMR spectrometer (Bruker; Karlsruhe, Germany) with CDCl3 or DMSO-d6 as the solvent and TMS as the internal standard. The Thermo Scientific Q Exactive (Thermo Scientific, Missouri, MO) instrument was utilized to obtain high-resolution mass spectrometry (HRMS) data of the compounds.
2.1.2. Chemicals
All chemical reagents utilized in this study were commercially available and were not subjected to further purification. The present study procured various reaction materials from Shanghai Titan Technology Co., Ltd. (Shanghai, China) and other chemical materials from Bositai Technology Co., Ltd. (Chongqing, China). Coumarin-3-carboxylic acid (3-CCA, 95%) was obtained from Bide Pharmatech Ltd. (Shanghai, China), while kasugamycin (65%) and a water solution (2%) were sourced from Shenzhen Novoxin Agrochemical Co., Ltd. (Shenzhen, China). All chemical reagents and solvents utilized in the study were of analytical purity.
2.1.3. Bacteria
In this study, three phytopathogenic bacterial strains assayed for in vitro antibacterial screening were Xoo (bacterial leaf blight of rice), Aac (bacterial fruit blotch), and Rs (tomato bacterial wilt). These strains were preserved for long-term use by storing them in 20% glycerol at −80 °C. The strains were cultured either on Luria–Bertani agar (LA) plates, which contained 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, 16 g of agar, and 1 L of distilled water, or in LB broth (without agar) at 28 °C in the dark.
2.2. Synthesis
2.2.1. General Synthetic Procedure for the Intermediate 1
α-Aminoacetophenone (150 mmol) and triethylamine (150 mmol) were added to 150 mL of anhydrous CH2Cl2. At 0 °C, various substitutes of benzoyl chloride (150 mmol) dissolved in 150 mL CH2Cl2 was added dropwise. The reaction continued at room temperature for about 3 h after dropping. The reaction was monitored by thin-layer chromatography (TLC) until it was completed. After the reaction was completed, an appropriate amount of water was added for washing. The organic phase was dried with anhydrous Na2SO4. After desolubilization, it was recrystallized with petroleum ether- ethyl acetate to obtain intermediate 1.28
2.2.2. General Synthetic Procedure for the Intermediate 2
Intermediate 1 (100 mmol) was added to 250 mL of 1,4-dioxane. NaOH (300 mmol) was added in batches; the temperature was slowly raised to 110 °C for 2 h. After the reaction was completed, the solvent was removed by the rotary evaporator. 300 mL of water was added to dissolve it, and then 1 mol/L HCl was used to adjust the solution pH to 7. After suction filtration, the filter cake was washed with water and dichloromethane-ethyl acetate mixed solution (v/v = 1:1) for several times to obtain intermediate 2.28
2.2.3. General Synthetic Procedure for the Intermediate 3
Intermediate 2 (90 mmol) was dissolved in 250 mL of anhydrous pyridine. Phosphorus pentasulfide (180 mmol) was slowly added at 0 °C, and the temperature was raised to 110 °C for 5 h. After the reaction was completed, the mixture was cooled to room temperature. A large amount of yellow precipitate appeared after adding an equal volume of water and stirring for a few minutes. The stirring continued for 0.5 h until the precipitation was complete, and then the mixture was filtered to obtain a crude product. The crude product was dissolved in 100 mL of NaOH solution with a mass fraction of 10% and acetic acid was added to adjust pH to 7. After suction filtration, the filter cake was washed with water and petroleum ether for several times to obtain intermediate 3.28
2.2.4. General Synthetic Procedure for the Intermediate 4
Water (100 mL) and ethanol (50 mL) were used as the reaction solvent, and 15 mol % n-bromosuccinimide was used as the catalyst. Salicylaldehyde (50 mmol) and 2,2-dimethyl-1,3-dioxane-4,6-dione acid (55 mmol) were reacted at room temperature for 10 h. After the end of the reaction, 50 mL of 20% ethanol was added to the reaction system and stirred at 100 °C for 10 min to remove impurities and catalysts. After sufficient cooling, suction filtration yields a pure intermediate 4.29
2.2.5. General Synthetic Procedure for the Intermediate 5
A mixture of coumarin-3-carboxylic acid (10.5 mmol) and DMF was added to a round-bottom flask containing a mixture of 1,2-dibromoethane (42 mmol) and triethylamine (21 mmol) for 6 h at room temperature. After the reaction was completed, the reaction mixture was poured into ice water and constantly stirred. When there was no solid precipitation, the obtained solid was dried at room temperature. And then the petroleum ether-ethyl acetate mixed solution (v/v = 3:1) was added for stirring overnight and filtered to obtain intermediate 5.30
2.2.6. General Synthetic Procedure for Compounds A1–A38
The intermediate 3 (6.43 mmol), K2CO3 (12.86 mmol), and 30 mL of DMF were sequentially added to a 100 mL round-bottom flask. After stirring for 15 min at room temperature, intermediate 5 (6.43 mmol) was added. Reaction was monitored by TLC. Once the reaction was completed, the liquid was poured into ice water. It was then filtered to obtain a crude product. The crude product was purified by column chromatography to obtain the final target compounds A1–A38.30
2.2.7. Antibacterial Activity Test In Vitro
The antibacterial activity of 3-CCA derivatives against Xoo, Rs, and Aac was assessed using the methodology outlined in a previously published research paper.31 Bacterial strains were cultivated at a temperature of 37 °C in LB broth on a rotary shaker operating at 180 rpm until an optical density of 0.6 at 600 nm (OD600) was attained. Subsequently, 10 μL of bacterial culture was combined with 190 μL of LB broth to achieve concentrations of 50 μg/mL. Kasugamycin and 3-CCA were used as the positive controls at the same concentration. As a control, equal volumes of DMSO were used as a vehicle control, with a maximum concentration of 1% DMSO. The 96-well plates were subjected to incubation at 28 °C, with shaking at 180 rpm for 12 h. The growth of bacteria was monitored by measuring the optical density at 600 nm, utilizing a microplate reader (Synergy H1, BioTek Instruments Inc., Vermont).
The virulences of potent antibacterial compounds against Xoo and Aac were tested at concentrations of 50, 25, 12.5, 6.25, and 3.125 μg/mL, with three replicates per concentration. The determination of the 50% effective concentration (EC50) involved conducting a regression analysis on the percentage growth inhibition data, which was log-transformed to account for bactericide concentration.32
2.2.8. Antibacterial Activities of A9 against AacIn Vivo
The protective and curative effects of compound A9 against Aac were tested in vivo using a potting method. The test compound A9, dissolved in DMSO, was then diluted with 0.1% Tween-20 distilled water to get the concentrations of 200 and 100 μg/mL. Seedlings of hybrid melon (cv. Yangjiaomi, obtained from China Vegetable Seed Technology Co., Ltd.) at the two true leaf stages were subjected to the potting test. To protect the leaves, the melon seedlings were subjected to spraying with either A9 or a control agent until complete saturation. Following a 24 h interval postspraying, the cotyledons were subsequently inoculated with Aac suspensions (with an OD600 of 1). The control seeds were treated with a solution of distilled water, DMSO, and Tween-20 in equal volumes. As positive controls, commercially available bactericides, namely 20% thiodiazole copper SC and 2% kasugamycin AS at a concentration of 100 μg/mL, were employed. For the evaluation of the curative effect, compound A9 was sprayed to melon cotyledons 24 h after the aforementioned spraying procedure. The treated seedlings were then cultivated for 7 days under standard conditions, which included 16 h of light at a temperature of 25 ± 2 °C and a relative humidity of 60 ± 5%, followed by 8 h of darkness at a temperature of 20 ± 2 °C and a relative humidity of 75 ± 5%. Each treatment was measured on six plants, and each experimental condition was replicated 3 times.33
2.2.9. Effect of A9 on the Growth of Aac
The effect of A9 on the growth of Aac was assessed using a method outlined by Silva-Angulo et al.34 with minor adjustments. Aac was cultivated in LB medium at 28 °C for 18 h with agitation at 180 rpm, followed by inoculation into LB medium supplemented with A9 to obtain final concentrations of 1/4EC50, 1/2EC50, EC50, 2EC50, and 4EC50. DMSO (1%) was used as a solvent control. Cultures were inoculated in 96-well plates and incubated at 28 °C with shaking at 180 rpm for 60 h. The optical density at 600 nm (OD600) was measured at 0, 1, 2, 4, 6, 8,10,12, and 24 h post-treatment. Each concentration was carried out in triplicate, and three independent experiments were performed.
2.2.10. Effect of A9 on the Ultrastructure of Aac
Scanning electron microscopy (SEM) was performed to evaluate the effect of compound A9 on the ultrastructure of Aac.35 Bacterial cultures (OD600 = 0.6) were harvested by centrifugation at 5000 rpm for 5 min at 4 °C, followed by three washes with 0.1 mol/L phosphate buffer (PBS, pH 7.2). Bacterial suspensions were then treated with A9 dissolved in dimethyl sulfoxide (DMSO) to obtain the concentrations of 2EC50. Control samples were prepared using solvent blanks of equal volume. The bacterial suspensions were incubated at 28 °C with shaking at 180 rpm for a duration of 24 h. The cells were harvested after centrifugation at 5000 rpm for 5 min at 4 °C. Subsequently, they were rinsed 3 times with a 0.1 mol/L PBS solution (pH 7.2). Following this, the samples were fixed for a period of 4 h in a 2.5% glutaraldehyde solution at a temperature of 4 °C. They were then washed 3 times for 5 min each in the same 0.1 mol/L PBS solution. The cells underwent dehydration using a sequential graded ethanol series (30, 50, 70, 80, 90, and 100%) for 15 min at each concentration, followed by a 20 min dehydration using 100% ethanol. After being freeze-dried for a duration of 8 h, the samples were coated with gold and examined for cell morphologies using a desktop scanning electron microscope (HITACHI SU8600).
2.2.11. Effect of A9 on the Membrane Permeability of Aac
The effect of A9 on the permeability of the cell membrane of Aac was examined using the method described by Ernst et al.36Aac cultures in the logarithmic growth phase (20 mL) were subjected to centrifugation at 2000 rpm for 5 min, followed by removal of the supernatant. Then, the cells were washed and resuspended in sterile water (20 mL). To get the concentrations of 1/2EC50, EC50, 2EC50, and 4EC50, compound A9 dissolved in DMSO was diluted with the cell suspensions The control measurements utilized DMSO as the solvent for the drugs, with a final concentration of 1%. Subsequently, the electric conductivity was measured and recorded at 0, 2, 4, 6, 8, and 12 h. To ensure accuracy and reliability, the experiment was repeated 3 times with triplicate samples for each concentration.
2.2.12. Effect of A9 on Motility of Aac
The effect of A9 on motility of Aac was previously described.37 The Aac cultures were prepared by adjusting the initial OD600 to 0.6 and dissolving them in LB with 0.3% agar using microwave heating. A9 was then added at concentrations of EC50, 2EC50, and 4EC50, with DMSO as the vehicle control. The media with A9 were poured into sterile Petri plates and allowed to solidify. A bacterial suspension (5 μL) was inoculated onto the center of the semisolid medium and incubated at 28 °C for 48 h. Swimming motility was assessed by measuring the diameter of the covered areas.
2.2.13. Effect of A9 on the Exopolysaccharide (EPS) Content of Aac
The effect of A9 on the exopolysaccharide (EPS) content of Aac was performed according to the means of Shi et al.38 The DMSO solutions containing A9 were diluted with bacterial suspensions (OD600 = 0.6) to achieve the final concentrations of EC50, 2EC50, and 4EC50, and subsequently incubated at 28 °C with continuous shaking at 180 rpm for 72 h. An equivalent concentration of the solvent (DMSO) was used as a control. The supernatant was collected following centrifugation at 3000 rpm for 20 min at 4 °C. The exopolysaccharides (EPS) were precipitated using three volumes of absolute ethanol and allowed to settle overnight. After subsequent centrifugation, the samples were oven-dried at 70 °C to a constant weight for weighing. This process was repeated 3 times with each assay being performed in triplicate.
2.2.14. Effect of A9 on the Biofilm Formation of Aac
The effect of A9 on the biofilm formation of Aac was assessed according to the method previously described by Du et al.39 with slight alteration. The bacterium was cultured in LB broth at 28 °C with shaking at 180 rpm for 18 h, followed by centrifugation and adjustment to an OD600 of 1. Gradient dilutions of A9 (EC50, 2EC50, and EC50) were introduced into sterile borosilicate glass tubes. Negative controls were established by adding bacterial cultures and an agent dilution buffer (100 μL of each). The test tubes were subsequently incubated at 28 °C for 2 days. Biofilms of Aac were stained with 0.1% crystal violet solution for 1 h and then washed 3 times with deionized water to remove excess crystal violet stain. The biofilms were diluted with 10% glacial acetic acid for a duration of 1 h. Optical density at 600 nm (OD600) was measured following the transfer of 200 μL of the culture into a 96-well plate. Each concentration treatment was conducted in sextuplicate, and the entire experimental procedure was replicated 3 times.
2.2.15. Statistical Analysis
The results were expressed as means ± standard error (SE) based on three independent repeated experiments. A variance analysis was conducted using SPSS software (version 20.0, IBM Corp., Armonk, NY) to analyze the data. To determine statistical significance, Duncan’s multiple range test was employed at a significance level of P < 0.05. Graphs were created using Sigma Plot (version 12.5, Systat Software Inc., San Jose, CA).
3. Results and Discussion
3.1. Chemistry
The synthetic route for the synthesis of coumarin-3-carboxylic acid derivatives containing a thioether quinoline moiety is depicted in Figure 1. The structures of these derivatives were characterized using 1H NMR,13C NMR, and HRMS techniques. Initially, 2-aminophenone was subjected to reaction with various substituted benzoyl chlorides to yield intermediate 1. Subsequently, intramolecular condensation of intermediate 1 was carried out employing Combes quinoline synthesis, resulting in the formation of intermediate 2. Furthermore, the utilization of phosphorus pentasulfide in the subsequent step led to the formation of intermediate 3. Additionally, intermediate 4 was synthesized through cyclization utilizing salicylaldehyde as the starting material, and intermediate 4 underwent a reaction with dibromoalkane to obtain intermediate 5. Ultimately, a substitution reaction between intermediate 3 and intermediate 5 achieved compounds A1-A38.
Figure 1.
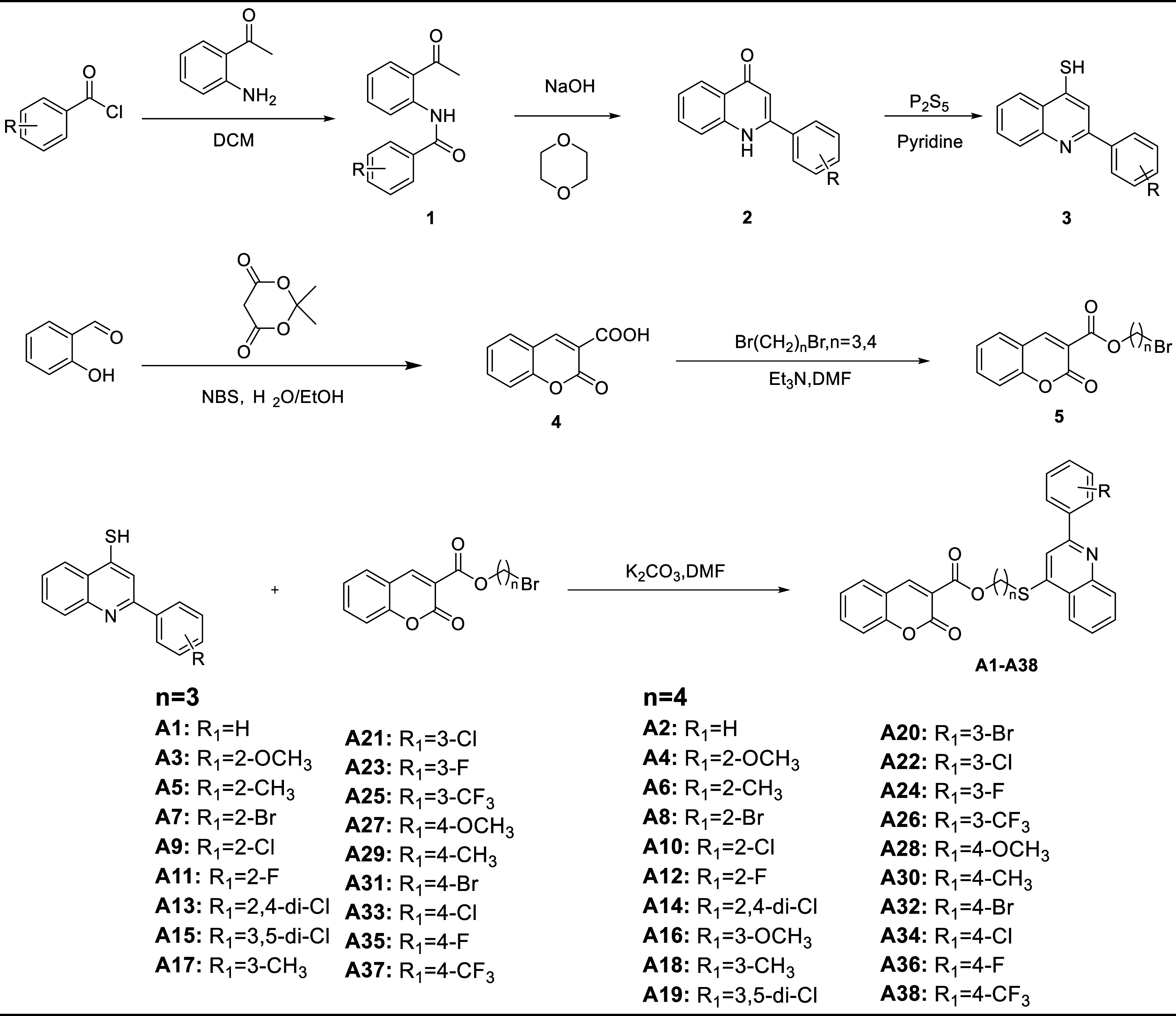
Synthesis route of the target compounds.
3.2. Antibacterial Activity of 3-CCA Derivatives against Xoo, Rs, and AacIn Vitro
The in vitro antibacterial activities of thirty-eight derivatives containing a thioether quinoline moiety were assessed against Xoo, Rs and Aac, as indicated in Table 1. The results revealed that these compounds exhibited greater efficacy against Xoo and Aac, while displaying lower antibacterial activity against Rs. Notably, compound A9 and A13 demonstrated the highest antibacterial potential against Xoo and Aac, with inhibition rates of 90.68, 89.29 and 93.71, 84.10% at 50 μg/mL, respectively. These rates were superior to those of 3-CCA and comparable to the positive control kasugamycin, which suggested promising antibacterial properties. Compound A35 exhibited superior antibacterial activity against Xoo and Aac in comparison to 3-CCA, albeit inferior to the positive control kasugamycin. Conversely, compound A25 demonstrated selectivity against Xoo, displaying enhanced antibacterial efficacy against Xoo relative to 3-CCA, yet still falling short of the positive control kasugamycin.
Table 1. Antibacterial Activity of 38 Compounds against Xoo, Rs, and Aac.
| inhibition
rate (%) |
|||||
|---|---|---|---|---|---|
| compound | n | R | Xoo | Rs | Aac |
| A1 | 3 | H | 76.05 | 35.57 | 60.21 |
| A2 | 4 | H | 78.18 | 34.43 | 54.78 |
| A3 | 3 | 2-OCH3 | 18.89 | 39.60 | 17.51 |
| A4 | 4 | 2-OCH3 | 1.36 | 40.87 | 14.62 |
| A5 | 3 | 2-CH3 | 9.50 | 8.10 | 25.38 |
| A6 | 4 | 2-CH3 | 22.38 | 18.60 | 19.05 |
| A7 | 3 | 2-Br | 44.2 | 30.93 | 67.22 |
| A8 | 4 | 2-Br | 39.03 | 22.6 | 54.1 |
| A9 | 3 | 2-Cl | 90.68 | 44.62 | 93.71 |
| A10 | 4 | 2-Cl | 76.64 | 35.8 | 71.32 |
| A11 | 3 | 2-F | 64.76 | 26.66 | 63.35 |
| A12 | 4 | 2-F | 58.12 | 40.05 | 61.14 |
| A13 | 3 | 2,4-di-Cl | 89.29 | 27.31 | 84.1 |
| A14 | 4 | 2,4-di-Cl | 65.47 | 18.89 | 2.72 |
| A15 | 3 | 3,5-di-Cl | 25.49 | 7.60 | 17.90 |
| A16 | 4 | 3-OCH3 | 30.75 | 40.3 | 69.37 |
| A17 | 3 | 3-CH3 | 42.62 | 37.28 | 67.26 |
| A18 | 4 | 3-CH3 | 38.26 | 36.05 | 46.91 |
| A19 | 4 | 3,5-di-Cl | 32.92 | 22.96 | 22.25 |
| A20 | 4 | 3-Br | 18.43 | 20.29 | 3.29 |
| A21 | 3 | 3-Cl | 47.88 | 33.72 | 20.25 |
| A22 | 4 | 3-Cl | 35.75 | 23.14 | 2.05 |
| A23 | 3 | 3-F | 73.30 | 15.00 | 18.99 |
| A24 | 4 | 3-F | 25.10 | 13.28 | 26.94 |
| A25 | 3 | 3-CF3 | 79.13 | 16.60 | 20.89 |
| A26 | 4 | 3-CF3 | 51.04 | 10.37 | 8.37 |
| A27 | 3 | 4-OCH3 | 72.87 | 42.29 | 47.65 |
| A28 | 4 | 4-OCH3 | 64.23 | 21.65 | 4.72 |
| A29 | 3 | 4-CH3 | 66.37 | 40.02 | 69.58 |
| A30 | 4 | 4-CH3 | 71.24 | 42.51 | 65.87 |
| A31 | 3 | 4-Br | 55.69 | 22.70 | 14.11 |
| A32 | 4 | 4-Br | 45.80 | 27.12 | 11.30 |
| A33 | 3 | 4-Cl | 72.51 | 24.52 | 53.86 |
| A34 | 4 | 4-Cl | 70.83 | 26.35 | 61.56 |
| A35 | 3 | 4-F | 82.76 | 34.76 | 72.87 |
| A36 | 4 | 4-F | 69.08 | 34.85 | 63.93 |
| A37 | 3 | 4-CF3 | 71.43 | 21.75 | 33.12 |
| A38 | 4 | 4-CF3 | 79.04 | 34.31 | 57.46 |
| 3-CCA | 64.92 | 51.24 | 56.44 | ||
| kasugamycin | 90.14 | 78.3 | 95.24 | ||
3.3. Structure–Activity Relationship Analysis
The antibacterial activity test data revealed that the biological activity of the compounds was greatly influenced by the length of the carbon chain and the type of substituent. Specifically, compounds with a carbon chain length of three exhibit significantly enhanced antibacterial activity compared to that of other compounds. For instance, compounds A9 (n = 3), A13 (n = 3), and A35 (n = 3) demonstrate this effect. Furthermore, the introduction of a single substituent, particularly an electron-withdrawing group, at position 2 of the benzene ring also affects the antibacterial activity. The bactericidal activity of compound A9 (R = 2-Cl) exhibits significant improvement. However, the introduction of single substituents at positions 3 and 4 did not contribute to the enhancement of activity. The relationship between structure and activity is intricate, and the impact of introduced substituents on activity was contingent on the bacterial category. In the case of Xoo, the inclusion of electron-withdrawing groups resulted in higher bactericidal activity, exemplified by compounds A25 (R = 3-CF3) and A35 (R = 4-F). The bactericidal activity of Rs was enhanced by the introduction of electron contributing groups, as observed in compounds A16 (R = 3-OCH3) and A30 (R = 4-CH3). Similarly, the bactericidal activity of Aac is increased when electron contributing groups were introduced, as demonstrated by compounds A17 (R = 3-CH3) and A29 (R = 4-CH3). Notably, the introduction of a double substituent on the terminal benzene ring, particularly at positions 2 and 4, significantly contributed to the observed activity enhancement, exemplified by compound A13 (R = 2,4-di-Cl).
3.4. Bacterial Virulence Assays of High-Activity Compounds
Based on the findings from Section 3.2, an assessment was conducted to evaluate the virulence of the high-activity compounds against Xoo and Aac, as indicated in Table 2. The outcomes revealed that A9 exhibited the most robust antibacterial activity against Xoo and Aac, as evidenced by the EC50 values of 11.05 and 8.05 μg/mL, respectively. This performance surpassed that of 3-CCA and was comparable to the antibacterial effect observed with positive control kasugamycin. Additionally, compound A35 demonstrated marked bactericidal activity against Xoo and Aac, with EC50 values of 14.87 and 15.42 μg/mL, respectively, which were equivalent to the antibacterial potency of A9. The remaining compounds had moderate antibacterial activity against Xoo and Aac, with EC50 values ranging from 11 to 32 μg/mL.
Table 2. Antibacterial Virulence of the High-Activity Compounds against Xoo and Aac.
| bacteria | compound | LC-P | r | EC50 (μg/mL) | 95%FL (μg/mL) |
|---|---|---|---|---|---|
| Xoo | A1 | 2.85 + 1.71x | 0.96 | 18.12 | 12.68–25.89 |
| A7 | 2.58 + 1.98x | 0.95 | 16.7 | 11.98–23.30 | |
| A9 | 3.42 + 1.52x | 0.97 | 11.05 | 7.03–17.36 | |
| A10 | 2.06 + 2.79x | 0.96 | 11.26 | 9.13–13.88 | |
| A11 | 2.58 + 2.03x | 0.99 | 15.55 | 11.20–21.58 | |
| A12 | 2.48 + 1.72x | 0.98 | 29.09 | 20.84–40.59 | |
| A13 | 3.59 + 1.17x | 0.99 | 16.34 | 10.73–24.87 | |
| A23 | 1.90 + 2.36x | 0.98 | 20.72 | 15.84–27.09 | |
| A27 | 2.26 + 2.03x | 0.97 | 22.42 | 16.65–30.19 | |
| A29 | 2.62 + 1.65x | 0.97 | 27.91 | 19.69–39.55 | |
| A35 | 3.29 + 1.46 | 0.99 | 14.87 | 10.51–21.05 | |
| A38 | 2.73 + 1.76x | 0.99 | 19.42 | 13.81–27.30 | |
| 3-CCA | 2.58 + 1.63x | 0.97 | 30.37 | 21.27–43.37 | |
| kasugamycin | 3.76 + 1.16x | 0.99 | 11.65 | 6.95–19.53 | |
| Aac | A1 | 1.45 + 2.34x | 0.97 | 32.65 | 25.15–42.38 |
| A9 | 3.42 + 1.74x | 0.99 | 8.05 | 5.75–11.27 | |
| A11 | 2.99 + 1.38x | 0.99 | 28.15 | 18.86–42.01 | |
| A13 | 2.87 + 1.52x | 0.98 | 24.95 | 17.59–35.40 | |
| A17 | 3.12 + 1.43x | 0.98 | 20.71 | 13.88–30.89 | |
| A35 | 2.79 + 1.86x | 0.99 | 15.42 | 11.63–20.45 | |
| 3-CCA | 3.23 + 1.1x | 0.98 | 39.48 | 24.51–63.58 | |
| kasugamycin | 3.45 + 1.37x | 0.97 | 13.55 | 8.81–20.83 |
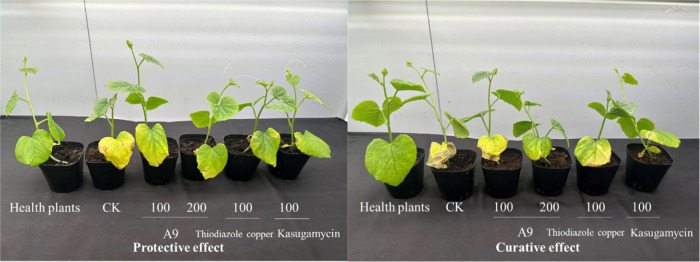
3.5. Bacterial Activity of A9 against AacIn Vivo
According to the findings presented in Table 3 and Figure 2, it is evident that A9 exhibits robust protective and curative effects against Aac in vivo. The efficacy rates for A9 are recorded at 61.50 and 54.86%, respectively, which are notably lower than the positive control kasugamycin (67.57, 60.71%), but superior to the positive control thiodiazole copper (51.89, 48.94%).
Table 3. Protective and Curative Effects of A9 against Aac In Vivoa.
| protective
effect |
curative
effect |
||||
|---|---|---|---|---|---|
| sample | concentration (μg/mL) | disease index | efficacy (%) | disease index | efficacy (%) |
| A9 | 200 | 10.56 ± 1.11c | 61.5b | 15.56 ± 0.96d | 54.86b |
| 100 | 15.37 ± 0.85b | 43.9d | 22.96 ± 0.64b | 33.32d | |
| thiodiazole copper | 100 | 13.15 ± 0.32c | 51.89c | 17.59 ± 0.85c | 48.94c |
| kasugamycin | 100 | 8.89 ± 0.56d | 67.57a | 13.52 ± 0.32e | 60.71a |
| CK | 27.41 ± 1.70a | 34.44 ± 1.11a | |||
Columns represent the mean ± SE. Different letters in the column represent significant difference at the P0.05 level, the same as following.
Figure 2.
Protective and curative effects of A9 against Aac in vivo.
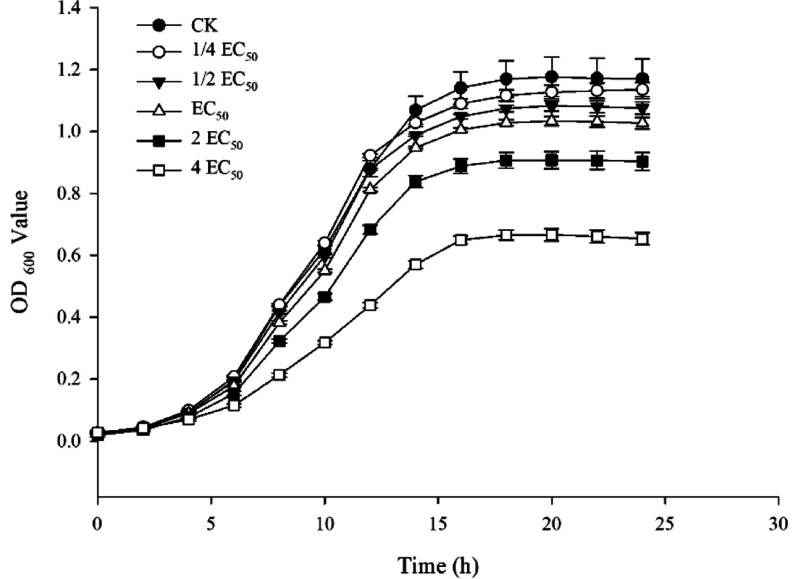
3.6. Growth Curve
The effect of varying concentrations of A9 on the growth curves of Aac was investigated, with the results depicted in Figure 3. Treatment with A9 exhibited a significant inhibitory effect on Aac growth, showing a concentration-dependent relationship across concentrations ranging from 1/4 to 4EC50.
Figure 3.
Effect of A9 concentration on the growth of Aac.
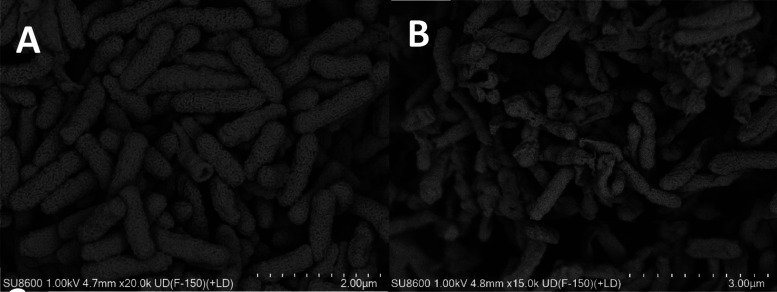
3.7. Bacterial Morphology
The effect of A9 on the ultramicrostructure of Aac is illustrated in Figure 4. The surface morphology of the control cells exhibited a rounded and full appearance, while the cells treated with A9 displayed an irregular shape with sunken and wrinkled surfaces.
Figure 4.
SEM observations of conAac treated with A9.
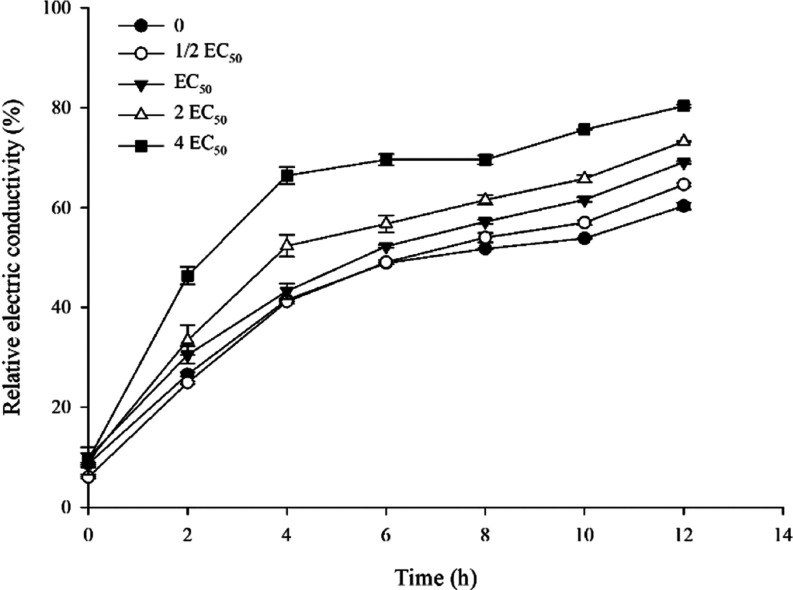
3.8. Cell Membrane Permeability
The results showed a positive correlation between A9 concentrations and electric conductivity, with a notable increase observed in relative electric conductivity within the first 4 h of treatment in Figure 5. Subsequently, the rate of increase slowed down, leading to a final relative conductivity of 81.13% at 4EC50 after 12 h of treatment, surpassing both the control and other treatments.
Figure 5.
Effect of compound A9 on cell membrane permeability of Aac.
3.9. Swimming Assay
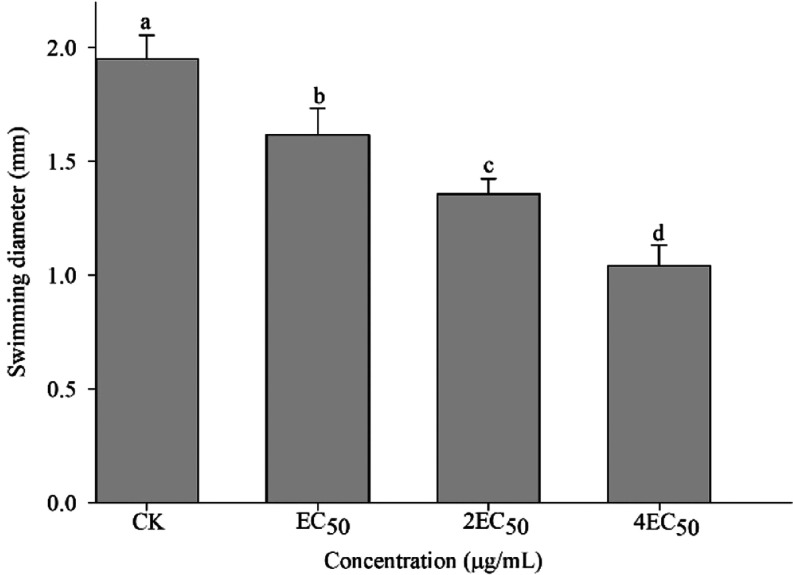
As showcased in Figure 6, the presence of A9 had a significant impact on the motility of Aac. The swimming diameter of A9 against Aac measured 1.04 mm at 4EC50, indicating a statistically superior performance compared with both the control group and other treatments.
Figure 6.
Effect of A9 on bacterial motility of Aac.
3.10. EPS Content
As demonstrated in Figure 7, A9 exhibits a significant impact on the production of exopolysaccharides from Aac. The production of exopolysaccharides from Aac decreased to 11.03, 10.65, and 9.60 mg at the concentrations of EC50, 2EC50, and 4EC50, resulting in reductions of 12.66, 15.70, and 24.01%, respectively, compared to the control.
Figure 7.
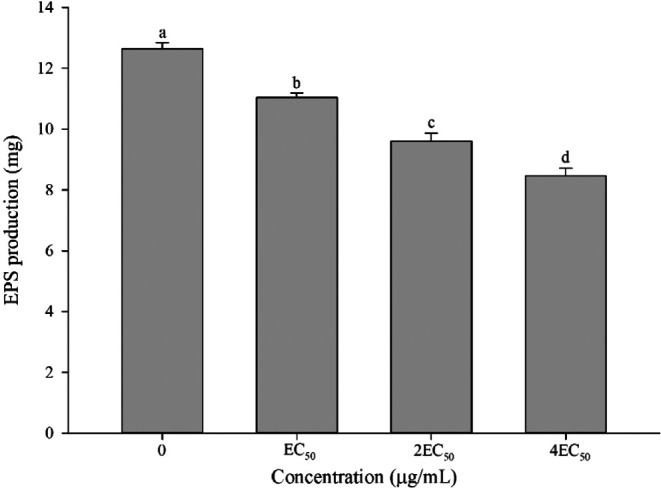
Effect of A9 on the EPS content of Aac.
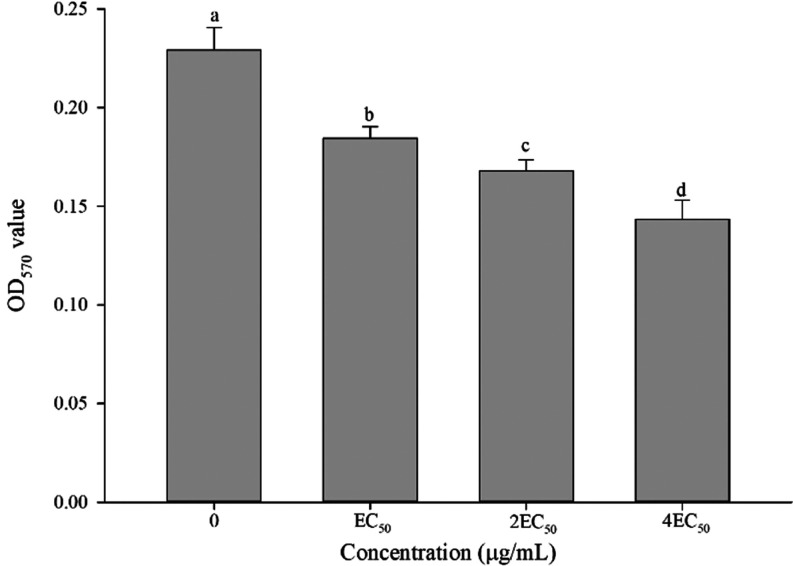
3.11. Biofilm Formation Assay
As shown in Figure 8, A9 exhibits inhibitory effects on the biofilm formation of Aac. These inhibitory effects were found to be positively associated with the concentrations at which the treatment was administered. Specifically, the biofilm formation of Aac reduced to 19.56, 26.69, and 36.15% at EC50, 2EC50, and 4EC50, respectively.
Figure 8.
Effect of A9 on the biofilm formation of Aac.
4. Conclusions
In summary, this study employed the active splicing principle to synthesize a range of coumarin-3-carboxylic acid derivatives featuring a thioether quinoline moiety. Subsequently, the biological activity of these derivatives was assessed. The results of the antibacterial assay indicated that the majority of the compounds displayed significant antibacterial activity against Xoo, Rs, and Aac. Particularly, compound A9 exhibited the most potent activity against Aac both in vitro and in vivo. Further toxicological investigations revealed that the inhibition of bacterial growth in Aac was attributed to induce alterations in cell morphology, damage to the bacterial cell membrane, decrease motility, reduce exopolysaccharide production, and prevention of biofilm formation. Consequently, these innovative derivatives of coumarin-3-carboxylic acid, incorporating a thioether quinoline moiety, hold promise as potential alternative frameworks for the development of novel antibacterial agents.
Acknowledgments
This work was supported financially by the Key Research Development Program of Hainan Province, China (ZDYF2024XDNY202), the Natural Science Foundation of China (32372598), Chinese Academy of Tropical Agricultural Sciences for Science and Technology Innovation Team of National Tropical Agricultural Science Center (CATASCXTD202410), Central Public-interest Scientific Institution Basal Research Fund (1630042024020).
Glossary
Abbreviations
- 1H NMR
1H nuclear magnetic resonance
- 13C NMR
13C nuclear magnetic resonance
- HRMS
high-resolution mass spectrometry
- EC50
median effective concentration
- 3-CCA
coumarin-3-carboxylic acid
- SEM
scanning electron microscopy
- DMSO
Dimethylsulfoxide
- Xoo
Xanthomonas oryzae pv.oryzae
- Rs
Ralstonia solanacearum
- Aac
Acidovorax citrulli
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c08627.
Characterizations data; 1H, 13C, and 19F NMR spectra and HRMS of title compounds are provided (PDF)
Author Contributions
§ Yuanquan Zhang, Zhiyuan Xu, and Minxiang Dou contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Huang X.; Liu H. W.; Long Z. Q.; Li Z. X.; Zhu J. J.; Wang P. Y.; Qi P. Y.; Liu L. W.; Yang S. Rational Optimization of 1,2,3-Triazole-Tailored Carbazoles As Prospective Antibacterial Alternatives with Significant In Vivo Control Efficiency and Unique Mode of Action. J. Agric. Food Chem. 2021, 69 (16), 4615–4627. 10.1021/acs.jafc.1c00707. [DOI] [PubMed] [Google Scholar]
- Ogunyemi S. O.; Abdallah Y.; Ibrahim E.; Zhang Y.; Bi J.; Wang F.; Ahmed T.; Alkhalifah D. H. M.; Hozzein W. N.; Yan C. Q.; Li B.; Xu L. H. Bacteriophage-mediated biosynthesis of MnO2NPs and MgONPs and their role in the protection of plants from bacterial pathogens. Front. Microbiol. 2023, 14, 1193206 10.3389/fmicb.2023.1193206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Hu D. Y.; Xie D. D.; Chen J. X.; Jin L. H.; Song B. A. Design, Synthesis, and Evaluation of New Sulfone Derivatives Containing a 1,3,4-Oxadiazole Moiety as Active Antibacterial Agents. J. Agric. Food Chem. 2018, 66 (12), 3093–3100. 10.1021/acs.jafc.7b06061. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Barman A.; Phukan T.; Kabyashree K.; Ray S. K.; et al. Ralstonia solanacearum virulence in tomato seedlings inoculated by leaf clipping. Plant Pathol. 2017, 66 (66), 835–841. 10.1111/ppa.12628. [DOI] [Google Scholar]
- Rahimi-Midani A.; Kim J. O.; Kim J. H.; Lim J.; Ryu J. G.; Kim M. K.; Choi T. J. Potential use of newly isolated bacteriophage as a biocontrol against Acidovorax citrulli. Arch. Microbiol. 2020, 202 (2), 377–389. 10.1007/s00203-019-01754-5. [DOI] [PubMed] [Google Scholar]
- Orfei B.; Moretti C.; Loreti S.; Tatulli G.; Onofri A.; Scotti L.; Aceto A.; Buonaurio R. Silver nanoclusters with Ag2+/3+ oxidative states are a new highly effective tool against phytopathogenic bacteria. Appl. Microbiol. Biotechnol. 2023, 107 (14), 4519–4531. 10.1007/s00253-023-12596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T.; Zhang T.; Zhou X.; Wang P. Y.; Gan J. H.; Song B. A.; Yang S.; Yang C. G. Dysregulation of ClpP by Small-Molecule Activators Used Against Xanthomonas oryzae pv. oryzae Infections. J. Agric. Food Chem. 2021, 69 (27), 7545–7553. 10.1021/acs.jafc.1c01470. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Bai Z.; Ling Y.; He L.; Huang P.; Gu H.; Hu R. F. Design, synthesis and biological evaluation of novel furoxan-based coumarin derivatives as antitumor agents. Med. Chem. Res. 2018, 27 (4), 1198–1205. 10.1007/s00044-018-2140-x. [DOI] [Google Scholar]
- Chimenti F.; Secci D.; Bolasco A.; Chimenti P.; Granese A.; Befani O.; et al. Inhibition of monoamine oxidases by coumarin-3-acyl derivatives: biological activity and computational study. Bioorg. Med. Chem. Lett. 2004, 14 (14), 3697–3703. 10.1016/j.bmcl.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Ji H.; Tan Y.; Gan N.; Zhang J.; Li S.; Zheng X.; et al. Synthesis and anticancer activity of new coumarin-3-carboxylic acid derivatives as potential lactatetransportinhibitors. Bioorg. Med. Chem. 2021, 29, 115870 10.1016/j.bmc.2020.115870. [DOI] [PubMed] [Google Scholar]
- Wei L.; Wang J.; Zhang X.; Wang P.; Zhao Y.; Li J.; et al. Discovery of 2H-chromen-2-one derivatives as G protein-coupled receptor-35 agonists. J. Med. Chem. 2017, 60 (1), 362–372. 10.1021/acs.jmedchem.6b01431. [DOI] [PubMed] [Google Scholar]
- Lin P. Y.; Yeh K. S.; Su C. L.; Sheu S. Y.; Chen T.; Ou K. L.; et al. Synthesis and antibacterial activities of novel 4-hydroxy-7-hydroxy- and 3-carboxycoumarin derivatives. Molecules 2012, 17 (9), 10846–10863. 10.3390/molecules170910846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Ren Z. L.; Wang W.; Gong J. X.; Chu M. J.; Ma Q. W.; et al. Novel coumarin-pyrazole carboxamide derivatives as potential topoisomerase II inhibitors: design, synthesis and antibacterial activity. Eur. J. Med. Chem. 2018, 157, 81–87. 10.1016/j.ejmech.2018.07.059. [DOI] [PubMed] [Google Scholar]
- Zhu F. D.; Fu X.; Ye H. C.; Ding H. X.; Gu L. S.; Zhang J.; Guo Y. X.; Feng G. Antibacterial activities of coumarin-3-carboxylic acid against Acidovorax citrulli. Front. Microbiol. 2023, 14 (14), 1207125 10.3389/fmicb.2023.1207125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.; Teng P.; Zhang Y. L.; Xu Z. J.; Zhang M. Z.; Zhang W. H. Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia 2018, 127, 387–395. 10.1016/j.fitote.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Liang J. C.; Fu X.; Zhang J.; Ding H. X.; Xu Z. Y.; Ye H. C.; Zhu F. D.; Yan C.; Gan X. H.; Feng G. Design, synthesis and antibacterial activity of coumarin-3-carboxylic acid derivatives containing acylhydrazone moiety. Arabian J. Chem. 2024, 17, 105389 10.1016/j.arabjc.2023.105389. [DOI] [Google Scholar]
- Wei L.; Wang J.; Zhang X.; Wang P.; Zhao Y.; Li J.; et al. Discovery of 2H-chromen-2-one derivatives as G protein-coupled receptor-35 agonists. J. Med. Chem. 2017, 60, 362–372. 10.1021/acs.jmedchem.6b01431. [DOI] [PubMed] [Google Scholar]
- El Shehry M. F.; Ghorab M. M.; Abbas S. Y.; Fayed E. A.; Shedid S. A.; Ammar Y. A. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur. J. Med. Chem. 2018, 143, 1463–1473. 10.1016/j.ejmech.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Zhang M. Z.; Jia C. Y.; Gu Y. C.; Mulholland N.; Turner S.; Beattie D.; Zhang W. H.; Yang G. F.; Clough J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. 10.1016/j.ejmech.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Jones R. A.; Panda S. S.; Hall C. D. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur. J. Med. Chem. 2015, 97, 335–355. 10.1016/j.ejmech.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Tseng C. H.; Tzeng C. C.; Yang C. L.; Lu P. J.; Chen H. L.; Li H. Y.; Chuang Y. C.; Yang C. N.; Chen Y. L. Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. Part 2. J. Med. Chem. 2010, 53, 6164–6179. 10.1021/jm1005447. [DOI] [PubMed] [Google Scholar]
- Liu F.; Zhang X.; Weisberg E.; Chen S.; Hur W.; Wu H.; Zhao Z.; Wang W.; Mao M.; Cai C.; Simon N. I.; Sanda T.; Wang J.; Look A. T.; Griffin J. D.; Balk S. P.; Liu Q.; Gray N. S. Discovery of a selective irreversible BMX inhibitor for prostate cancer. ACS Chem. Biol. 2013, 8, 1423–1428. 10.1021/cb4000629. [DOI] [PubMed] [Google Scholar]
- Adsule S.; Barve V.; Chen D.; Ahmed F.; Dou Q. P.; Padhye S.; Sarkar F. H. Novel schiff base copper complexes of quinoline-2 carboxaldehyde as proteasome inhibitors in human prostate cancer cells. J. Med. Chem. 2006, 49, 7242–7246. 10.1021/jm060712l. [DOI] [PubMed] [Google Scholar]
- Karad S. C.; Purohit V. B.; Thakor P.; Thakkar V. R.; Raval D. K. Novel morpholinoquinoline nucleus clubbed with pyrazoline scaffolds: Synthesis, antibacterial, antitubercular and antimalarial activities. Eur. J. Med. Chem. 2016, 112, 270–279. 10.1016/j.ejmech.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Thomas K. D.; Adhikari A. V.; Telkar S.; Chowdhury I. H.; Mahmood R.; Pal N. K.; Row G.; Sumesh E. Design, synthesis and docking studies of new quinoline-3-carbohydrazide derivatives as antitubercular agents. Eur. J. Med. Chem. 2011, 46, 5283–5292. 10.1016/j.ejmech.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Chioua M.; Sucunza D.; Soriano E.; Hadjipavlou-Litina D.; Alcazar A.; Ayuso I.; Oset-Gasque M. J.; Gonzalez M. P.; Monjas L.; Rodriguez-Franco M. I.; Marco-Contelles J.; Samadi A. Alphaaryl-N-alkyl nitrones, as potential agents for stroke treatment: synthesis, theoretical calculations, antioxidant, anti-inflammatory, neuroprotective, and brain-blood barrier permeability properties. J. Med. Chem. 2012, 55, 153–168. 10.1021/jm201105a. [DOI] [PubMed] [Google Scholar]
- Wang Q. F.; Xing L.; Zhang Y. Q.; Gong C. Y.; Zhou Y. X.; Zhang N.; He B. C.; Xue W. Antiviral activity evaluation and action mechanism of myricetin derivatives containing thioether quinoline moiety. Mol. Diversity 2023, 3, 1039–1055. 10.1007/s11030-023-10631-9. [DOI] [PubMed] [Google Scholar]
- Yang R.; Ma Y. N.; Huang T.; Xie W.; Zhang X.; Huang G. S.; Liu X. D. Synthesis and Antifungal Activities of 4-Thioquinoline Compounds. Chin. J. Org. Chem. 2018, 38 (8), 2143–2150. 10.6023/cjoc201801024. [DOI] [Google Scholar]
- Ruan H. L.; Zhang J. Y.; Sun S.; Yang Y.; Zhu X. L.; Lü C. W. N-Bromosuccinimide Mediated the Reaction of 2-Hydroxyaryl Aldehydes with Meldrum’s Acid for Synthesis of Coumarin-3-carboxylic Acids. Chin. J. Org. Chem. 2017, 37 (8), 2139–2144. 10.6023/cjoc201701003. [DOI] [Google Scholar]
- Peng F.; Liu T. T.; Cao X.; Wang Q. F.; Liu F.; Liu L. W.; He M.; Xue W. Antiviral activities of novel myricetin derivatives containing 1,3,4-oxadiazole bisthioether. Chem. Biodiversity 2022, 19 (3), e202100939 10.1002/cbdv.202100939. [DOI] [PubMed] [Google Scholar]
- Zhiyuan X.; Haixin D.; Xin F.; Yan X.; Fadi Z.; Huochun Y.; Yuan Z. Y.; Yongxia G.; Jing Z.; Gang F. Antibacterial activity and possible mechanism of action of isoquinoline-3-carboxylic acid. Nat. Prod. Commun. 2024, 10.1177/1934578x241226562. [DOI] [Google Scholar]
- Li G. H.; Qiao M. Y.; Guo Y.; Wang X.; Xu Y. F.; Xia X. D. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog. Dis. 2014, 11, 677–683. 10.1089/fpd.2013.1731. [DOI] [PubMed] [Google Scholar]
- Li B.; Shi Y.; Shan C. L.; Zhou Q.; Ibrahim M.; Wang Y. L.; Wu G. X.; Li H. Y.; Xie G. L.; Sun G. C. Effect of chitosan solution on the inhibition of Acidovorax citrulli causing bacterial fruit blotch of watermelon. J. Sci. Food Agric. 2013, 93, 1010–1015. 10.1002/jsfa.5812. [DOI] [PubMed] [Google Scholar]
- Silva-Angulo A. B.; Zanini S. F.; Rosenthal A.; Rodrigo D.; Klein G.; Martínez A. Comparative study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS One. 2015, 10, e0114026 10.1371/journal.pone.0114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. L.; Guan W.; Huang Q.; Yang Y. W.; Yan W. R.; Sun B. X.; Zhao T. C. Quorum-sensing contributes to virulence, twitching motility, seed attachment and biofilm formation in the wild type strain Aac-5 of Acidovorax citrulli. Microb. Pathog. 2016, 100, 133–140. 10.1016/j.micpath.2016.08.039. [DOI] [PubMed] [Google Scholar]
- Ernst W. A.; Thoma-Uszynski S.; Teitelbaum R.; Ko C.; Hanson D. A.; Clayberger C.; Krensky A. M.; Leippe M.; Bloom B. R.; Ganz T.; Modlin R. L. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 2000, 165, 7102–7108. 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura G.; Piccolomini R.; Paludi D.; D’Orio V.; Vergara A.; Conter M.; Lanieri A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. 10.1111/j.1365-2672.2007.03688.x. [DOI] [PubMed] [Google Scholar]
- Shi L. L.; Wang W. L.; Gao M. N.; Wu Z. X.; et al. Antibacterial activity and mechanism of action of sulfone derivatives containing 1,3,4-oxadiazole moieties on rice bacterial leaf blight. Molecules 2015, 20, 11660–11675. 10.3390/molecules200711660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W.; Zhou M.; Liu Z.; Chen Y.; Li R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2018, 85, 119–126. 10.1016/j.foodcont.2017.09.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.