Abstract
Recently, there has been immense interest in using biodegradable polymers to replace petro-derived polymers. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), which is gaining popularity due to its biodegradability, is used in developing blends and composites for a variety of applications. To enhance the miscibility between different components of a material with PHBV, functionalization of the PHBV chain can be done. In this study, grafting of maleic anhydride (MA) onto PHBV was performed using organic peroxide also, called Luperox [2,5-dimethyl-2,5-di(tert-butylperoxy)hexane], as an initiator. The effects of different initiator and MA concentrations on the grafting percentage, gel content, rheology, and thermal properties were evaluated. Additionally, a chain extender (Joncryl ADR 4468) was added during the grafting process to prevent chain scission of PHBV during processing and to promote long-chain branching. Higher initiator concentrations played a significant role in increasing the grafting percentage. Adding the chain extender further enhanced the grafting percentage of the compatibilizers. Compatibilizers with chain extenders increased the grafting percentage by up to 91% compared with their counterparts without chain extenders. Differential scanning calorimetry analysis demonstrated that the melting temperature of the compatibilizers decreased by up to 8 °C with increasing initiator concentration. Furthermore, the thermal stability of maleic anhydride grafted PHBV (MA-g-PHBV) reduced at higher initiator content, likely attributed to the formation of low-molecular-weight species during processing. The maleated PHBV with a chain extender exhibited improved thermal stability, with an increase of up to 6 °C, attributed to an increase in molecular weight because of the chain extender. This can be corroborated by the rheological studies, which showed higher viscosity, particularly for MA-g-PHBV with chain extender. Therefore, this is the first ever scientific study to improve and analyze the grafting percentage of MA-g-PHBV using a combination of chain extender and varying initiator concentrations. This maleated PHBV with a higher grafting percentage will then facilitate the development of composites and blends with improved performance, even at low MA-g-PHBV concentrations.
1. Introduction
In the present time, plastic pollution is widespread across all terrestrial biomes, with the production of plastics outpacing nearly all other industrial materials. Annually, Canada produces 3 million tonnes of plastic waste, equivalent to $8 billion per year lost in energy and wasted resources. The European Union recycled approximately 30% of its plastic waste, while for Canada, it was only ∼9%, significantly less than the global mean of ∼15%.1 As a result, biodegradable polymers have become very popular in recent decades due to global concerns over plastic waste generation, increasing dependence on petroleum-derived products, and the generation of secondary microplastics.
Polyhydroxyalkanoates (PHAs) are a class of polyesters that are derived from microbial fermentation of biomass and have exhibited immense potential to replace petro-derived polymers. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), one of the most well-known bioplastics belonging to PHAs, is an aliphatic copolyester derived from the bacterial fermentation of lipids and sugar.2 PHBV exhibits mechanical properties comparable to polyolefins like polypropylene and is considered both industrial and home compostable and shows marine biodegradability as well.3 Because of these superb qualities, PHBV is a promising material used in different applications, including packaging and the biomedical industry. Nevertheless, the thermal instability and brittleness of PHBV are its primary disadvantages. It is established in the literature that PHBV has high crystallinity, and the brittleness originates due to the crack progression in the large spherulites of the polymer.4 Moreover, PHBV has a narrow processing window, making it thermally sensitive to degradation. As such, careful attention is required while processing PHBV.5 Therefore, PHBV is often combined with other polymers and natural fillers owing to its high price and the need to tailor its properties depending on the application. However, PHBV-based blends and composites have moderate properties because of poor interfacial adhesion among the different phases, as established previously in the literature. For example, Berthet et al.6 reported a considerable reduction in mechanical properties and moisture barrier properties of PHBV/wheat straw fiber composites due to higher filler content and poor compatibility between the matrix and the fiber. Also, natural fiber incorporation at higher concentrations leads to difficulty in processing, which includes higher viscosity and poor fiber dispersion, among others. But even with considerable research attempts to improve PHBV properties in the past decades by direct and postpolymerization techniques, limited progress has been made.7 As such, compatibilization is necessary to develop a finished product having comparative mechanical performance, as most of the blends and composites are known to be immiscible.8
Chemical modification of the PHBV backbone by introducing new functional groups such as amine, epoxide, oxazoline, carboxylic acid, anhydride, and isocyanate allows us to develop laminates, multilayer films, blends/alloys, and composites for industrial applications having enhanced performance and cost-effectiveness.9 Graft polymerization is a widely recognized technique for modifying the chemical and physical characteristics of polymers for specific applications. Maleic anhydride (MA) grafting onto polymer chains has been carried out extensively to improve compatibility among polar and nonpolar polymers, interaction between polymer and fillers, and adhesion to metals.10 MA-functionalized PHBV can also be used as a tie layer in multilayer extrusion. The grafted MA might react with the specific functional groups of the reinforcing bio-based filler/fiber, leading to better interaction between the two phases. Graft polymerization is preferably done in the presence of an initiator such as a peroxide and can be carried out in the solid state, in solution, or in the polymer melt. Organic peroxides are widely used for this process since they decompose to generate free radicals. These radicals then react with the hydrogen groups in the polymer chains to alter the polymer properties. Multiple factors can impact the efficiency of this process; hence, the optimized processing temperature and residence time are critical parameters that control the decomposition of peroxides into free radicals.11 Therefore, the most ideal method for conducting these reactions in the polymer melt is done by the process of reactive extrusion due to its cost-effectiveness and simple method.10 The widespread use of MA to functionalize biopolymers is because of its desirable combination of high activity of the succinic anhydride moiety, low cost of MA, and better processing of the MA-grafted polymers.7
During reactive extrusion, the grafting of MA may result in the chain scission of PHBV into much shorter chains. Chain extenders are often used to lengthen broken chains during processing, resulting in enhanced melt strength, viscosity, and thermal stability of the polymer. Epoxy group-based chain extenders are frequently used for polyesters where a higher concentration of such chain extenders can result in the effective chain extension of the polymer with itself.12 These chain extenders, therefore, result in the increased molecular weight of the polymer, in conjunction with chain branching based on the additive’s functionality.13 Joncryl ADR grade, an epoxide styrene-acrylic-based compound, is a well-known reactive chain extender having a low molecular weight (Mn < 3000 g/mol). The high reactivity of Joncryl is because of the presence of multiple epoxy groups which can react with carboxyl and hydroxyl groups of different polyesters.14 Najafi et al.15 reported that using Joncryl can result in chain extension leading to long-chain branching in PLA. Duangphet et al.16 also used Joncryl to counter the processing shortcomings of PHBV.
By far, grafting of MA onto polyolefins like polypropylene and polyethylene is among the most common methods used to synthesize functionalized polymers for industrial applications. But research into MA grafting of PHAs, especially PHBV in the presence and absence of a chain extender, has not been explored to the best of our knowledge. Therefore, this study aims at developing MA-grafted-PHBV compatibilizers using a reactive extrusion technique with different concentrations of MA and the initiator in the presence and absence of a chain extender followed by characterizing and comparing them based on their thermal behavior, viscosity, percentage grafting, and chemical interactions. The study provides insight into the grafting mechanism of MA and the role of chain extenders and initiators in the grafting of MA onto the PHBV backbone. The rationale behind this study is that MA-grafted-PHBV with a higher grafting percentage, both with and without a chain extender, will be able to enhance the compatibility between immiscible materials. This leads to improved material performance and allows for tailoring the material property to specific requirements with only a small quantity of MA-grafted-PHBV. Furthermore, producing such compatibilizers using a large-scale twin-screw extruder makes them suitable for industrial-scale production and supports widespread commercialization as a functional additive used in different manufacturing sectors.
2. Experimental Section
2.1. Materials
PHBV polymer (ENMAT Y1000P) with a hydroxyvalerate content of 3 mol % was provided by Tianan Biologic Material Co., Ltd. (China). The preparation of the compatibilizer involved the use of both MA and an initiator. The free radical initiator, Luperox 101 [2,5-dimethyl-2,5-di(tert-butylperoxy)hexane], was obtained from Arkema (USA). The MA was purchased from Thermo Fischer Scientific Inc. (Canada). Joncryl (ADR 4468), an epoxy-functionalized acrylic resin-based chain extender, was procured from BASF (Germany). To determine the % grafting of the grafted materials, ethyl alcohol and phenolphthalein were obtained from Thermo Fisher Scientific Inc. (Canada), while potassium hydroxide (0.1 N) and chloroform were purchased from Sigma-Aldrich (Canada). Luperox 101 and Joncryl will be termed as LUP and JON, respectively hereinafter.
2.2. Maleation of PHBV Using Reactive Extrusion
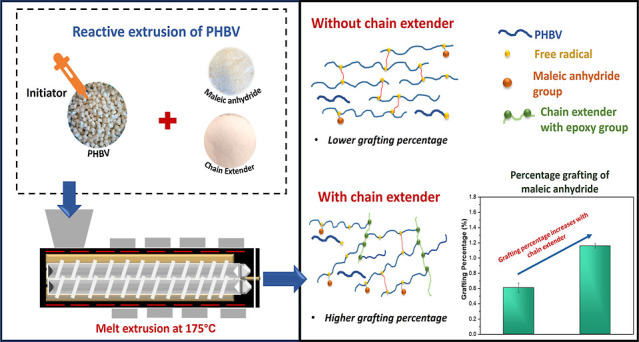
The grafting of MA onto the PHBV backbone in this study was performed by using the reactive extrusion process with an initiator. Before the reactive extrusion process, the PHBV pellets were placed in an oven overnight at 80 °C to dry. The compatibilizers were processed by adding different concentrations of MA at 2.5 and 5.0 wt % (taken with respect to the mass of polymer) into neat PHBV at various LUP contents [0.25, 0.5, and 1 part per hundred (phr)]. Additionally, JON, a chain extender, was added to some formulations at 0.5 phr to prevent chain scission of PHBV during processing. The properties of these compatibilizer formulations were also characterized to examine the impact of MA, initiator, and chain extender. The formulation table for MA-grafted-PHBV is presented in Table 1. To produce the compatibilizers, the dried PHBV pellets were first thoroughly mixed with an appropriate amount of LUP in ziplock bags to ensure that the PHBV pellets were coated with LUP. Before LUP was incorporated into PHBV, it was diluted with acetone to facilitate homogeneous coating onto PHBV pellets. The next step involves the addition of the required amount of MA into the PHBV-LUP mixture, followed by thorough shaking of the mixture, which ensures that the MA adheres to the pellets. Subsequently, the blend was then fed into a co-rotating twin-screw extruder (Micro-27 Model, Leistritz, Germany) at the processing temperature of 175 °C with a feed rate of 5 kg/h and a screw speed of 60 rpm. This extruder has a length-to-diameter ratio (L/D) of 48:1, in which the screws rotate within 12 heating zones. After extrusion, the strands were directed through a water bath for cooling and then converted into pellets using a pelletizer (Bullet 64 model from Reduction Engineering, USA). Finally, the pellets were placed in a vacuum oven at 70 °C for 48 h to eliminate any unreacted MA. The developed MA-grafted-PHBV is referred to as MA-g-PHBV from now.
Table 1. Formulation Table for Different Types of MA-g-PHBV Developed with Different MA, LUP, and JON Concentrations.
| Formulation | Sample ID | PHBV (wt %) | MA (wt %) | LUP (phr) | JON (phr) |
|---|---|---|---|---|---|
| PHBV/2.5%MA/0.25 phr LUP | Comp_1 | 97.5 | 2.5 | 0.25 | - |
| PHBV/2.5%MA/0.25 phr LUP/0.5 phr JON | Comp_2 | 97.5 | 2.5 | 0.25 | 0.5 |
| PHBV/2.5%MA/0.5 phr LUP | Comp_3 | 97.5 | 2.5 | 0.5 | - |
| PHBV/2.5%MA/0.5 phr LUP/0.5 phr JON | Comp_4 | 97.5 | 2.5 | 0.5 | 0.5 |
| PHBV/5%MA/1 phr LUP | Comp_5 | 95.0 | 5.0 | 1.0 | - |
The processing of MA-g-PHBV showed that increasing the concentrations of MA and LUP reduced the melt viscosity due to the reactive extrusion process, which was confirmed by the decline in the extruder torque during processing. Continuous strands without breakage were consistently obtained during extrusion, and they were easily pelletized without forming clumps. The addition of JON to the formulation further helped in processing, where it was easier to collect the strands, and they were more stable because of the presence of JON. Additionally, the maleated PHBV exhibited a more yellowish color compared to neat PHBV pellets, with the yellowish intensity increasing with higher LUP concentrations. This trend in color change in biopolymers after grafting has been observed in other studies and is mainly attributed to side reactions occurring during the reactive extrusion process.17
For the initiator, the technical data sheet for LUP organic peroxide shows that the half-life data are 1 min at 180 °C and 6 min at 164.4 °C.18 The half-life of the initiator is the loss of one-half of the peroxide’s active oxygen content at a specific time and temperature, and it is correlated to the peroxide decomposition rate and therefore to the formation of radicals. This rate can be affected by the temperature and the solubility of the polymer in the molten state. De Roover et al.19 emphasize that the half-life time of the peroxide depends basically on temperature. However, the half-life of the peroxide also depends on the time at which it is exposed to that specific temperature. In this study, the residence time in the barrel at the specified conditions (175 °C/60 rpm) is 2 to 3 min, which is in accordance with the half-life of the initiator.
2.3. Characterization of the Compatibilizers
2.3.1. Purification of MA-g-PHBV
The vacuum oven-dried MA-g-PHBV is purified to determine the % grafting of MA onto the PHBV backbone. First, ∼5 g of compatibilizer was dissolved in chloroform (50 mL) by stirring for 3 h under heat on a hot stage. After the samples were completely dissolved, MA-g-PHBV was precipitated by adding ethyl alcohol and subsequently filtered. The precipitated MA-g-PHBV was washed with an excess of ethyl alcohol to remove any unreacted MA or initiator followed by drying in a vacuum oven at 60 °C for 24 h. The oven-dried sample was subsequently used for the determination of % grafting.
2.3.2. Calculation of Percentage Grafting of MA
The % grafting of MA onto the PHBV backbone was calculated using the back-titration method mentioned elsewhere.20 Continuing the previous procedure, the dried sample of known weight was again dissolved in 50 mL of chloroform under ambient temperature for 1 h. After the complete dissolution of the purified MA-g-PHBV into chloroform, 3 drops of deionized water and 8 drops of phenolphthalein indicator were added to the solution under stirring. The solution was then titrated against alcoholic KOH (0.1 N) until the end point was reached. At this stage, MA-g-PHBV was completely dissolved in chloroform, and precipitation was not observed during titration. The % grafting was determined as follows [eq 1]20,21
| 1 |
where VKOH (liters) and NKOH represent the volume of KOH used and the normality of KOH, respectively, while W is the weight of purified MA-g-PHBV taken for titration. The molecular weight of MA was 98.06 g/mol.
2.3.3. Gel Content
The gel content of the MA-g-PHBV compatibilizers was calculated using the Soxhlet extraction method mentioned elsewhere.22 Both grafted and ungrafted PHBV were soluble in chloroform, while cross-linked PHBV was insoluble. The extraction involved refluxing chloroform at 90 °C for 72 h. After 72 h, the insoluble PHBV obtained in the thimble was dried in a vacuum oven at 85 °C for ∼48 h to eliminate residual chloroform. The dried thimble weight was determined, and the gel content was measured according to eq 2(22)
| 2 |
where Wi represents the weight of residues obtained after extraction and Wo is the initial weight of the sample. The gel content experiment was conducted in duplicates.
2.3.4. Fourier Transform Infrared Spectroscopy
Fourier transform infrared (FTIR) analysis of different MA-g-PHBV samples was conducted using the Nicolet Summit X FTIR spectrophotometer (Thermo Scientific, USA) in attenuated total reflectance mode. Purified MA-g-PHBV samples were used for this experiment. The infrared spectra of the samples were collected from 4000 to 500 cm–1 at a resolution of 4 cm–1 and 256 scans. This study was done to determine the presence of MA in the PHBV backbone and to observe the changes in functional groups and interactions within the samples.
2.3.5. Rheology
The rheological analysis of the samples was conducted with a stress-controlled Anton Paar MCR-302 rheometer with a parallel plate setup having a 25 mm diameter. The experiment was done at 175 °C in a N2 atmosphere having a plate gap of 1 mm. The test was conducted in dynamic frequency sweep mode within the range of 100 to 0.1 rad/s having a constant strain of 1%, which ensures that the experiment was executed within the linear viscoelastic region of the material.
2.3.6. Thermal Properties
A Q200 differential scanning calorimeter (DSC) by TA Instruments (USA) was used to determine the melting temperature (Tm), crystallization temperature (Tc), and the % crystallinity of the samples. To perform the DSC test, ∼10 mg of the sample was placed inside a hermetically sealed aluminum pan and put inside the DSC equipment. The samples were subjected to heat–cool–heat cycles under a N2 atmosphere. The first heating cycle, ranging from −70 to 190 °C at 10 °C/min, was performed to remove the thermal history of the samples. This was followed by the cooling cycle ranging from 190 to −70 °C at a cooling rate of 5 °C/min. The second heating cycle involved heating of the specimen from −70 to 190 °C at 10 °C/min. The data obtained from the cooling cycle and the second heating cycle was used for analyzing the thermal events. The % crystallinity of the samples (% Xc) was obtained using eq 3.23
| 3 |
where ΔHf = heat of fusion of PHBV, ΔH0f = heat of fusion of 100% crystalline PHBV [109 J/g24], and Wp = weight fraction of PHBV in the compatibilizer.
The thermal stability of the compatibilizers was performed with a thermogravimetric analyzer (TGA) (Q500, TA Instruments, USA). The experiment involved heating of samples (∼15 mg) from ambient temperature to 600 °C with a 10 °C/min heating rate in a N2 environment having a 50 mL/min flow rate. TGA gave information regarding the onset temperature (T2%) using the thermogravimetric (TG) curve, while the derivative thermogravimetric (DTG) curve was used to calculate the maximum degradation temperatures (Tmax).
3. Results and Discussion
3.1. Theory of the Grafting Process
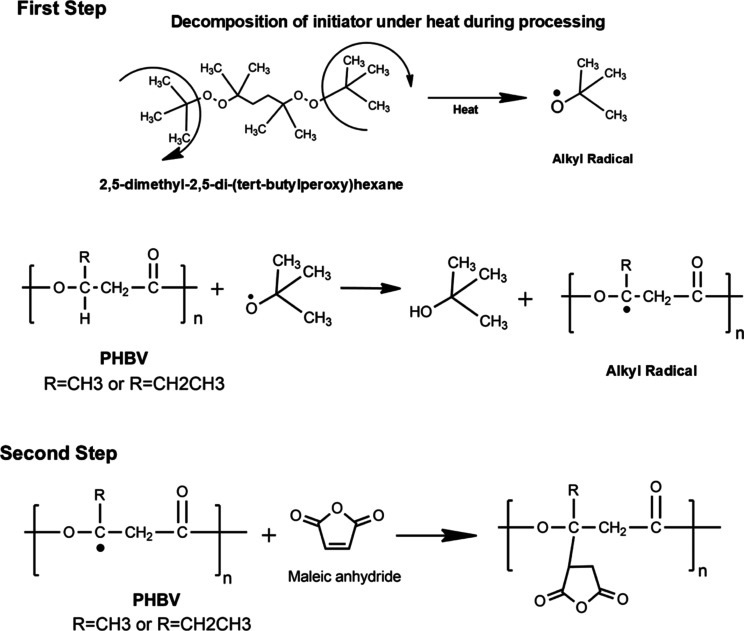
A probable mechanism of the grafting reaction of MA onto PHBV is provided based on literature findings19,25 and is presented in Figure 1.
Figure 1.
Schematics for the grafting of MA onto the PHBV backbone. The first step involves the decomposition of the initiator under heat into a reactive alkyl radical. The reactive radical extracts a hydrogen atom from the PHBV backbone making the PHBV chain reactive. In second step, the MA reacts with the PHBV chain to form MA-g-PHBV.
Due to the heat and residence time inside the extruder, the organic peroxide initiator undergoes homolytic decomposition, which attracts secondary hydrogens in the PHBV. In the case of LUP, the formation could lead to at least 2 free radicals per molecule. In this step, the double bond located in the ring of the MA breaks, forming MA radicals. Thus, by nucleophilic reactions, the hydrogen atoms are removed from the surface of the polymer backbone, and the MA radicals attached to those radicals formed on the surface of the polymer, giving rise to the grafting process or the attachment of the MA onto the PHBV backbone.
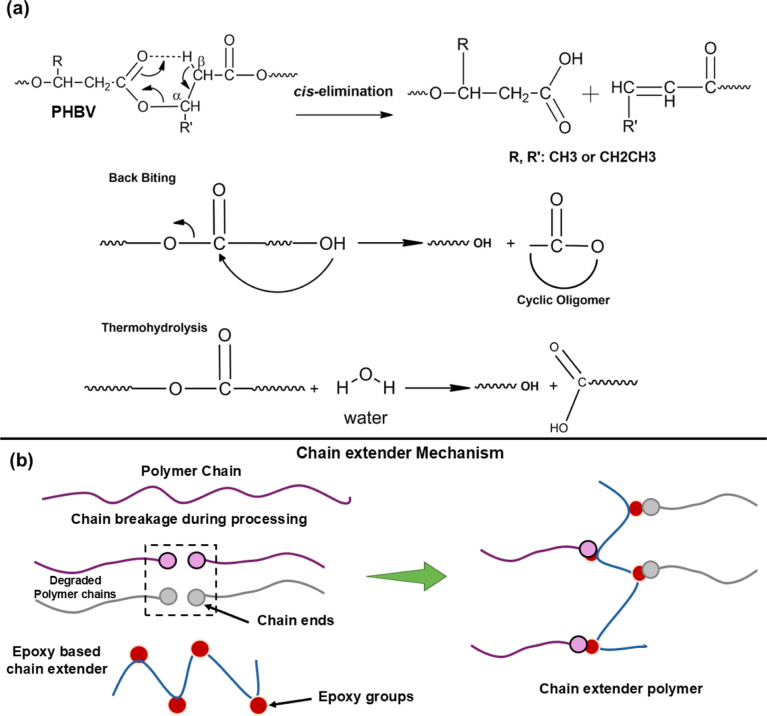
On the other hand, there is another possibility that the reaction proceeds also by scission of the polymer chains, which is well-documented in the specialized literature.19 Carbon–carbon bonds are more susceptible to breaking due to the lower energy compared to carbon–oxygen bonds. It is also probable that after the replacement of the proton, the reaction continues with the carbon–carbon bond. According to the literature, the chain scission in PHBV can take place by a β-chain scission process at ester groups involving a six-membered ring ester as a transition state; chain scission in polyesters might also take place by an intramolecular transesterification process (like backbiting or thermohydrolysis) as mentioned in the literature [Figure 2a].9,26,27
Figure 2.
(a) Probable mechanisms of degradation of PHBV; (b) schematic representation of the chain extension mechanism of polymers incorporated with JON [Redrawn with permission from Standau et al.14 Copyright 2021, Taylor and Francis (License no.: 5872110208913)]. The end group of the chain extender, JON, reacts with the reactive hydroxyl and carboxyl end groups of PHBV. This can help in the formation of the long-chain branching of PHBV.
Additionally, the chain extender, JON, was also added during the reactive extrusion process since it has the ability to react with the end groups of low-molecular-weight PHBV obtained during the chain scission reaction. The probable reaction mechanism is presented in Figure 2b. The reaction occurs between the chain extender and the carboxyl or hydroxyl reactive end groups of PHBV, which are capable of developing branching. The reactivity of these reactive groups is crucial for the formation of long chains.14,28,29 Therefore, the chain extension of grafted MA can help enhance the rheological properties and thermal stability by increasing the molecular weight caused by rebonding the degraded chains of the MA-g-PHBV compatibilizers.
3.2. Percentage Grafting of MA
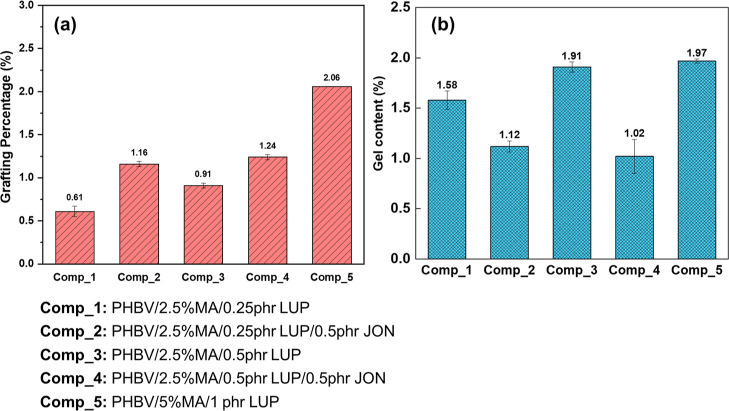
The effect of initiator concentration, in conjunction with the presence or absence of a chain extender, on the % grafting of MA is depicted in Figure 3a. As observed in this figure, the % grafting is greatly dependent on the initiator concentration. An increase in initiator concentration from 0.25 to 0.5 phr LUP at 2.5 wt % MA leads to a notable increment in the % grafting of MA (from 0.61% to 0.91%). The highest % grafting was for Comp_5 (5 wt % MA with 1 phr LUP), which is obvious due to higher MA and initiator concentration. The increment in the LUP concentration enhances the likelihood of hydrogen abstraction from the PHBV chain. LUP under heat forms more free radicals from the degradation of LUP which facilitates active site formation on the PHBV backbone, leading to increased graft reaction. Some studies have reported a reduction in % grafting at higher initiator concentration due to the presence of excess initiator, which might trigger the free radical to undergo termination reactions in PHB macroradicals or a combination reaction among them, leading to % grafting reduction.4 Therefore, it is advisable to optimize the initiator concentration to obtain maximum % grafting.
Figure 3.
Change in (a) grafting percentage of MA onto the PHBV backbone and (b) gel content of prepared formulations. The grafting percentage of the compatibilizers increased with increase in MA and LUP concentrations and this improved further upon addition of JON. The gel content also increased at higher MA and LUP concentrations with Comp_5 showing the highest value. The addition of JON reduced the gel content as compared to its counterpart without JON.
On the other hand, the addition of 0.5 phr JON increased the % grafting of the compatibilizers. As shown in Figure 3a, Comp_2 and Comp_4 exhibited a higher percentage of grafting compared to their counterparts without JON (Comp_1 and Comp_3). This is probably because of the long chain branching in MA-g-PHBV caused by the rebonding of low-molecular-weight PHBV under the influence of the chain extender.
3.3. Gel Content
To evidence the cross-linking in MA-g-PHBV in the presence of an initiator and with a combination of chain extenders, the gel content study has been conducted. The Figure 3b results showed that the increase in initiator and MA had increased the cross-linking density. The gel content increased as the initiator increased from 0.25 to 0.5 phr for Comp_1 and Comp_3, and with an increase in MA from 2.5 to 5% for Comp_3 and Comp_5, respectively. This can be corroborated by the increase in grafting percentage with an increase in the concentration of initiator and MA [Figure 3a]. The study conducted by Chen et al.4 has further shown that the initiator is sensitive to graft degree where an increase in initiator concentration from 0.1 to 0.2% has increased the graft degree from 0.24 to 0.85%. This is because, with the increment in the initiator content, more free radicals are formed by the dissociation of the initiator which could form more active sites in the polymer backbone through hydrogen abstraction.4 The free radicals in PHBV could form radical-induced cross-linking or branching which increases the gel content.30
The increase in MA concentration in Comp_5 has increased the percentage of grafting; however, the gel content remained comparable with Comp_3. This may be because of the increased β-scission reaction between free radicals trapped on MA and the polymer chain that forms alkoxy radicals or macroradicals.9,30 The addition of JON reduced the gel content which indicates that it reduced the PHBV–PHBV radical cross-linking. This further supported the formation of a branched structure with the PHBV end group and chain extender to form long-chain molecules. The evidence for this observation is also shown in the increase in grafting percentage, with Comp_2 and Comp_4 exhibiting higher MA grafting compared to Comp_1 and Comp_3.
The importance of gel content was highlighted in a disclosure patent by Mohanty et al.,31 who stated that higher gel content reduces the flowability and reduces its ability to be processed by an extrusion or injection molding technique, thereby increasing the manufacturing cost and energy consumption. Thus, the application of JON as a chain extender in this study can be looked upon as an efficient technique to reduce the gel content with increased % grafting.
3.4. FTIR Spectroscopy
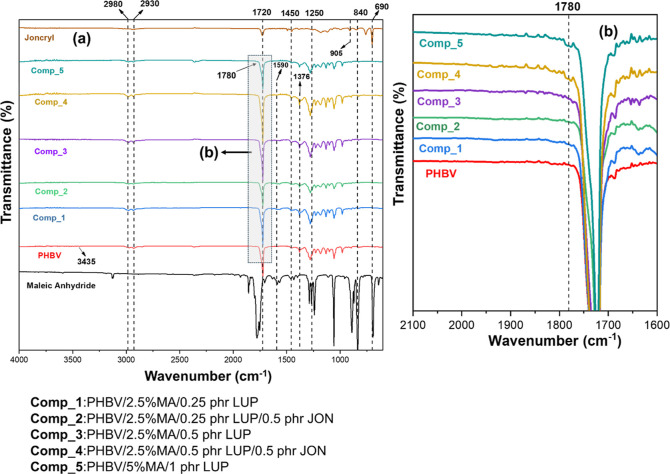
FTIR study was performed to investigate the grafting reactions of MA and PHBV. FTIR spectra of neat PHBV and purified MA-g-PHBV samples are shown in Figure 4a,b. Neat PHBV showed an absorption band at 2980 cm–1, which is related to the asymmetric elongation vibrations of the methyl groups (−CH3). Another absorption at 2930 cm–1 is associated with the elongation asymmetric vibrations of −CH2 groups. The sharp absorption signal at 1720 cm–1 represents the aliphatic carbonyl elongation vibrations (C=O), and the absorption bands between 1050 and 1280 cm–1 are associated with the C–O bond. Additionally, PHBV showed distinctive peaks at ∼1450 and ∼1376 cm–1 which corresponds to the stretching and bending vibrations of the methyl group (−CH3), respectively.32 The weak absorption signal at 3435 cm–1 is because of the stretching vibrations of the hydroxyl groups (−OH). These peaks are in good agreement with the previous literature.21,33 The peak observed in the range of 1500 to 900 cm–1 is related to −CH3 and −CH vibrations and C–O–C and C–C stretching vibrations.34
Figure 4.
(a) FTIR spectra of PHBV, purified MA-g-PHBV samples, JON, and MA. (b) The highlighted region from Figure 4a (2000–1600 cm–1) is expanded, in which Comp_4 and Comp_5 are showing small peak at 1780 cm–1. This peak indicates the grafting of MA onto the PHBV backbone, associated with the succinic anhydride group of MA, and is absent in neat PHBV. [For other samples, the peak at 1780 cm–1 was absent due to lower grafting percentage]. Additionally, the FTIR spectra have been brought closer along the Y axis in Figure 4b using Origin software to enhance clarity and make it easier to distinguish between the peaks in the spectra.
For the MA-g-PHBV samples, we noticed the same absorption bands, which were discussed above for PHBV in the FTIR spectra. The small new peak at 1780 cm–1 was observed in MA-g-PHBV samples, which is associated with the saturated cyclic anhydride carbonyl ring (succinic anhydride group) [Figure 4b]. This peak confirms that the MA monomer is grafted to the PHBV backbone, during which MA is converted to a saturated anhydride (succinic anhydride).35 This small peak is more prominent in Comp_4 and Comp_5 [presented in Figure 4b] compared to other MA-g-PHBV samples, which is due to the higher percentage of grafting in Comp_4 and Comp_5. Similar weak absorption peaks were reported in the previous literature as evidence of grafting MA on the polymer backbone.35−37 Also, PHBV is a polyester having ester –COO groups, and therefore, the ester groups of a small molecule such as MA may be masked by the ester groups of PHBV, resulting in a small wide peak at 1780 cm–1.
Moreover, the absorption peak at 1720 cm–1 was wider than that of pure PHBV due to the superposition of the ester peak (C=O) of PHBV and the ester peak (C=O) of MA. This is the reason for the wider absorption peak around 1720 cm–1 in the FTIR spectra of MA-g-PHBV samples in comparison with that of pure PHBV. A similar observation was reported for MA-g-PLA with a wider absorption peak of C=O at around 1750 cm–1 compared to that of pure PLA.38 The information presented above showed that the MA was successfully grafted onto the PHBV backbone.
On the other hand, in the MA-g-PHBV samples, the elimination of unreacted MA can be confirmed by the absence of the MA ring at around 690 cm–1 in the FTIR signals of all MA-g-PHBV samples [Figure 4a]. In addition, the FTIR spectra of MA exhibited a signal at 1590 cm–1 which corresponds to C=C stretching of the MA,21 which was not observed in the spectra of MA-g-PHBV samples [Figure 4a], which confirms that the unsaturated cyclic ring of MA is converted to a saturated succinic anhydride ring after grafting to the PHBV backbone. Overall, the appearance of a small peak at 1780 cm–1, the absence of peaks at 690 and 1590 cm–1, and a wider peak at 1720 cm–1 in MA-g-PHBV FTIR spectra together with the percentage of MA grafting data reported in Figure 3a are evidence of successful grafting of MA on the PHBV backbone by the initiation of LUP.
Finally, in the case of neat JON, the peaks at 905, 840, and 1250 cm–1 correspond to symmetric and asymmetric ring deformation of cyclic epoxide (−C–O stretching).39,40 These peaks are not observed in the MA-g-PHBV FTIR spectra, suggesting that interaction could take place among epoxy groups of JON and terminal hydroxyl or carboxyl groups of the PHBV backbone [Figure 4a]. A similar observation was reported in FTIR analysis of the previous literature for the reaction of JON with other polyesters such as PLA39,41 and PLA/PBAT blend.40
3.5. Rheology
The behavior of molten polymer chains between the matrix and additives is closely linked to the melt rheology of any polymer. Additionally, rheology offers insights into processing parameters like operating temperature, shear stress, and their impact on the other analytical properties of the polymer, such as mechanical and thermal properties.42
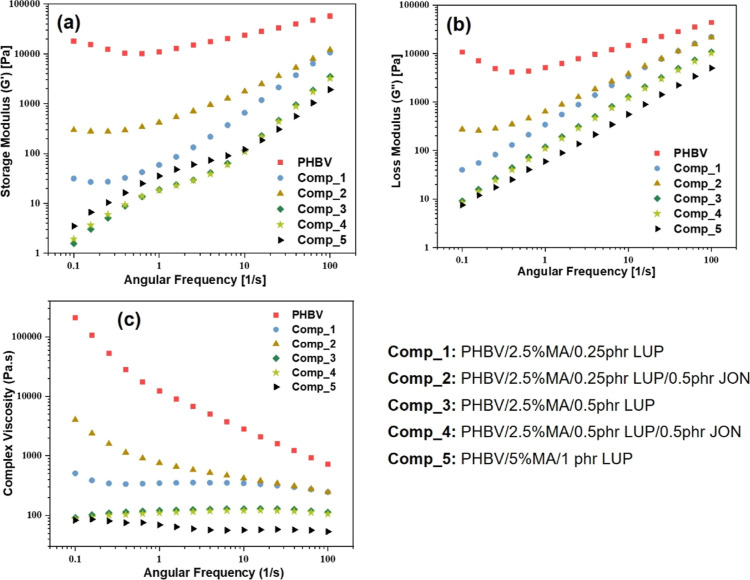
In this research, the rheological behavior of maleated PHBV was examined, and the obtained results in the form of moduli (storage and loss) and viscosity are presented in Figure 5(a–c), respectively. The values belonging to storage (G′) and loss modulus (G″) of all the formulations increase as the angular frequency (1/s) progresses toward higher values in the range of 1–100 1/s, which satisfies the linear viscoelastic theory.3 However, in the lower angular frequency range (lower than 1), Comp_1 and Comp_2 displayed a plateau in the storage modulus, representing an internal polymer structure that was difficult to relax within a long time scale.
Figure 5.
Rheological behavior of different maleated PHBV samples; (a) storage modulus, (b) loss modulus, and (c) complex viscosity. For MA-g-PHBV, the increase in LUP concentration reduced the complex viscosity and storage modulus with Comp_5 showing the lowest values. The addition of JON, however, increased the rheological properties (especially in Comp_2).
The effect of maleation was clearly observed on the storage modulus of pristine PHBV, as shown in Figure 5a. The storage modulus of pristine PHBV was reduced significantly after the addition of a coupling agent (MA) and peroxide (LUP) in the absence or presence of a chain extender (JON). It signifies the chain scission phenomena of longer PHBV polymer chains due to thermal degradation and hydrolysis34 during reactive extrusion and, hence, the molecular weight of PHBV was reduced significantly.43 The polydispersity index of maleated PHBV was increased due to generating a wide range of molecular weight distribution. It also means that the elastic nature of PHBV was reduced significantly after maleation.34
The storage modulus of Comp_2 was observed to be the highest among all other combinations except pristine PHBV. It is due to the effect of the chain extender present in Comp_2, which restricts the scission of PHBV chains compared with the counterpart without a chain extender. It is also due to the suppressed relaxation of the polymer chains, as the function of a chain extender is to combine two or more polymer chains. However, the effect of the chain extender was not visible when the amount of initiator was increased from 0.25 to 0.5 phr. However, it is crucial to note that the impact of the initiator dominates among other additives, such as MA and JON.
The complex viscosity (η*; Pa·s) data was plotted against angular frequency, and the graph is shown in Figure 5c. With increasing angular frequency, the η* values were reduced for pristine PHBV, representing a shear-thinning behavior.32 The polymer chain disentanglement at a higher shear rate can describe this phenomenon. Similar behavior of the complex viscosity was also observed for Comp_1 and Comp_2. Interestingly, the other formulations (Comp_3/Comp_5) showed almost the same complex viscosity over the entire angular frequency range. It means that the shear-thinning phenomena were suppressed in these formulations (Comp_3 to Comp_5) due to the reduced complex viscosity range. It may also be due to the formation of gel content in higher amounts, as the gel formation does not follow viscoelastic behavior. Comparing Comp_1 and Comp_2, it is evident from Figure 5c that Comp_2 had higher η* values than Comp_1. This increment can be attributed to the increase in chain length of maleated PHBV upon addition of JON leading to increased molecular weight. This is a common behavior where complex viscosity increases after JON incorporation, as observed in numerous studies with bioplastics and polyolefins.14
3.6. Thermal Properties
3.6.1. Differential Scanning Calorimetry
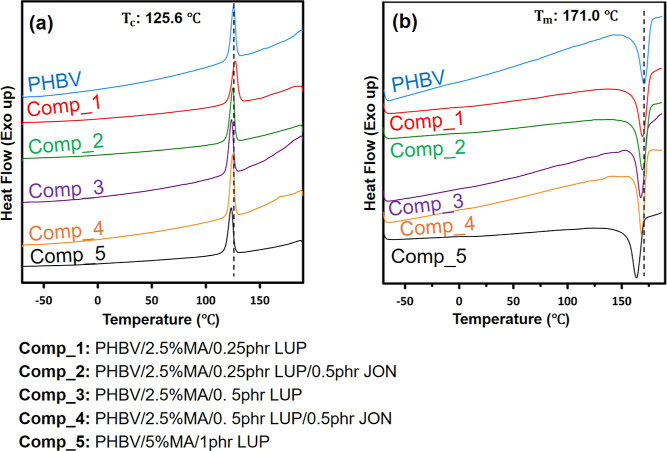
DSC curves of PHBV and MA-g-PHBV samples showing thermal properties in the first cooling and second heating cycles are presented in Figure 6, and DSC data analysis is listed in Table 2. Because of high crystallinity of PHBV, the glass-transition temperature for PHBV and all MA-g-PHBV samples was not observed, which was also reported in the previous literature.44 A single melting temperature and crystallization temperature were observed for neat PHBV at 171.0 and 125.6 °C, which coincides with the previous literature.45
Figure 6.
DSC thermograms of PHBV and MA-g-PHBV samples; (a) first cooling cycle and (b) second heating cycle showing the changes in thermal properties of PHBV with addition of different concentrations of MA and initiator in the presence or absence of JON. The dotted lines were added in the figures to compare the melting and crystallization temperatures of maleated PHBV. The melting temperature of maleated PHBV decreased with an increase in the MA and LUP concentration. The % crystallinity of the MA-g-PHBV samples decreased with an increase in MA and LUP concentrations except for Comp_5 where % crystallinity increased.
Table 2. Thermal Properties of PHBV and MA-g-PHBVa.
| Sample | Tm (°C) | Tc (°C) | ΔHm (J/g) | % Xc (%) | T2% (°C) | Tmax (°C) |
|---|---|---|---|---|---|---|
| PHBV | 171.0 | 125.6 | 94.00 | 86.23 | 272.1 | 300.8 |
| Comp_1 | 169.4 | 127.1 | 86.71 | 81.59 | 255.7 | 303.7 |
| Comp_2 | 169.2 | 125.0 | 78.12 | 73.50 | 258.4 | 301.4 |
| Comp_3 | 167.7 | 123.7 | 74.76 | 70.34 | 234.2 | 300.7 |
| Comp_4 | 168.4 | 124.6 | 73.05 | 68.73 | 240.8 | 300.2 |
| Comp_5 | 163.6 | 123.4 | 77.63 | 74.96 | 210.8 | 299.9 |
Comp_1: PHBV/2.5%MA/0.25 phr LUP, Comp_2: PHBV/2.5%MA/0.25 phr LUP/0.5 phr JON, Comp_3: PHBV/2.5%MA/0.5 phr LUP, Comp_4: PHBV/2.5%MA/0.5 phr LUP/0.5 phr JON, and Comp_5: PHBV/5%MA/1 phr LUP; Tm: melting temperature, Tc: crystallization temperature, ΔHm: enthalpy of fusion, % Xc: percentage crystallinity, T2%: temperature at which mass loss of 2% takes place, Tmax: temperature at which maximum degradation takes place.
As seen in Table 2, the melting temperature of PHBV decreased by increasing the content of the initiator and MA. The reduction in melting temperature of PHBV after grafting by MA in the presence of an initiator has been reported in the literature.34 Also, by increasing the content of initiator and MA, a decrease in % crystallinity can be noticed compared to neat PHBV. Grafting of MA bulky groups onto the PHBV backbone reduces the polymer chain regularity and hinders the growth of crystals, and as the grafting percentage increases in the presence of higher MA and initiator, a lower degree of crystallinity can be expected. Such a trend in the crystallinity of grafted biopolymers was also reported by other researchers.4,21 However, in the case of Comp_5, with a higher percentage of grafting, we observed an increase in the degree of crystallinity. It can be suggested that the low-molecular-weight polymer chains in this sample may contribute to the formation of more crystals, which resulted in the higher crystallinity of this composition as compared to other grafted samples.
Increasing the initiator amount resulted in a reduction of crystallization temperature when comparing Comp_1 with Comp_3 and Comp_2 with Comp_4. The same trend was observed for Comp_5 with increasing initiator and MA content. The introduction of an initiator can induce the formation of a polymer network and restrict the crystallization process, which was also reported in the previous literature.46 The addition of JON, however, did not show a considerable change in the crystallization temperature comparing Comp_1 with Comp_2 and Comp_3 with Comp_4. Here, the role of the JON as the chain extender might have been limited to a minor contribution in balancing or counteracting thermal degradation caused by the addition of an initiator. Consequently, this could result in polymer chains with similar properties to those of PHBV with no significant variations in chain packing. A similar observation was reported for the effect of adding a chain extender to PHAs in the literature.47
3.6.2. Thermogravimetric Analysis
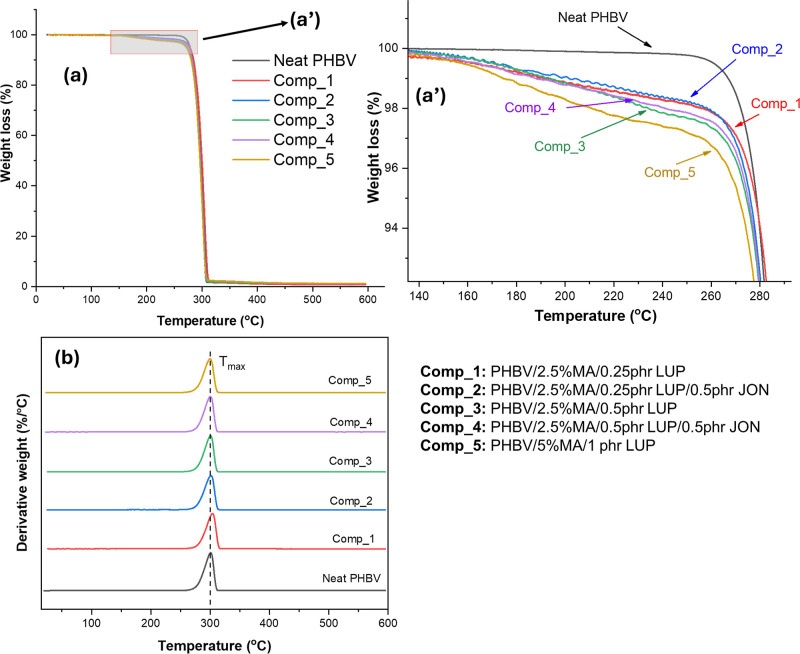
Figure 7a,b explains the maleation process’s effect on the thermal stability of PHBV. As can be seen in Figure 7a, neat PHBV and all maleated PHBV materials demonstrate very similar behavior showing a single major mass loss step initiating at a temperature close to 300 °C. The maximum thermal degradation temperature (Tmax), representing the thermal degradation temperature of the polymer, is very similar in all samples as shown in the DTG curve [Figure 7b]. Such a trend explains the good thermal stability of PHBV during reactive extrusion. However, when comparing the mass loss of the samples at a temperature lower than 300 °C, we can notice a difference between neat PHBV and maleated PHBV. As can be seen in Figure 7a′, more mass loss occurred at this region for grafted samples as compared to neat PHBV, and this mass loss was more significant in Comp_5 containing the highest content of initiator and MA.
Figure 7.
Thermal stability trend in PHBV and maleated PHBV; (a) mass loss behavior of different samples; (a′) focused area of mass loss curve; (b) derivative weight graph of PHBV and maleated PHBV (the dotted lines were added to compare the Tmax of maleated PHBV). The T2% of the maleated PHBV was enhanced after the inclusion of JON compared to its counterpart without JON. The highest T2% value was observed for Comp_2, while Comp_5 was the lowest. As such, JON helped enhance the thermal stability of the maleated PHBV.
Table 2 also shows the onset temperature (T2%) of the samples. The onset temperature decreased after grafting with MA. The increase in the content of the initiator clearly contributed to the lower onset temperature, with the minimum value in Comp_5. Higher mass loss in grafted samples can be attributed to the fact that polymer chain scission can happen in the presence of an initiator during melt processing, which results in the formation of low-molecular-weight species with lower thermal stability. Such a trend after grafting a biopolymer with MA during reactive extrusion was also reported by other researchers.9,48 Adding JON enhanced the thermal stability of the samples, which is also reflected in the onset degradation temperature of the compatibilizers. The onset degradation temperature (T2%) of Comp_2 and Comp_4 (both containing the chain extender) is 3 and 6 °C higher compared to their counterparts without the chain extender (Comp_1 and Comp_3). This data is also presented in Table 2. It is hypothesized that macromolecular chain extension took place after JON addition by its reaction with the polymer chain ends, thereby forming a branched structure ultimately contributing to an increased molecular weight.16 The effect of JON as a chain extender on the thermal stability of PHBV has been studied in the work by Nuchanong et al.49 who reported a nominal improvement in thermal stability after the addition of JON.
4. Conclusions
MA-grafted bioplastics have shown great promise in enhancing the performance of bioplastic-based materials by enhancing the interfacial adhesion between matrix–matrix and matrix–filler. Hence, MA-g-PHBV can similarly be utilized to enhance the properties of PHBV-based composites and blends for various applications by improving their miscibility. The grafting of MA onto PHBV chains was performed by a reactive extrusion process using organic peroxide as an initiator. The effect of different concentrations of MA and initiator in the presence and absence of a chain extender was investigated. The successful grafting of MA onto the PHBV backbone was confirmed using FTIR and a chemical titration process. The results showed that the initiator and MA content played a major part in the grafting reaction, where the MA grafting increased as the initiator and MA content increased. Also, the presence of a chain extender further improved the % grafting of MA because of the probable formation of branched chains of maleated PHBV chains. Moreover, at higher initiator concentrations, the rheological properties decreased, which suggests that chain scission of PHBV might have occurred during processing. The addition of a chain extender acted as a remedy to this behavior, where the viscosity improved, suggesting that the increase in molecular weight of the grafted materials was facilitated by branching. This infers that adding a chain extender can mitigate the issue of chain scission while improving the % grafting of MA-g-PHBV. Furthermore, the thermal properties of the maleated PHBV samples were studied by TGA and DSC. The maleated PHBV exhibited lower crystallinity, melting, and crystallization temperature compared to neat PHBV. This suggests that the MA grafting disrupts the regularity of PHBV chains and considerably hampers the crystallization process. As expected, the onset of thermal degradation temperature (T2%) of maleated PHBV was much lower than that of neat PHBV due to the formation of low-molecular-weight PHBV species during processing. This was, however, slightly improved by a maximum of ∼6 °C after the incorporation of the chain extender, which suggests macromolecular chain extension leading to improved thermal stability. From the analysis, we can conclude that adding a chain extender could successfully enhance the thermal stability, rheological properties, and % grafting of the maleated PHBV, probably by the phenomenon of macromolecular chain extension, which is a common action of chain extenders. Hence, maleated PHBV can be of significant interest to both industry and researchers as it promotes improved miscibility between PHBV resins and organic fillers. Maleated PHBV with a higher percentage of grafting can also be used to form PHBV-based blends and composites having competitive performance to market-available products.
Acknowledgments
The authors would like to thank the financial support of (i) the Ontario Agri-Food Innovation Alliance—Bioeconomy for Industrial Uses Research Program (Project nos. 030648, 030671, and 030699); the Gryphon’s Leading to the Accelerated Adoption of Innovative Research (LAAIR) Program (Project No. 030736); and the Ontario Agri-Food Research Initiative (Project No. 056442); (ii) the Ontario Ministry of Colleges and Universities (MCU), Ontario, Canada Research Fund—Research Excellency, ORF-RE-11 (Project no. 056106); (iii) the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chair (CRC) program project no. 460788; and the NSERC Discovery grants project no. 401716; and (iv) NSERC Alliance Grants Program (Project no. 401769) along with the partner industry Competitive Green Technologies, Leamington, Ontario, Canada (Project no. 055427) to carry out this research.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available to prevent data misuse.
Author Contributions
Debarshi Nath: methodology, investigation, formal analysis, data curation, and writing—original draft. Ehsan Pesaranhajiabbas: methodology, investigation, formal analysis, data curation, and writing—original draft. Fatemeh Jahangiri: methodology, investigation, formal analysis, data curation, and writing—original draft. Aarsha Surendren: methodology, investigation, formal analysis, data curation, and writing—original draft. Akhilesh K. Pal: methodology, investigation, formal analysis, data curation, and writing—original draft. Arturo Rodriquez-Uribe: methodology, investigation, formal analysis, data curation, and writing—original draft. Manjusri Misra: conceptualization, investigation, validation, resources, writing—review and editing, funding acquisition, project administration, and supervision. Amar K. Mohanty: conceptualization, investigation, resources, validation, funding acquisition, supervision, and writing—review and editing. All authors contributed to the discussion, reviews, and approval of the manuscript for publication.
The authors declare no competing financial interest.
References
- Semple K. E.; Zhou C.; Rojas O. J.; Nkeuwa W. N.; Dai C. Moulded Pulp Fibers for Disposable Food Packaging: A State-of-the-Art Review. Food Packag. Shelf Life 2022, 33, 100908. 10.1016/j.fpsl.2022.100908. [DOI] [Google Scholar]
- Zhao X.; Ji K.; Kurt K.; Cornish K.; Vodovotz Y. Optimal Mechanical Properties of Biodegradable Natural Rubber-Toughened PHBV Bioplastics Intended for Food Packaging Applications. Food Packag. Shelf Life 2019, 21, 100348. 10.1016/j.fpsl.2019.100348. [DOI] [Google Scholar]
- Pal A. K.; Wu F.; Misra M.; Mohanty A. K. Reactive Extrusion of Sustainable PHBV/PBAT-Based Nanocomposite Films with Organically Modified Nanoclay for Packaging Applications: Compression Moulding vs. Cast Film Extrusion. Composites, Part B 2020, 198, 108141. 10.1016/j.compositesb.2020.108141. [DOI] [Google Scholar]
- Chen C.; Peng S.; Fei B.; Zhuang Y.; Dong L.; Feng Z.; Chen S.; Xia H. Synthesis and Characterization of Maleated Poly(3-Hydroxybutyrate). J. Appl. Polym. Sci. 2003, 88 (3), 659–668. 10.1002/app.11771. [DOI] [Google Scholar]
- Bossu J.; Le Moigne N.; Dieudonné-George P.; Dumazert L.; Guillard V.; Angellier-Coussy H. Impact of the Processing Temperature on the Crystallization Behavior and Mechanical Properties of Poly[R-3-Hydroxybutyrate-Co-(R-3-Hydroxyvalerate)]. Polymer 2021, 229, 123987. 10.1016/j.polymer.2021.123987. [DOI] [Google Scholar]
- Berthet M. A.; Angellier-Coussy H.; Chea V.; Guillard V.; Gastaldi E.; Gontard N. Sustainable Food Packaging: Valorising Wheat Straw Fibres for Tuning PHBV-Based Composites Properties. Composites, Part A 2015, 72, 139–147. 10.1016/j.compositesa.2015.02.006. [DOI] [Google Scholar]
- Zhang M.; Colby R. H.; Milner S. T.; Chung T. C. M.; Huang T.; Degroot W. Synthesis and Characterization of Maleic Anhydride Grafted Polypropylene with a Well-Defined Molecular Structure. Macromolecules 2013, 46 (11), 4313–4323. 10.1021/ma4006632. [DOI] [Google Scholar]
- Calderón B. A.; Soule J.; Sobkowicz M. J. Synthesis and Characterization of Compatibilizers for Blends of Polypropylene Carbonate and Polybutylene Succinate via Free-Radical Grafting of Maleic Anhydride. J. Appl. Polym. Sci. 2019, 136 (21), 47553. 10.1002/app.47553. [DOI] [Google Scholar]
- Carlson D.; Nie L.; Narayan R.; Dubois P. Maleation of Polylactide (PLA) by Reactive Extrusion. J. Appl. Polym. Sci. 1999, 72 (4), 477–485. . [DOI] [Google Scholar]
- Bettini S. H. P.; Agnelli J. A. M. Grafting of Maleic Anhydride onto Polypropylene by Reactive Extrusion. J. Appl. Polym. Sci. 2002, 85 (13), 2706–2717. 10.1002/app.10705. [DOI] [Google Scholar]
- Przybysz-Romatowska M.; Haponiuk J.; Formela K. Reactive Extrusion of Biodegradable Aliphatic Polyesters in the Presence of Free-Radical-Initiators: A Review. Polym. Degrad. Stab. 2020, 182, 109383. 10.1016/j.polymdegradstab.2020.109383. [DOI] [Google Scholar]
- Feijoo P.; Mohanty A. K.; Rodriguez-Uribe A.; Gámez-Pérez J.; Cabedo L.; Misra M. Biodegradable Blends from Bacterial Biopolyester PHBV and Bio-Based PBSA: Study of the Effect of Chain Extender on the Thermal, Mechanical and Morphological Properties. Int. J. Biol. Macromol. 2023, 225, 1291–1305. 10.1016/j.ijbiomac.2022.11.188. [DOI] [PubMed] [Google Scholar]
- Corre Y. M.; Duchet J.; Reignier J.; Maazouz A. Melt Strengthening of Poly (Lactic Acid) through Reactive Extrusion with Epoxy-Functionalized Chains. Rheol. Acta 2011, 50 (7–8), 613–629. 10.1007/s00397-011-0538-1. [DOI] [Google Scholar]
- Standau T.; Nofar M.; Dörr D.; Ruckdäschel H.; Altstädt V. A Review on Multifunctional Epoxy-Based Joncryl® ADR Chain Extended Thermoplastics. Polym. Rev. 2021, 62 (2), 296–350. 10.1080/15583724.2021.1918710. [DOI] [Google Scholar]
- Najafi N.; Heuzey M. C.; Carreau P. J.; Wood-Adams P. M. Control of Thermal Degradation of Polylactide (PLA)-Clay Nanocomposites Using Chain Extenders. Polym. Degrad. Stab. 2012, 97 (4), 554–565. 10.1016/j.polymdegradstab.2012.01.016. [DOI] [Google Scholar]
- Duangphet S.; Szegda D.; Song J.; Tarverdi K. The Effect of Chain Extender on Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate): Thermal Degradation, Crystallization, and Rheological Behaviours. J. Polym. Environ. 2014, 22 (1), 1–8. 10.1007/s10924-012-0568-5. [DOI] [Google Scholar]
- Pesaranhajiabbas E.; Mohanty A. K.; Al-Abdul-Wahid M. S.; Misra M. A New Approach for Grafting Plasticized Cellulose Acetate Biodegradable Plastic with Maleic Anhydride: Processing and Characterization. SPE Polymers 2024, 5 (2), 217–227. 10.1002/pls2.10121. [DOI] [Google Scholar]
- Luperox® 101 for Polypropylene - Arkema | Arkema Global. https://www.arkema.com/global/en/products/product-finder/product/organicperoxide/luperox/luperox101/#otherDocAnchor (accessed Jan 26, 2024).
- De Roover B.; Sclavons M.; Carlier V.; Devaux J.; Legras R.; Momtaz A. Molecular Characterization of Maleic Anhydride-Functionalized Polypropylene. J. Polym. Sci., Part A: Polym. Chem. 1995, 33 (5), 829–842. 10.1002/pola.1995.080330509. [DOI] [Google Scholar]
- Root K. P.; Pal A. K.; Pesaranhajiabbas E.; Mohanty A. K.; Misra M. Injection Moulded Composites from High Biomass Filled Biodegradable Plastic: Properties and Performance Evaluation for Single-Use Applications. Compos., Part C: Open Access 2023, 11, 100358. 10.1016/j.jcomc.2023.100358. [DOI] [Google Scholar]
- Muthuraj R.; Misra M.; Mohanty A. K. Injection Molded Sustainable Biocomposites from Poly(Butylene Succinate) Bioplastic and Perennial Grass. ACS Sustain. Chem. Eng. 2015, 3 (11), 2767–2776. 10.1021/acssuschemeng.5b00646. [DOI] [Google Scholar]
- Le Delliou B.; Vitrac O.; Benihya A.; Dole P.; Domenek S. Film-Blown Blends of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) by Compatibilization with Poly(Butylene-Co-Succinate-Co-Adipate) with a Free Radical Initiator. Polym. Test. 2023, 124, 108072. 10.1016/j.polymertesting.2023.108072. [DOI] [Google Scholar]
- Nath D.; Pal A. K.; Misra M.; Mohanty A. K. Biodegradable Blown Film Composites from Bioplastic and Talc: Effect of Uniaxial Stretching on Mechanical and Barrier Properties. Macromol. Mater. Eng. 2023, 308, 2300214. 10.1002/mame.202300214. [DOI] [Google Scholar]
- Meereboer K. W.; Pal A. K.; Misra M.; Mohanty A. K. Sustainable PHBV/Cellulose Acetate Blends: Effect of a Chain Extender and a Plasticizer. ACS Omega 2020, 5 (24), 14221–14231. 10.1021/ACSOMEGA.9B03369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Uribe A.; Wang T.; Pal A. K.; Wu F.; Mohanty A. K.; Misra M. Injection Moldable Hybrid Sustainable Composites of BioPBS and PHBV Reinforced with Talc and Starch as Potential Alternatives to Single-Use Plastic Packaging. Compos., Part C: Open Access 2021, 6, 100201. 10.1016/j.jcomc.2021.100201. [DOI] [Google Scholar]
- Liu Q. S.; Zhu M. F.; Wu W. H.; Qin Z. Y. Reducing the Formation of Six-Membered Ring Ester during Thermal Degradation of Biodegradable PHBV to Enhance Its Thermal Stability. Polym. Degrad. Stab. 2009, 94 (1), 18–24. 10.1016/j.polymdegradstab.2008.10.016. [DOI] [Google Scholar]
- Carlson D.; Dubois P.; Nie L.; Narayan R. Free Radical Branching of Polylactide by Reactive Extrusion. Polym. Eng. Sci. 1998, 38 (2), 311–321. 10.1002/pen.10192. [DOI] [Google Scholar]
- Yahyaee N.; Javadi A.; Garmabi H.; Khaki A. Effect of Two-Step Chain Extension Using Joncryl and PMDA on the Rheological Properties of Poly (Lactic Acid). Macromol. Mater. Eng. 2020, 305 (2), 1900423. 10.1002/mame.201900423. [DOI] [Google Scholar]
- Bikiaris D. N.; Karayannidis G. P. Chain Extension of Polyesters PET and PBT with N,N′-bis (Glycidyl Ester) Pyromellitimides. I. J. Polym. Sci., Part A: Polym. Chem. 1995, 33 (10), 1705–1714. 10.1002/pola.1995.080331017. [DOI] [Google Scholar]
- Takamura M.; Nakamura T.; Takahashi T.; Koyama K. Effect of Type of Peroxide on Cross-Linking of Poly(l-Lactide). Polymer Degradation and Stability 2008, 93 (10), 1909–1916. 10.1016/j.polymdegradstab.2008.07.001. [DOI] [Google Scholar]
- Mohanty A. K.; Misra M.; Wu F.. Biodegradable Nanostructured Composites. US Patent 11,279,823 B2, March 22, 2022. www.sabic-ip.com.
- Pal A. K.; Misra M.; Mohanty A. K. Silane Treated Starch Dispersed PBAT/PHBV-Based Composites: Improved Barrier Performance for Single-Use Plastic Alternatives. Int. J. Biol. Macromol. 2023, 229, 1009–1022. 10.1016/j.ijbiomac.2022.12.141. [DOI] [PubMed] [Google Scholar]
- Hamour N.; Boukerrou A.; Djidjelli H.; Beaugrand J. In Situ Grafting Effect of a Coupling Agent on Different Properties of a Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Olive Husk Flour Composite. Polym. Bull. 2019, 76 (12), 6275–6290. 10.1007/s00289-019-02725-y. [DOI] [Google Scholar]
- Montanheiro T. L. D. A.; Passador F. R.; Oliveira M. P. d.; Duran N.; Lemes A. P. Preparation and Characterization of Maleic Anhydride Grafted Poly(Hydroxybutirate-CO-Hydroxyvalerate) - PHBV-g-MA. Mater. Res. 2016, 19 (1), 229–235. 10.1590/1980-5373-MR-2015-0496. [DOI] [Google Scholar]
- Detyothin S.; Selke S. E. M.; Narayan R.; Rubino M.; Auras R. Reactive Functionalization of Poly(Lactic Acid), PLA: Effects of the Reactive Modifier, Initiator and Processing Conditions on the Final Grafted Maleic Anhydride Content and Molecular Weight of PLA. Polym. Degrad. Stab. 2013, 98 (12), 2697–2708. 10.1016/j.polymdegradstab.2013.10.001. [DOI] [Google Scholar]
- Du J.; Wang Y.; Xie X.; Xu M.; Song Y. Styrene-Assisted Maleic Anhydride Grafted Poly(Lactic Acid) as an Effective Compatibilizer for Wood Flour/Poly(Lactic Acid) Bio-Composites. Polymers 2017, 9 (11), 623. 10.3390/polym9110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.; Zhang Y.; Hu H.; Li J.; Zhou W.; Zhao X.; Peng S. Effect of Maleic Anhydride Grafted Poly(Lactic Acid) on Rheological Behaviors and Mechanical Performance of Poly(Lactic Acid)/Poly(Ethylene Glycol) (PLA/PEG) Blends. RSC Adv. 2022, 12 (49), 31629–31638. 10.1039/D2RA03513H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linghu C.; Xie L.; Yang L.; Li X.; Tao Y.; Xu Y.; Luo Z. Preparation and Characterization of Maleic Anhydride-Based Double-Monomer Grafted Polylactic Acid Compatibilizer. J. Appl. Polym. Sci. 2022, 139 (22), 52234. 10.1002/app.52234. [DOI] [Google Scholar]
- Makwakwa D.; Ojijo V.; Bandyopadhyay J.; Ray S. S. Flow Characteristics, Mechanical, Thermal, and Thermomechanical Properties, and 3d Printability of Biodegradable Polylactide Containing Boehmite at Different Loadings. Polymers 2021, 13 (12), 2019. 10.3390/polym13122019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Huang A.; Fan J.; Xu R.; Liu P.; Li G.; Yang S. Effect of Blending Procedures and Reactive Compatibilizers on the Properties of Biodegradable Poly(Butylene Adipate-Co-Terephthalate)/Poly(Lactic Acid) Blends. J. Polym. Eng. 2021, 41 (2), 95–108. 10.1515/polyeng-2020-0161. [DOI] [Google Scholar]
- Wang Y.; Fu C.; Luo Y.; Ruan C.; Zhang Y.; Fu Y. Melt Synthesis and Characterization of Poly(L-Lactic Acid) Chain Linked by Multifunctional Epoxy Compound. J. Wuhan Univ. Technol., Mater. Sci. Ed. 2010, 25 (5), 774–779. 10.1007/s11595-010-0090-3. [DOI] [Google Scholar]
- Ugartemendia J. M.; Muñoz M. E.; Sarasua J. R.; Santamaria A. Phase Behavior and Effects of Microstructure on Viscoelastic Properties of a Series of Polylactides and Polylactide/Poly(ε-Caprolactone) Copolymers. Rheol. Acta 2014, 53 (10–11), 857–868. 10.1007/s00397-014-0797-8. [DOI] [Google Scholar]
- Krishnamoorti R.; Banik I.; Xu L. Rheology and Processing of Polymer Nanocomposites. Rev. Chem. Eng. 2010, 26 (1–2), 3–12. 10.1515/REVCE.2010.003. [DOI] [Google Scholar]
- Tomano N.; Boondamnoen O.; Aumnate C.; Potiyaraj P. Enhancing Impact Resistance and Biodegradability of PHBV by Melt Blending with ENR. Sci. Rep. 2022, 12 (1), 22633. 10.1038/s41598-022-27246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zytner P.; Pal A. K.; Wu F.; Rodriguez-Uribe A.; Mohanty A. K.; Misra M. Morphology and Performance Relationship Studies on Poly(3-Hydroxybutyrate- Co-3-Hydroxyvalerate)/Poly(Butylene Adipate- Co-Terephthalate)-Based Biodegradable Blends. ACS Omega 2023, 8, 1946–1956. 10.1021/acsomega.2c04770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Cornish K.; Vodovotz Y. Synergistic Mechanisms Underlie the Peroxide and Coagent Improvement of Natural-Rubber-Toughened Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Mechanical Performance. Polymers 2019, 11 (3), 565. 10.3390/polym11030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura H.; Laguna-Gutiérrez E.; Rodriguez-Perez M. A.; Ardanuy M. Effect of Chain Extender and Water-Quenching on the Properties of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Foams for Its Production by Extrusion Foaming. Eur. Polym. J. 2016, 85, 14–25. 10.1016/j.eurpolymj.2016.10.001. [DOI] [Google Scholar]
- Muthuraj R.; Misra M.; Mohanty A. K. Biodegradable Biocomposites from Poly(Butylene Adipate-Co-Terephthalate) and Miscanthus: Preparation, Compatibilization, and Performance Evaluation. J. Appl. Polym. Sci. 2017, 134 (43), 45448. 10.1002/app.45448. [DOI] [Google Scholar]
- Nuchanong P.; Seadan M.; Khankrua R.; Suttiruengwong S. Thermal Stability Enhancement of Poly(Hydroxybutyrate-Co-Hydroxyvalerate) through in Situ Reaction. Des. Monomers Polym. 2021, 24 (1), 113–124. 10.1080/15685551.2021.1914406. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available to prevent data misuse.