Abstract
Propranolol is a heavily prescribed, nonspecific beta-adrenoceptor (bAR) antagonist frequently found in wastewater effluents, prompting concern over its potential to adversely affect exposed organisms. In the present study, the transcriptional responses of 4, 5, and 6 days postfertilization (dpf) ±1 h fathead minnow, exposed for 6, 24, or 48 h to 0.66 or 3.3 mg/L (nominal) propranolol were characterized using RNA sequencing. The number of differentially expressed genes (DEGs) was used as an estimate of sensitivity. A trend toward increased sensitivity with age was observed; fish >7 dpf at the end of exposure were particularly sensitive to propranolol. The DEGs largely overlapped among treatment groups, suggesting a highly consistent response that was independent of age. Cluster analysis was performed using normalized count data for unexposed and propranolol-exposed fish. Control fish clustered tightly by age, with fish ≥7 dpf clustering away from younger fish, reflecting developmental differences. When clustering was conducted using exposed fish, in cases where propranolol induced a minimal or no transcriptional response, the results mirrored those of the control fish and did not appreciably cluster by treatment. In treatment groups that displayed a more robust transcriptional response, the effects of propranolol were evident; however, fish <7 dpf clustered away from older fish, despite having similar numbers of DEGs. Increased sensitivity at 7 dpf coincided with developmental milestones with the potential to alter propranolol pharmacokinetics or pharmacodynamics, such as the onset of exogenous feeding and gill functionality as well as increased systemic expression of bAR. These results may have broader implications because toxicity testing often utilizes fish <4 dpf, prior to the onset of these potentially important developmental milestones, which may result in an underestimation of risk for some chemicals.
Keywords: Aquatic toxicology, Contaminants, Developmental toxicity, Transcriptomics, Propranolol, Fathead minnow, Early development
INTRODUCTION
Wastewater effluents are a major source of pharmaceutical contamination in aquatic ecosystems and surface waters (Batt et al., 2016; Carpenter & Helbling, 2018; Glassmeyer et al., 2005; Zhi, 2020). Pharmaceuticals are designed to be bioactive at low concentrations and, because they often target physiological systems that are broadly conserved across taxa, have the potential to affect aquatic organisms. Though some pharmaceutical use varies seasonally, such as allergy medications, the presence of chronically prescribed pharmaceuticals is expected to be more consistent (Kot-Wasik et al., 2016), suggesting that these pharmaceutical classes may be of particular concern. Anti-hypertensives are among the most commonly prescribed drug classes (Fuentes et al., 2018) and may pose a particular ecological risk (Donnachie et al., 2016). As a group, anti-hypertensives include a diverse set of modes of action and molecular targets, including diuretics, beta-adrenergic blocking agents, Ca2+ channel blocking agents, angiotensin II receptor antagonists, and angiotensin-converting enzyme inhibitors (Laurent, 2017).
Propranolol, a nonselective beta-adrenoceptor (bAR) antagonist, is among the most commonly prescribed drugs in the United States (Kane, 2022) and is frequently found in surface waters and wastewater effluent (Sumpter et al., 2021). Adrenoceptors are G-coupled protein receptors that are responsive to the catecholamines epinephrine and norepinephrine. Adrenoceptors are divided into two major classes, a and b, which are further divided into subtypes that differ in their tissue distribution and ligand affinities. In humans and mammalian models, propranolol binds both b1 and b2-adrenoceptors (b1AR and b2AR) with similar affinity and b3-adrenoceptors (b3AR) with a lower affinity (Daly & McGrath, 2011).
The bARs are relatively well conserved across vertebrate species; however, as a result of a whole-genome duplication, teleosts have multiple copies of b2AR and b3AR (Zavala et al., 2017). Orthologs of the bARs have been identified in a number of fish species, including fathead minnow (Pimephales promelas; FHM) and zebrafish (Danio rerio). The sequences of FHM b1AR, b2bAR, and b3AR are highly similar to those of zebrafish (88%, 86%, and 74%, respectively) but less so to human orthologs, 51%, 55%, and 40%, respectively (Fabbri & Moon, 2016). Propranolol has been shown to bind bAR in several fish species and to affect cardiovascular endpoints consistent with their pharmacological mechanisms (Finn et al., 2012).
Despite the conservation of the bARs across taxa, direct read-across from mammalian effects to fish is complicated by physiological differences, as well as differences in the number of bAR subtypes and their tissue distribution. Though the onset of bAR expression occurs at <12 h postfertilization (hpf; Steele et al., 2011), they differ in the developmental timing of their maximal expression as well as their tissue distributions, which suggests that the timing of adrenergic disruption relative to development may be a determinant of sensitivity. Over the last decade, there has been an increased interest in the use of gene expression as an alternative to organism-level endpoints typically used in toxicity testing for chemical risk assessment (Thomas et al., 2013; Villeneuve et al., 2023). The present study expands on this by using the magnitude of the transcriptional response as a measure of sensitivity with the aim of characterizing differences in sensitivity and response at different age intervals of the free-swimming larval stage. We subsequently aim to identify developmentally related explanatory factors for observed differences in sensitivity across selected age classes by characterizing the gene expression response of 4, 5, or 6 days postfertilization (dpf) larval FHM exposed for 6, 24, or 48 h to two different nominal concentrations of propranolol (0.66 and 3.3 mg/L).
MATERIALS AND METHODS
Exposure organisms
Larval FHM were obtained from the on-site culture at the US Environmental Protection Agency’s Andrew W. Breidenbach Environmental Research Center in Cincinnati, Ohio, as described (US Environmental Protection Agency, 2002). All experiments were conducted in accordance with the approved animal care and use protocol. Eggs of FHM were collected within 1 h of placing spawning tiles in breeding tanks. Eggs and larvae were maintained in an incubator at 25 °C in dechlorinated tap water supplemented with CaCO3 to a hardness of 180 mg/L.
Exposures
Nominal concentrations were determined based on a range-finder study, with the high concentration being the highest concentration resulting in no observable mortality and the low concentration being 20% of the high. Experimental design was based on three factors: (1) larval age class at the onset of exposure (4, 5, or 6 dpf ± 1 h), (2) duration of exposure (6, 24, or 48 h), and (3) concentration (control, low [0.66 mg propranolol/L], or high [3.3 mg propranolol/L]). All concentrations were nominal, and exposures were conducted in moderately hard reconstituted water. Each combination of age class and concentration consisted of five replicate exposure vessels, each containing 12 FHM larvae. Exposures were conducted in 150-ml beakers containing 130 ml exposure solution, resulting in a loading rate of 1 mg/10 ml (0.1 g/L) at 25 °C under a 16:8–h light:dark cycle. Mortality was assessed at 6, 24, and 48 h; and dead larvae were removed. No significant mortality was observed in any treatment. At each of the three exposure durations, three larvae were randomly selected from each exposure vessel, while the remaining larvae in each exposure vessel were used for subsequent time points. Larvae were placed into separate 1.5-ml microcentrifuge tubes containing a 3.2-mm stainless steel bead and snap-frozen in liquid nitrogen for RNA-sequencing (RNA-seq) analysis. Two of these were used for subsequent analysis, while the third served as an alternative sample in case of RNA degradation or another technical reason for exclusion. For the 6-dpf age group, two of the remaining larvae from the high exposures for each duration were analyzed for differential small noncoding (snc) RNA expression (n = 8 per duration). Samples were subsequently stored at −80 °C. As a result, n = 10 for each combination of age, duration, and concentration.
RNA isolation, library preparation, and sequencing
RNA isolation.
The MagMAX-96 Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA) was used to extract RNA from larvae following the manufacturer’s protocol. Samples were homogenized in lysis/binding buffer using a Bullet Blender Storm 24 homogenizer (Next Advance). A Synergy HTX Multi-Mode Microplate Reader with a Take3 Micro-Volume Plate (Biotek) was used to quantify RNA spectrophotometrically. The quality of RNA was assessed using a 4200 TapeStation (Agilent).
Gene expression profiling.
Each treatment group contained an equal number of samples from each replicate exposure experiment. Libraries were prepared from 250 ng RNA using the SENSE mRNA [messenger RNA]–Seq Library Prep Kit V2 (Lexogen) according to the supplied protocol. The concentration of each library was determined using the Qubit dsDNA HS Assay with a Qubit 2.0 Fluorometer (Thermo Fisher Scientific). Equimolar amounts of libraries from 16 samples were pooled and loaded onto one lane of an Illumina HiSeq 4000 flow cell and sequenced in the 1 × 50 bp single read format using HiSeq 4000 SBS reagents (Illumina). Base calling was done by Illumina Real Time Analysis (RTA, Ver 2.7.7), and output of RTA was demultiplexed and converted to FastQ format with Bcl2fastq ([2017] Ver 2.19.1, Illumina, San Diego, CA, USA).
sncRNA sequencing.
Total RNA (1000 ng) was used as input for library preparation using the TruSeq Small RNA Library preparation kit (Illumina) following the manufacturer’s protocol. Sequencing was performed as described above with the exception that these samples were split evenly into two pools for sequencing in two different lanes. Treatments were split across sequencing lanes to avoid batch effects.
mRNA mapping
The 50-base-long reads in the RNA-seq fastq(.gz) file for each sample were mapped to indexed transcripts from each of the 47,578 gene models (which include isoforms) from the newly updated FHM genome (WIOS00000000; BioSample SAMN12875914; Martinson et al., 2022) using the STAR alignment software (Ver 2.7.1a; Dobin et al., 2013). Following STAR mapping, sam output was filtered to only preserve primary mappings in the reverse strand and only keeping target_id and any XS or AS tags. A feature X sample matrix was generated from filtered sam files and a target fasta file. Count matrices were filtered so that only those features for which at least three samples had at least 10 counts were included. All analyses were based on the reverse strand count matrix. An initial quality control assessment was done at this stage involving generation of density plots, a box plot, and multidimensional scaling (MDS) plots for each toxicant/control combination. Density and box plots showed no unusual count distributions, but MDS plots revealed four outlier samples, which were removed from analysis of the 6-dpf group. Outlier identification and removal were based on visual inspection of principal components analysis plots and an informal fourfold leading fold change threshold. The MDS plots were generated via limma’s plotMDS function, which calculates distance between each pair of samples as the root mean square of the 1000 largest log2-fold gene expression changes. Sequencing data have been uploaded to the National Center for Biotechnology Information’s Gene Expression Omnibus database (GSE197442) and are freely available.
Differential expression analysis and feature selection
Differential expression analysis was performed using the R limma package (Ver 3.40.6; Ritchie et al., 2015). Between-sample normalization was conducted using the trimmed mean of M values method (Robinson & Oshlack, 2010) implemented by the calcNormFactors function from the R edgeR package (Ver 3.26.8; Robinson et al., 2010). Precision weights (Law et al., 2014) were calculated using the voom function from the limma package. The limma lmFit function was used to fit a generalized linear model with a term for chemical treatment effects and a term for experimental batches. Moderated t statistics (Phipson et al., 2016) were calculated for chemical treatment effects using the limma eBayes function. The limma topTable function was used to sort features (i.e., genes) based on their nominal p values. The Benjamini-Hochberg procedure was used to correct raw p values for multiple testing (Hochberg & Benjamini, 1990). Genes whose false discovery rate (FDR)–adjusted p values were <0.05 were annotated using GCA_016745375.1 (Martinson et al., 2022). Volcano plots of differentially expressed genes (DEGs) were generated using the EnhancedVolcano R package (Blighe et al., 2023).
Enrichment analyses
Ingenuity Pathway Analysis enrichment analysis.
Differentially expressed genes (FDR < 0.05) were mapped to human orthologs and uploaded into Ingenuity Pathway Analysis (IPA; Ver 81348237). The Core Analysis module was used to determine enriched canonical pathways, upstream analysis, and diseases and functions. Significantly enriched canonical pathways were exported into R for cluster analysis. Cluster analysis was conducted using the pvclust R package (Suzuki & Shimodaira, 2006) on the enrichment p values for the complete lists of all significantly enriched pathways identified in IPA. Clustering parameters were as follows: complete linkage, correlation as the distance measure, and 1000 bootstraps. For graphing purposes, for each treatment group, enriched canonical pathways were sorted by the –log (p value). The 25 most significant pathways were selected for display. Heatmaps were made using the complexHeatmap R package (Gu et al., 2016).
Gene ontology enrichment.
Enrichment of gene ontology (GO) terms (cellular compartment [CC], molecular function [MF], and biological process [BP]) was conducted on human orthologs of FHM DEGs using the gprofiler2 R package (Kolberg et al., 2020).
Semantic similarity analysis was applied to GO enrichment results to aid in interpretability using the simplifyEnrichment R package (Gu & Hubschmann, 2022). For each GO category, treatment groups with fewer than five significant terms were removed from analysis. Clustering of CC, BP, and MF GO terms was done using kmeans.
MicroRNA identification
MicroRNAs (miRNAs) of FHM were identified previously using the command-line version of miRDeep*, MDS_command_line_V37 (An et al., 2013) as described in Martinson et al. (2022). Using the miRDeep* log-odds probability score for being a genuine miRNA precursor, 620 miRNAs resulted after applying a score filter of 100 (only two of these received “no hits” when compared to the miRBase.org mature miRNA database).
Correspondence of DEG lists among treatment groups
Statistically significant overlap of gene sets among treatment conditions was determined using the SuperExactTest R package (Wang et al., 2015). The background set of unique genes used to determine enrichment was 26,150, which corresponds to the total number of unique, nonpsuedogene gene models in the FHM genome assembly (Martinson et al., 2022).
RESULTS AND DISCUSSION
Propranolol is among the top 100 most commonly prescribed drugs (Kane, 2022) and is commonly found in surface waters. This, coupled with its ability to affect a broad and diverse array of tissues and tissue functions, has prompted concern over its potential to adversely affect aquatic organisms. Though a recent risk assessment has suggested that propranolol poses little direct risk when considering apical endpoints of regulatory concern (Sumpter et al., 2021), this assessment did not account for the potential of coexposure of similarly acting compounds, a not unlikely scenario given the estimation that 8% of pharmaceuticals found in aquatic environments are beta-blockers (Santos et al., 2010). Exposures during early life stages may be of particular concern because of the potential to alter normal development. Though many studies have evaluated propranolol’s effects in early development, the relative sensitivity of different developmental windows has not been fully considered. In the present study, we evaluated the effects of propranolol over different exposure durations and ages at the onset and conclusion of exposure, using the magnitude of the transcriptomic response as a proxy for sensitivity. We further compared the nature of the transcriptomic response among durations and age classes in an attempt to identify putative factors explaining differences in sensitivity as well as impacted tissues and cellular functions.
Concentration and duration of exposure
Though having confirmational chemistry of the concentrations would be preferred, the absence of measured values should not meaningfully affect the conclusions, given that the goal was to characterize the transcriptomic responses of FHM to two well-separated sublethal concentrations of propranolol, not to benchmark the responses to precise exposure concentrations.
Very few to no DEGs were identified in any of the low-concentration exposures (Table 1), suggesting that this concentration was at or below the bioactive levels of propranolol for the tested exposure durations. The single exception to this was the 6dpf_24hr_Low treatment group; however, several factors suggest that gene expression response observed in the 6dpf_24hr_Low treatment group was not driven by the propranolol exposure (discussed below). For this reason, this treatment group will be largely excluded from discussion; however, the full list of DEGs can be found in Supporting Information, S1.
TABLE 1:
Number of differentially expressed genes across age classes and concentrations
| Low (0.66 mg/L) | High (3.3 mg/L) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 6 h | 24 h | 48 h | 6h | 24 h | 48 h | |
| 4 dpf | 0 | 0 | 0 | 0 | 0 | 190 |
| 5 dpf | 0 | 10 | 0 | 0 | 15 | 252 |
| 6 dpf | 0 | 1099 | 0 | 0 | 113 | 706 |
dpf = days postfertilization.
The magnitude of expression within age classes increased with increasing duration of exposure. No DEGs were observed in any of the 6-h exposure durations, few were seen at 24 h, and maximal expression was observed after 48 h in the high concentration (Table 1). Though fewer genes were found to be differentially expressed after 24 h, the majority of those identified were also found at the 48-h exposure durations for both the 5- and 6-dpf age classes (Figure 1). This was most obvious for the 6-dpf age class exposed to the high concentration, where 70% (113 DEGs 6dpf_24 hr_High/706 DEGs 6dpf_48 hr_High) of the DEGs identified at 24 h overlapped with those found at 48 h (Figure 1). The most significant of all comparisons was the overlap of the 6dpf_24 hr_High, the only 24-h exposure group with a reasonably large gene expression response, with the 5dpf_48 hr_High and 6dpf_48hr_High (47 DEGs; Figure 1). Among the 48-h exposure groups, 38 genes overlapped across all three age classes. In pairwise comparisons, both the 4- and 5-dpf age classes overlapped to a greater degree with the 6-dpf age class (88/190 and 123/252, respectively) than they did with each other (53 overlapping genes; Figure 1), suggesting a qualitative difference in their transcriptional response. It is notable that overlaps with the 6dpf_24 hr_Low treatment group were generally the least significant and that only a small fraction (maximum of 51) of the 1099 DEGs identified in that group overlapped with any other group. With that exception, the high degree of overlap among treatment groups underscores the biological relevance of the identified DEGs to the propranolol response.
FIGURE 1:
Results of Fisher’s super exact test (SET). The SET was used to determine whether the overlap of differentially expressed genes (DEGs) among treatment groups was statistically significant. The SET calculates the significance based on the likelihood of DEG lists of a given size overlapping to this extent given a fixed population size (i.e., total number of genes in the complete gene list). In this case, 26,150 was the total number of unique gene models identified in the fathead minnow genome assembly. The height of the bars and the numbers on top of the bars indicate the number of genes overlapping. The color of the bars indicates the –log10 of the p value. Green circles indicate which treatment groups are being compared. The numbers at the end of each row indicate the number of DEGs identified in that treatment group. dpf = days postfertilization.
The consistency of the gene expression across treatment groups was also evident in enrichment analysis. Enrichment analysis using both IPA, which is built around mammalian model organisms and relies on a large proprietary database of annotations, and gprofiler2 was conducted on treatment groups with >100 DEGs (five treatment groups in total). Again, with the exception of 6dpf_24hr_Low, results overlapped across treatments. In IPA, all four groups had similar top-ranked enriched pathways (Figure 2). The coordinated lysosomal expression and regulation (CLEAR) signaling pathway was the most significant in all but the 4dpf_48hr_High exposure group, where it was the fourth most significant enriched pathway. In addition to CLEAR enrichment, several other pathways with relevance to lysosomal regulation, such as autophagy and phagosome maturation, were among the most significantly enriched pathways. Lysosome- and vesicularly related terms were also identified in enrichment analysis based on CC GO terms (Figure 3, Clusters 3, 5, and 7). For the 6dpf_24hr_Low treatment group, few terms related to lysosomes or vesicles were identified as enriched in either IPA or GO enrichment analysis. The 6dpf_24hr_Low group displayed a strikingly different pattern of enrichment, where it was singularly enriched in several clusters (Figure 3, Clusters 1, 6, 12, and 14).
FIGURE 2:
Comparison analysis of enriched canonical pathways. Enrichment analysis was conducted using Ingenuity Pathway Analysis for each treatment group. Clustering was done using all significantly enriched pathways. For graphing purposes, nonsignificant pathways for a given treatment group were assigned a value of 0.5; only the top 25 pathways are displayed. CLEAR = coordinated lysosomal expression and regulation; TR/RXR = thyroid hormone receptor/retinoid X receptor; NRF1 = nuclear respiratory factor 1; MAPK = mitogen-activated protein kinase; LPS/IL-1 = lipopolysaccharide/interleukin 1; LXR = liver X receptor; PXR = pregnane X receptor; FXR = farnesoid X receptor; dpf = days postfertilization.
FIGURE 3:
Semantic similarity analysis of enriched cellular compartment gene ontology (GO) terms: GO enrichment of differentially expressed genes from each treatment was conducted and subjected to semantic similarity analysis to identify key themes. Coloring represents the –log10 p value. Cluster indices are indicated by the numbering on the left of the heatmap. Term font size indicates relative prevalence within each cluster. Sim = similarity; CC = cellular compartment; atpase = adenosine triphosphatase; atp = adenosine triphosphate; abc = atp binding cassette; dpf = days postfertilization.
The likely cause of duration-related effects may be that propranolol fails to reach effective plasma concentrations until 24 or 48 h. The main route of exposure in fish is absorption across the gills and direct transport to the tissues (Owen et al., 2007). Because of its low octanol–water partition coefficient and relatively small size, propranolol is expected to move through the gill epithelium through both passive and active mechanisms (Stott et al., 2015), where approximately 90% of it would partition into the plasma (Henneberger et al., 2020; Owen et al., 2007). Based on an in vitro liver spheroid model derived from trout, approximately 40% of propranolol would be expected to be metabolized within 24 h (t½ = 39.4 h; Baron et al., 2017). In juvenile rainbow trout exposed to approximately 1 mg/L of propranolol, plasma concentrations reached approximately 20% and 56% of the measured water concentration at 25 and 98 h, respectively (Bartram et al., 2012). In the present study, internal concentrations of propranolol were not measured; however, assuming similar pharmacokinetics, this would suggest plasma concentrations of 0.66 and 0.132 μg/L for the high and low exposure groups at the 24-h time point, respectively, and a maximum of 1.8 and 0.37 μg/L at the 48-h time point. Stanley et al. (2006) reported a 7-day growth lowest-observed effect concentration of 128.2 μg/L, which would equate to plasma concentrations of 25.6 and 71.8 μg/L at 25 and 96 h, respectively—well below the predicted plasma concentrations in the present study.
Age-dependent sensitivity
The present study focused on posthatch developmental time points; however, previous studies have observed propranolol effects in zebrafish which are consistent with its documented effects on cardiovascular and neurological function in humans at much earlier developmental stages (Fraysse et al., 2006; Li et al., 2022; Mitchell & Moon, 2016). Though this demonstrates that propranolol is bioactive throughout early development, it does not address the potential for propranolol sensitivity to change across development, which has the potential to affect risk estimates.
Within each exposure duration, age appeared to be a determinant in the magnitude of the expression response, with very limited transcriptional response prior to 6 dpf and a much greater response at 7 dpf, suggesting an interaction between age and sensitivity (Table 1). Though age and duration are at least partially confounded, comparisons across age classes exposed for the same duration support the influence of age. This was most noticeable in the 6-dpf age class (Table 1), which was the only age class with fish 7 dpf at the 24-h exposure time point (Table 2). Significantly more DEGs were identified relative to the younger age classes at both exposure durations (Table 1). At the 24-h exposure time point, 113 DEGs were identified in the 6-dpf age class, compared to none and 15 for the 4- and 5-dpf treatment groups, respectively (Table 1). A similar trend was observed in the 48-h exposure groups, where again a much larger response was observed for the 6-dpf age class (Table 1) relative to either the 4- or 5-dpf age class. The magnitude of the DEG response was similar between the 4- and 5-dpf age classes (Table 1).
TABLE 2:
Age classes at the beginning and end of exposures
| Age at end of exposure | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Age at start of exposure | 4 dpf | 5 dpf | 6 dpf | 7 dpf | 8 dpf | |
| 4 dpf | 6 h | 24 h | 48 h | ← Duration | ||
| 4dpf_6hr | 4dpf_24hr | 4dpf_48hr | ← Group ID | |||
| 5 dpf | 6 h | 24 h | 48 h | ← Duration | ||
| 5dpf_6hr | 5dpf_24hr | 5dpf_48hr | ← Group ID | |||
| 6 dpf | 6 h | 24 h | 48 h | ← Duration | ||
| 6dpf_6hr | 6dpf_24hr | 6dpf_48hr | ← Group ID | |||
dpf = days postfertilization.
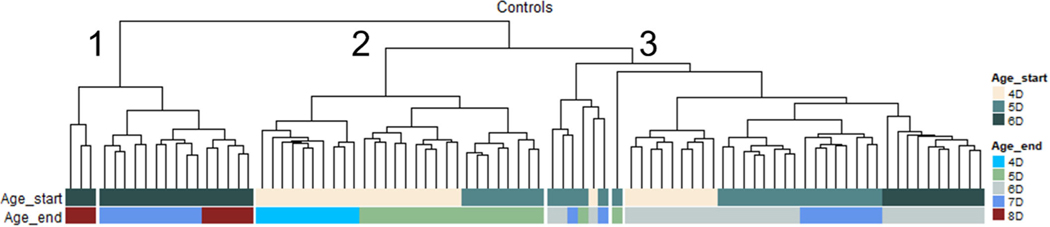
Developmental differences among age classes potentially explain the relationship between sensitivity and organism age. To evaluate this, cluster analysis was performed on control fish to determine whether cluster structure in the absence of propranolol reflected sensitivity (i.e., less sensitive age classes would cluster together). This approach assumes that, in the absence of propranolol, gene expression would largely reflect developmental processes. The combinations of age class at the onset and the three different exposure durations resulted in five age classes included in the analysis (Table 2), ranging from 4 (4 dpf + 6-h exposure) to 8 dpf (6 dpf + 48-h exposure), where all but the youngest and oldest age classes were composed of organisms from separate egg collections. Control samples clustered into three distinct branches by age class: ≥7 dpf, 6 dpf, and <6 dpf (Figure 4; Clusters 1, 3, and 2). The ≥7-dpf cluster was comprised of the age classes that responded to propranolol to the greatest degree, 6dpf_48hr, 6dpf_24 hr, and 5dpf_48 hr.
FIGURE 4:
Hierarchical clustering of control samples across age classes. Top row coloring indicates age class at the start of the exposure, and the bottom row is the age at the end of the exposure. D = days.
Though the magnitude of the transcriptional responses of the 4dpf_48hr_High and 5dpf_48hr_High groups was similar, the 4dpf_48hr control fish clustered with other 6-dpf samples, suggesting that these two age classes differed developmentally. To evaluate this, cluster analysis was performed on propranolol-treated samples (Figure 5). Independent of the propranolol exposure, the influence of age was clearly apparent across age classes in both the 24- and 48-h exposure durations. For the 24-h exposures, where propranolol effects on transcription were minimal, this was much more pronounced, where the major branching structure exactly mirrored what was observed in the clustering of control fish. Not surprisingly, propranolol had a relatively minor effect on clustering, but its influence did appear to increase with fish age. In the 4-dpf age class (Cluster 2), control and propranolol-treated samples were largely intermixed, whereas some clustering of the high-propranolol treatment could be seen in the 6-dpf age class (Cluster 1) and to a lesser extent the 5-dpf age class (Cluster 3).
FIGURE 5:
Hierarchical cluster analysis of samples exposed for either 24 h (left) or 48 h (right) to low or high concentrations of propranolol or controls. Coloring of the top row indicates treatment level, and bottom row indicates age class at the start of the exposure. TRT = treatment; C = control; H = high; L = low.
At 48 h, the effect of propranolol was much more evident across all three age classes (Figure 5, right panel). Tight clusters were observed for all three age classes exposed to the high propranolol concentration. However, despite similar numbers of DEGs in the 4- and 5-dpf age classes, both of which overlapped to a similar extent with the 6-dpf age class (Figure 1), the 4-dpf age class clustered distinctly away from older age classes (Figure 4, Cluster 1 vs. 2 and 3). This may indicate that 6 dpf may coincide with a developmental milestone relevant to the propranolol response. An alternative explanation for this observation is that clustering simply reflects a higher degree of developmental similarity between the 5- and 6-dpf age classes. However, in cases where propranolol effects are minimal (Figure 5, left panel) or nonexistent (Figure 4), the 4- and 5-dpf age classes consistently cluster together and away from the 6-dpf age class. This further supports the influence of an age-related determinant of propranolol sensitivity.
The mechanism underlying age-related sensitivity differences is unclear; however, it may suggest a developmental milestone that affects either the pharmacokinetics (PK) or pharmacodynamics (PD) of propranolol. One possibility is that age-related differences in sensitivity are due to the developmental timing and tissue distribution of bAR expression. The timing of bAR expression during development in FHM has not been characterized; however, in zebrafish, initial expression of b1AR and b2AR occurs very early in development (<12 hpf). For bARa1, expression rapidly increases 200-fold by 12 hpf and reaches levels statistically significantly higher than the initial expression (1 hpf) at 6 and 8 dpf (Steele et al., 2011), and is maximally expressed by 10 dpf (Wang et al., 2009). Though less drastic, b2AR exhibits similar trends to both b2AR1 and b2AR2, increasing significantly above early expression levels by 8 dpf (Steele et al., 2011) and reaching maximal expression in adulthood (Wang et al., 2009). The coincident timing of increased bAR expression levels and increased sensitivity suggests that bAR expression may contribute to age-related differences in sensitivity.
Another potential explanation is that increased propranolol sensitivity is due to an anatomical structure either becoming functional or reaching a level of maturity where it serves as a new target for propranolol. The FHM age classes evaluated in the present study largely correspond to distinct developmental stages identified in zebrafish, which are defined by the appearance of specific anatomical features. For zebrafish, anatomical features have been annotated with the earliest and latest development stages where they have been observed, providing a means to identify structures coincident with increased sensitivity. The posthatch stages identified in zebrafish differ in resolution across age classes; 4, 5, and 6 dpf are each distinct stages, but 7 to 13 dpf are binned into a single stage. Only 14 anatomical features begin development at 6 dpf, six of which are components of the optic tectum, a structure in the teleost brain involved in coordinating motor responses to visual sensory information received from the retinal ganglion cells of the eye that requires a spatial map, for example, prey movement (Nevin et al., 2010). In the 7 to 13 dpf group, 99 terms were identified, 66% of which were related to the development of the skeletal system, while the remaining terms were involved in the development of the mature mesonephros, which does not begin development until after the observed increase in sensitivity (10–12 dpf). Though bARs populate both the optic tectum and skeletal system (Ampatzis & Dermon, 2010; Huang et al., 2009) and propranolol is known to affect bone remodeling (Folwarczna et al., 2011; Minkowitz et al., 1991; Rodrigues et al., 2012), it is unlikely that either is driving the gene expression response. Results from both IPA and GO analysis (Figures 2 and 5) were relatively consistent across treatment and age groups, again with the exception of the 6dpf_24hr_Low treatment group; and no enrichment of bone or visual functions was observed. This suggests that age-related effects are not due to altered PD resulting from propranolol interactions with anatomical targets that arise starting at 7 dpf.
The large degree of overlap of DEGs among age classes indicates that age-dependent factors alter sensitivity without affecting the nature of the expression response, as would be expected if a new propranolol target had reached a critical developmental stage. This suggests that the observed increase in sensitivity is related to changes in the PK of propranolol. Similar increases in sensitivity during this same developmental period have been previously reported in zebrafish exposed to diphenhydramine and diazinon (Kristofco et al., 2015, 2016), which was also found to coincide with increased uptake for diphenhydramine (Kristofco et al., 2018). Increases in sensitivity among the three different unrelated chemicals during this developmental window suggest that a generalized mechanism is affecting PK. This period of increased sensitivity coincides with important milestones in gill and gastrointestinal development, both of which are important in chemical uptake. Propranolol uptake has been shown to occur, at least in part, through the gills by both passive and active means (Stott et al., 2015). Though gills do not become the primary route of gas exchange until approximately 2 weeks postfertilization, their role in ionoregulation begins at approximately 7 dpf (Rombough, 2002), suggesting a link between the onset of gill function and increased sensitivity. Similarly, this period coincides with onset of gastrointestinal function because the FHM larvae transition from endogenous to exogenous feeding at approximately 7.5 dpf (Devlin et al., 1996). Interpreting the connection between increased functionality and sensitivity is somewhat confounded by the possibility that the absence of exogenous sources of nutrition during the study may have acted as a second stressor. This is somewhat supported by the enrichment of IPA terms associated with autophagy (Figure 2), which would be expected as energy stores become depleted (Levine & Klionsky, 2004). However, autophagy-related terms were also enriched in the 4- and 5-dpf age classes exposed for 48 h, which would be prior to the transition to exogenous feeding. In addition, propranolol is known to affect autophagy in a number of different model systems of disease (Lorusso et al., 2022; Zhao et al., 2020), which is thought to be a potential mode of action by which propranolol promotes the involution of IH (Lorusso et al., 2022) and reduces tumor growth (Zhao et al., 2020). Together, the facts that this period of development has been shown to coincide with increased sensitivity in previous studies, that autophagy-related terms are enriched prior to the depletion of the yolk sac, and that propranolol has been shown to regulate autophagy argue against the likelihood of that increased sensitivity observed in the present study are due to the energy status of larvae.
Anomalous transcriptional response of the 6dpf_24hr_Low exposure group
Transcriptional results across treatment groups were highly consistent, displaying a high degree and statistically significant overlap of DEGs among treatments. This consistency was likewise observed in enrichment analyses. Further, there was a clear trend toward a more robust expression response with increased concentrations, longer exposures, and older ages. The single exception to this was the 6dpf_24hr_Low treatment group, which had the greatest number of DEGs among all treatment groups. The DEGs from this group overlapped minimally with any other treatment group, including the 6dpf_24hr_High group, which is the most direct comparator (Figure 1). Given the consistency of the transcriptional response of treatment groups with adjacent exposure conditions (same concentration but different age, same age but different duration, different starting age but the same exposure conditions, etc.), it seems highly unlikely that the transcriptional response of this group results from the propranolol exposure. No obvious technical explanatory factor has been identified. Fish in the 6dpf_24hr_Low group were from the same brood and shared the same exposure vessels as other fish from the 6-dpf age class. The same exposure solutions were used in other groups, the replicate exposure beakers were blocked positionally during the exposure, and RNA isolation and library preparations were conducted concurrently with other treatment groups. Sequencing depth and quality were also found to be comparable to other groups (data not shown). Qualitatively, the nature of the 6dpf_24hr_Low group appeared somewhat different from the other treatments (Figure 6). The magnitude of both the level of expression as well as the significance of DEGs from the 6dpf_24hr_Low group was muted relative to other treatment groups. Despite having a much larger number of DEGs, the number of DEGs that changed in expression >log2-fold was less than or equal to all but the 4dpf_48hr_High group, which had one less gene changing of this magnitude but 909 fewer DEGs overall. Though no obvious explanatory factor could be identified, we cannot conceive of a biological explanation for this result; and its divergence from all other treatment groups, coupled with qualitative differences in the expression profile, makes it suspect.
FIGURE 6:
Volcano plots of the 5dfp_48hr_High group and all members of the 6dpf age class with significant gene expression results. Volcano plots of differentially expressed genes were generated using the EnhancedVolcano R package. dpf = days postfertilization; FC = fold change.
Analysis of miRNA.
Early developmental stages may be at heightened risk from chemical effects because of the potential for disruption of normal epigenetic regulation, which may permanently alter the normal developmental trajectory of exposed organisms. Small non-coding RNAs are one of the three recognized epigenetic mechanisms, along with histone modification and DNA methylation (Brander et al., 2017). Small RNAs generally reduce protein levels of target genes through direct interference with transcription, through translation, or by targeting nascent mRNA for degradation. In mammalian models, the differential expression of miRNAs has been implicated in the positive therapeutic effects of propranolol on the cardiovascular system and in the treatment of IH (Li et al., 2017; Lu et al., 2009). Comparatively fewer studies have been published evaluating sncRNA expression in response to environmental toxicants in early life stage fish; however, existing evidence suggests that miRNA expression is affected (Ji et al., 2019; Zhao et al., 2017). To determine if propranolol exposure affects the transcriptional levels of sncRNA, small-RNA-seq analyses were conducted on samples from the 6dpf_48hr_Low and 6dpf_48hr_High groups. The RNA reads were mapped to the 620 FHM miRNAs described in Martinson et al. (2022). No differentially expressed miRNA genes were detected at an FDR of 0.05, suggesting that propranolol does not alter sncRNA transcriptional levels. This result mirrors that of another one of our recent FHM larval studies (Toth et al., 2021), where small-RNA-seq analyses showed no significantly affected miRNA following ethinylestradiol exposure. Given that these are the only two studies to evaluate sncRNA expression in FHM, it is possible that negative results reflect the immaturity of the FHM miRNAome or may result from the use of whole organisms and the averaging of tissue-specific miRNA levels across tissues.
CONCLUSIONS
Using the number of DEGs as an indicator of sensitivity to propranolol, the present study demonstrates a positive relationship among the age at onset and the completion of propranolol exposure, exposure duration, and sensitivity. Although the relationship between exposure duration and sensitivity is not surprising, the distinct age-related differences in the magnitude of response observed at ≥7 dpf were somewhat unexpected. We have identified several putative mechanistic explanations for these differences that would affect either propranolol PD (developmental timing and tissue distribution of bAR expression) or PK (developmental onset of gill and gastrointestinal function) that coincide with the sharp increase in sensitivity. No obvious explanation was identified for the divergent expression observed in the 6dpf_24hr_Low treatment group; however, DEGs were highly consistent among all other treatment groups, as were age- and concentration- related trends. The divergent nature of the 6dpf_24hr_Low treatment group is further underscored by the fact that each age class was compared with its own set of controls and can be considered somewhat independent. The consistency in gene expression across age classes suggests two things: (1) targets of propranolol remain consistent across developmental stages and exposure durations, and (2) expression results are biologically relevant to the propranolol response. The identification of windows of increased sensitivity corresponding to later developmental stages may have implications for chemical risk assessment because early life stages (<3 dpf) are being increasingly utilized to reduce the reliance on the use of organisms for chemical testing.
Supplementary Material
Acknowledgments—
The authors declare no conflicts of interest. The present study was directly funded by the US Environmental Protection Agency’s Office of Research and Development.
Footnotes
Supporting Information—The Supporting Information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5814.
Disclaimer—The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the US Environmental Protection Agency.
Data Availability Statement—
Sequencing data have been uploaded to the National Center for Biotechnology Information’s Gene Expression Omnibus database (GSE197442) and are freely available.
REFERENCES
- Ampatzis K, & Dermon CR (2010). Regional distribution and cellular localization of beta2-adrenoceptors in the adult zebrafish brain (Danio rerio). Journal of Comparative Neurology, 518, 1418–1441. 10.1002/cne.22278 [DOI] [PubMed] [Google Scholar]
- An J, Lai J, Lehman ML, & Nelson CC (2013). miRDeep*: an integrated application tool for miRNA identification from RNA sequencing data. Nucleic Acids Research, 41, 727–737. 10.1093/nar/gks1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron MG, Mintram KS, Owen SF, Hetheridge MJ, Moody AJ, Purcell WM, Jackson SK, & Jha AN (2017). Pharmaceutical metabolism in fish: Using a 3-D hepatic in vitro model to assess clearance. PLOS ONE, 12, Article e0168837. 10.1371/journal.pone.0168837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram AE, Winter MJ, Huggett DB, McCormack P, Constantine LA, Hetheridge MJ, Hutchinson TH, Kinter LB, Ericson JF, Sumpter JP, & Owen SF (2012). In vivo and in vitro liver and gill EROD activity in rainbow trout (Oncorhynchus mykiss) exposed to the beta-blocker propranolol. Environmental Toxicology, 27, 573–582. 10.1002/tox.20684 [DOI] [PubMed] [Google Scholar]
- Batt AL, Kincaid TM, Kostich MS, Lazorchak JM, & Olsen AR (2016). Evaluating the extent of pharmaceuticals in surface waters of the United States using a national-scale rivers and streams assessment survey. Environmental Toxicology and Chemistry, 35, 874–881. 10.1002/etc.3161 [DOI] [PubMed] [Google Scholar]
- Bcl2fastq (Version 2.19.1) [Computer software]. (2017). Illumina. [Google Scholar]
- Blighe K, Rana S, & Lewis M. (2023). EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. Bioconductor. https://bioconductor.org/packages/EnhancedVolcano [Google Scholar]
- Brander SM, Biales AD, & Connon RE (2017). The role of epigenomics in aquatic toxicology. Environmental Toxicology and Chemistry, 36, 2565–2573. 10.1002/etc.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CMG, & Helbling DE (2018). Widespread micropollutant monitoring in the hudson river estuary reveals spatiotemporal micropollutant clusters and their sources. Environmental Science & Technology, 52, 6187–6196. 10.1021/acs.est.8b00945 [DOI] [PubMed] [Google Scholar]
- Daly CJ, & McGrath JC (2011). Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends in Pharmacological Sciences, 32, 219–226. 10.1016/j.tips.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Devlin E, Brammer J, Puyear R, & McKim J. (1996). Prehatching development of the fathead minnow Pimephales promelas Rafinesque. US Environmental Protection Agency. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, & Gingeras TR (2013). STAR: Ultrafast universal RNA-seq aligner. Bioinformatics, 29, 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnachie RL, Johnson AC, & Sumpter JP (2016). A rational approach to selecting and ranking some pharmaceuticals of concern for the aquatic environment and their relative importance compared with other chemicals. Environmental Toxicology and Chemistry, 35, 1021–1027. 10.1002/etc.3165 [DOI] [PubMed] [Google Scholar]
- Fabbri E, & Moon TW (2016). Adrenergic signaling in teleost fish liver, a challenging path. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 199, 74–86. 10.1016/j.cbpb.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Finn J, Hui M, Li V, Lorenzi V, de la Paz N, Cheng SH, Lai-Chan L, & Schlenk D. (2012). Effects of propranolol on heart rate and development in Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquatic Toxicology, 122–123, 214–221. 10.1016/j.aquatox.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Folwarczna J, Pytlik M, Sliwinski L, Cegiela U, Nowinska B, & Rajda M. (2011). Effects of propranolol on the development of glucocorticoid-induced osteoporosis in male rats. Pharmacological Reports, 63, 1040–1049. 10.1016/s1734-1140(11)70620-x [DOI] [PubMed] [Google Scholar]
- Fraysse B, Mons R, & Garric J. (2006). Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicology and Environmental Safety, 63, 253–267. 10.1016/j.ecoenv.2004.10.015 [DOI] [PubMed] [Google Scholar]
- Fuentes AV, Pineda MD, & Venkata KCN (2018). Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy, 6, Article 43. 10.3390/pharmacy6020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassmeyer ST, Furlong ET, Kolpin DW, Cahill JD, Zaugg SD, Werner SL, Meyer MT, & Kryak DD (2005). Transport of chemical and microbial compounds from known wastewater discharges: Potential for use as indicators of human fecal contamination. Environmental Science & Technology, 39, 5157–5169. 10.1021/es048120k [DOI] [PubMed] [Google Scholar]
- Gu Z, Eils R, & Schlesner M. (2016). Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics, 32, 2847–2849. 10.1093/bioinformatics/btw313 [DOI] [PubMed] [Google Scholar]
- Gu Z, & Hubschmann D. (2022). simplifyEnrichment: A Bioconductor package for clustering and visualizing functional enrichment results. Genomics, Proteomics & Bioinformatics/Beijing Genomics Institute, 21, 190–202. 10.1016/j.gpb.2022.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger L, Kluver N, Muhlenbrink M, & Escher B. (2020). Trout and human plasma protein binding of selected pharmaceuticals informs the fish plasma model. Environmental Toxicology and Chemistry, 41, 559–568. 10.1002/etc.4934 [DOI] [PubMed] [Google Scholar]
- Hochberg Y, & Benjamini Y. (1990). More powerful procedures for multiple significance testing. Statics in Medicine, 9, 811–818. 10.1002/sim.4780090710 [DOI] [PubMed] [Google Scholar]
- Huang HH, Brennan TC, Muir MM, & Mason RS (2009). Functional α1- and β2-adrenergic receptors in human osteoblasts. Journal of Cellular Physiology, 220, 267–275. 10.1002/jcp.21761 [DOI] [PubMed] [Google Scholar]
- Ji C, Guo X, Ren J, Zu Y, Li W, & Zhang Q. (2019). Transcriptomic analysis of microRNAs–mRNAs regulating innate immune response of zebrafish larvae against Vibrio parahaemolyticus infection. Fish and Shellfish Immunology, 91, 333–342. 10.1016/j.fsi.2019.05.050 [DOI] [PubMed] [Google Scholar]
- Kane SP (2022). The top 200 of 2020: ClinCalc DrugStats Database (Version 2022.08). https://clincalc.com/DrugStats/Top200Drugs.aspx
- Kolberg L, Raudvere U, Kuzmin I, Vilo J, & Peterson H. (2020). gprofiler2—An R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Research, 9, ELIXIR-709. 10.12688/f1000research.24956.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot-Wasik A, Jakimska A, & Sliwka-Kaszynska M. (2016). Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants. Environmental Monitoring and Assessment, 188, Article 661. 10.1007/s10661-016-5637-0 [DOI] [PubMed] [Google Scholar]
- Kristofco LA, Cruz LC, Haddad SP, Behra ML, Chambliss CK, & Brooks BW (2016). Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquatic Toxicology, 170, 344–354. 10.1016/j.aquatox.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristofco LA, Du B, Chambliss CK, Berninger JP, & Brooks BW (2015). Comparative pharmacology and toxicology of pharmaceuticals in the environment: Diphenhydramine protection of diazinon toxicity in Danio rerio but not Daphnia magna. AAPS Journal, 17, 175–183. 10.1208/s12248-014-9677-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristofco LA, Haddad SP, Chambliss CK, & Brooks BW (2018). Differential uptake of and sensitivity to diphenhydramine in embryonic and larval zebrafish. Environmental Toxicology and Chemistry, 37, 1175–1181. 10.1002/etc.4068 [DOI] [PubMed] [Google Scholar]
- Laurent S. (2017). Antihypertensive drugs. Pharmacological Research, 124, 116–125. 10.1016/j.phrs.2017.07.026 [DOI] [PubMed] [Google Scholar]
- Levine B, & Klionsky DJ (2004). Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell, 6, 463–477. 10.1016/s1534-5807(04)00099-1 [DOI] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, & Smyth GK (2014). voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biology, 15, R29. 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li P, Guo Z, Wang H, & Pan W. (2017). Downregulation of miR- 382 by propranolol inhibits the progression of infantile hemangioma via the PTEN-mediated AKT/mTOR pathway. International Journal of Molecular Medicine, 39, 757–763. 10.3892/ijmm.2017.2863 [DOI] [PubMed] [Google Scholar]
- Li X, Liao X, Chen C, Zhang L, Sun S, Wan M, Liu J, Huang L, Yang D, Hu H, Ma X, Zhong Z, Liu F, Xiong G, Lu H, Chen J, & Cao Z. (2022). Propranolol hydrochloride induces neurodevelopmental toxicity and locomotor disorders in zebrafish larvae. Neurotoxicology, 93, 337–347. 10.1016/j.neuro.2022.10.016 [DOI] [PubMed] [Google Scholar]
- Lorusso B, Cerasoli G, Falco A, Frati C, Graiani G, Madeddu D, Nogara A, Corradini E, Roti G, Cerretani E, Gherli A, Caputi M, Gnetti L, Pilato FP, Quaini F, & Lagrasta C. (2022). Β-blockers activate autophagy on infantile hemangioma–derived endothelial cells in vitro. Vascular pharmacology, 146, Article 107110. 10.1016/j.vph.2022.107110 [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Shan H, Pan Z, Li X, Li B, Xu C, Zhang B, Zhang F, Dong D, Song W, Qiao G, & Yang B. (2009). MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: A new mechanism for ischaemic cardioprotection. Cardiovascular Research, 84, 434–441. 10.1093/cvr/cvp232 [DOI] [PubMed] [Google Scholar]
- Martinson JW, Bencic DC, Toth GP, Kostich MS, Flick RW, See MJ, Lattier D, Biales AD, & Huang W. (2022). De novo assembly of the nearly complete fathead minnow reference genome reveals a repetitive but compact genome. Environmental Toxicology and Chemistry, 41, 448–461. 10.1002/etc.5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkowitz B, Boskey AL, Lane JM, Pearlman HS, & Vigorita VJ (1991). Effects of propranolol on bone metabolism in the rat. Journal of Orthopaedic Research, 9, 869–875. 10.1002/jor.1100090613 [DOI] [PubMed] [Google Scholar]
- Mitchell KM, & Moon TW (2016). Behavioral and biochemical adjustments of the zebrafish Danio rerio exposed to the beta-blocker propranolol. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 199, 105–114. 10.1016/j.cbpb.2015.10.009 [DOI] [PubMed] [Google Scholar]
- Nevin LM, Robles E, Baier H, & Scott EK (2010). Focusing on optic tectum circuitry through the lens of genetics. BMC Biology, 8, Article 126. 10.1186/1741-7007-8-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Giltrow E, Huggett DB, Hutchinson TH, Saye J, Winter MJ, & Sumpter JP (2007). Comparative physiology, pharmacology and toxicology of beta-blockers: Mammals versus fish. Aquatic Toxicology, 82, 145–162. 10.1016/j.aquatox.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Phipson B, Lee S, Majewski IJ, Alexander WS, & Smyth GK (2016). Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. The Annals of Applied Statistics, 10, 946–963. 10.1214/16-AOAS920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, & Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research, 43, e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, & Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26, 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, & Oshlack A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology, 11, R25. 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues WF, Madeira MF, da Silva TA, Clemente-Napimoga JT, Miguel CB, Dias-da-Silva VJ, Barbosa-Neto O, Lopes AH, & Napimoga MH (2012). Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. British Journal of Pharmacology, 165, 2140–2151. 10.1111/j.1476-5381.2011.01686.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombough P. (2002). Gills are needed for ionoregulation before they are needed for O2 uptake in developing zebrafish, Danio rerio. Journal of Experimental Biology, 205, 1787–1794. 10.1242/jeb.205.12.1787 [DOI] [PubMed] [Google Scholar]
- Santos LH, Araujo AN, Fachini A, Pena A, Delerue-Matos C, & Montenegro MC (2010). Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. Journal of Hazardous Materials, 175, 45–95. 10.1016/j.jhazmat.2009.10.100 [DOI] [PubMed] [Google Scholar]
- Stanley JK, Ramirez AJ, Mottaleb M, Chambliss CK, & Brooks BW (2006). Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magna and Pimephales promelas. Environmental Toxicology and Chemistry, 25, 1780–1786. 10.1897/05-298r1.1 [DOI] [PubMed] [Google Scholar]
- Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, Tiberi M, & Perry SF (2011). In vivo and in vitro assessment of cardiac β-adrenergic receptors in larval zebrafish (Danio rerio). Journal of Experimental Biology, 214, 1445–1457. 10.1242/jeb.052803 [DOI] [PubMed] [Google Scholar]
- Stott LC, Schnell S, Hogstrand C, Owen SF, & Bury NR (2015). A primary fish gill cell culture model to assess pharmaceutical uptake and efflux: Evidence for passive and facilitated transport. Aquatic Toxicology, 159, 127–137. 10.1016/j.aquatox.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter JP, Runnalls TJ, Donnachie RL, & Owen SF (2021). A comprehensive aquatic risk assessment of the beta-blocker propranolol, based on the results of over 600 research papers. Science of the Total Environment, 793, Article 148617. 10.1016/j.scitotenv.2021.148617 [DOI] [PubMed] [Google Scholar]
- Suzuki R, & Shimodaira H. (2006). Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics, 22, 1540–1542. 10.1093/bioinformatics/btl117 [DOI] [PubMed] [Google Scholar]
- Thomas RS, Wesselkamper SC, Wang NC, Zhao QJ, Petersen DD, Lambert JC, Cote I, Yang L, Healy E, Black MB, Clewell HJ 3rd, Allen BC, & Andersen ME (2013). Temporal concordance between apical and transcriptional points of departure for chemical risk assessment. Toxicological Sciences, 134, 180–194. 10.1093/toxsci/kft094 [DOI] [PubMed] [Google Scholar]
- Toth GP, Bencic DC, Martinson JW, Flick RW, Lattier DL, Kostich MS, Huang W, & Biales AD (2021). Development of omics biomarkers for estrogen exposure using mRNA, miRNA and piRNAs. Aquatic Toxicology, 235, Article 105807. 10.1016/j.aquatox.2021.105807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. (2002). Method 1000.0: Fathead minnow, Pimephales promelas, larval survival and growth; Chronic toxicity. In Office of Water (Ed.), US Environmental Protection Agency. [Google Scholar]
- Villeneuve DL, Le M, Hazemi M, Biales A, Bencic DC, Bush K, Flick R, Martinson J, Morshead M, Rodriguez KS, Vitense K, & Flynn K. (2023). Pilot testing and optimization of a larval fathead minnow high throughput transcriptomics assay. Current Research in Toxicology, 4, Article 100099. 10.1016/j.crtox.2022.100099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao Y, & Zhang B. (2015). Efficient test and visualization of multi-set intersections. Scientific Reports, 5, Article 16923. 10.1038/srep16923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Nishimura Y, Shimada Y, Umemoto N, Hirano M, Zang L, Oka T, Sakamoto C, Kuroyanagi J, & Tanaka T. (2009). Zebrafish β-adrenergic receptor mRNA expression and control of pigmentation. Gene, 446, 18–27. 10.1016/j.gene.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Zavala K, Vandewege MW, Hoffmann FG, & Opazo JC (2017). Evolution of the β-adrenoreceptors in vertebrates. General and Comparative Endocrinology, 240, 129–137. 10.1016/j.ygcen.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Zhao J, Xu T, Yin D, Zhang B, & Bai J. (2017). The regulatory roles of microRNA in effects of 2,2′4,4′-tetrabromodiphenyl ether (BDE47) on the transcriptome of zebrafish larvae. PLOS ONE, 12, Article e0169599. 10.1371/journal.pone.0169599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Fan S, Shi Y, Ren H, Hong H, Gao X, Zhang M, Qin Q, & Li H. (2020). Propranolol induced apoptosis and autophagy via the ROS/ JNK signaling pathway in human ovarian cancer. Journal of Cancer, 11, 5900–5910. 10.7150/jca.46556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H, Kolpin DW, Klaper RD, Iwanowicz LR, Meppelink SM, & LeFevre GH (2020). Occurrence and Spatiotemporal Dynamics of Pharmaceuticals in a Temperate-Region Wastewater Effluent-Dominated Stream: Variable Inputs and Differential Attenuation Yield Evolving Complex Exposure Mixtures. Environmental Science and Technology, 54, 12967–12978. 10.1021/acs.est.0c02328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data have been uploaded to the National Center for Biotechnology Information’s Gene Expression Omnibus database (GSE197442) and are freely available.