Abstract
Background:
Obstructive sleep apnea (OSA) is a severe condition associated with numerous cardiovascular complications, including heart failure. The complex biological and morphological relationship between OSA and atherosclerotic cardiovascular disease (ASCVD) poses challenges in predicting adverse cardiovascular outcomes. While artificial intelligence (AI) has shown potential for predicting cardiovascular disease (CVD) and stroke risks in other conditions, there is a lack of detailed, bias-free, and compressed AI models for ASCVD and stroke risk stratification in OSA patients. This study aimed to address this gap by proposing three hypotheses: (i) a strong relationship exists between OSA and ASCVD/stroke, (ii) deep learning (DL) can stratify ASCVD/stroke risk in OSA patients using surrogate carotid imaging, and (iii) including OSA risk as a covariate with cardiovascular risk factors can improve CVD risk stratification.
Methods:
The study employed the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) search strategy, yielding 191 studies that link OSA with coronary, carotid, and aortic atherosclerotic vascular diseases. This research investigated the link between OSA and CVD, explored DL solutions for OSA detection, and examined the role of DL in utilizing carotid surrogate biomarkers by saving costs. Lastly, we benchmark our strategy against previous studies.
Results:
(i) This study found that CVD and OSA are indirectly or directly related. (ii) DL models demonstrated significant potential in improving OSA detection and proved effective in CVD risk stratification using carotid ultrasound as a biomarker. (iii) Additionally, DL was shown to be useful for CVD risk stratification in OSA patients; (iv) There are important AI attributes such as AI-bias, AI-explainability, AI-pruning, and AI-cloud, which play an important role in CVD risk for OSA patients.
Conclusions:
DL provides a powerful tool for CVD risk stratification in OSA patients. These results can promote several recommendations for developing unique, bias-free, and explainable AI algorithms for predicting ASCVD and stroke risks in patients with OSA.
Keywords: obstructive sleep apnea, cardiovascular disease, stroke, cardiac autonomic dysfunction, artificial intelligence, recommendations, deep learning, bias, pruning, explainable, cloud
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) accounted for approximately 800,000 deaths in the United States (US) in 2020, representing 36% of total mortality, with 647,000 of these deaths occurring in individuals over the age of 65 [1, 2]. By 2030, the direct medical expenses associated with ASCVD are projected to exceed USD 920 billion [3]. According to the Sleep Apnea Association (SAA), 38,000 Americans die annually due to the combined effects of ASCVD and obstructive sleep apnea (OSA) [4]. Meanwhile, countries such as China, the US, Brazil, and India report the highest prevalence of OSA globally [5]. OSA affects 34% of males and 17% of females but is often underdiagnosed, with only 10% of OSA patients receiving an appropriate diagnosis and treatment [6]. Severe OSA contributes to an increased mortality rate from ASCVD and is associated with intermittent hypoxia, hypercapnia, and sympathetic overactivity [7].
OSA-related strokes occur when the brainstem fails to effectively communicate with the upper airway or lower thoracic muscles, leading to brain activation, intrathoracic pressure shifts, hypoxia, and reoxygenation due to upper airway collapse [8]. These events decrease oxyhemoglobin saturation and cause electroencephalogram (EEG) arousals [2]. During sleep, these cycles initiate pathways that increase the risk of atherosclerosis [9, 10, 11]. Previous studies have utilized polysomnographic data to predict OSA using machine learning (ML) and deep learning (DL) techniques [1, 2]. Traditional signal-processing methods for predicting cardiovascular outcomes in OSA patients have several limitations [12]. These methods often struggle with the complexity and variability of biological signals and may not effectively capture the multifactorial nature of OSA and ASCVD interactions [13]. Furthermore, they can be biased and fail to provide a comprehensive and explainable risk assessment [14, 15].
However, no study has focused on ASCVD risk stratification in OSA patients. Artificial intelligence (AI)-based systems have been employed to analyze heart rate variability (HRV) events, yet several reports indicate a biological link between OSA and ASCVD. Therefore, including OSA risk as a covariate in cardiovascular risk assessments could enhance the accuracy of ASCVD risk stratification [16, 17].
DL leverages convolution, max-pooling, and attention mechanisms (spatial and temporal attention maps) to extract features and characterize OSA–ASCVD relationships in both signal-based and image-based frameworks [18, 19]. Given the complexity of biological non-linear phenomena, developing robust, accurate, real-time DL paradigms is crucial for detecting OSA and stratifying ASCVD risk [20, 21]. Since evaluating ASCVD risk in OSA patients is challenging due to biological and morphological changes, surrogate biomarkers such as carotid artery disease can be used as indicators for coronary artery disease (CAD) [22, 23, 24].
This study proposes a DL approach to detect high-risk OSA and predict ASCVD risk using a carotid window [25, 26, 27]. We also address clinical evaluation and validation challenges, which can introduce bias and overfitting in DL prediction models [28]. With the trend towards miniaturized medical devices, such as edge devices, reducing the size of DL-based training systems is essential [29, 30]. Therefore, we explored pruned or compressed AI models for assessing ASCVD risk in OSA patients [29, 30]. Additionally, we emphasized the importance of understanding AI “black boxes” through explainability paradigms and extended this into a cloud-based framework [31, 32].
This study aimed to review DL systems for the joint detection of OSA and ASCVD risk stratification [33] while ensuring lower bias, higher compression, and clinical explainability within a cloud/telemedicine framework.
2. Search Strategy
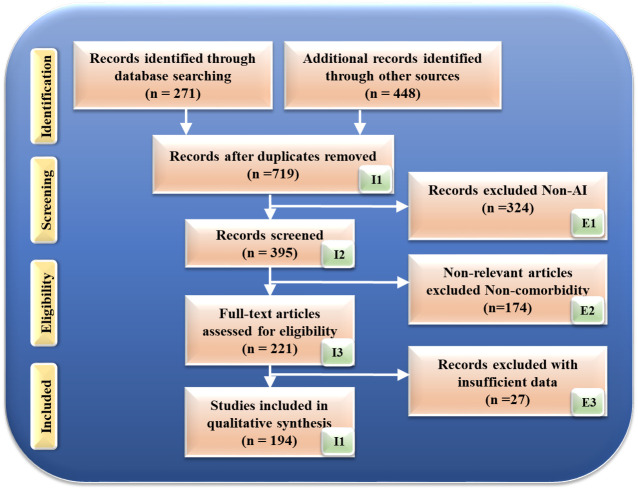
Fig. 1 illustrates the search technique based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. This process began with a comprehensive search using specific keywords related to OSA and its associations with cardiovascular disease, stroke, carotid imaging, AI, and deep learning. The search terms included combinations such as “obstructive sleep apnea and cardiovascular disease”, “obstructive sleep apnea and stroke”, “obstructive sleep apnea and carotid imaging”, “obstructive sleep apnea and AI”, “ASCVD atherosclerotic tissue classification and characterization”, “obstructive sleep apnea and deep learning”, “carotid plaque tissue characterization in sleep apnea”, and the conjunction of “obstructive sleep apnea” with databases, including PubMed and Google Scholar, to screen pertinent papers.
Fig. 1.
Search strategy based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) model. AI, artificial intelligence.
Initially, this search yielded 271 records from the specified databases and an additional 448 records from other sources, resulting in 719 records after accounting for quality-specific factors, such as timeliness and relevance. These factors ensured that the included studies were up-to-date and pertinent to the research topic.
The review process involved several stages of filtering. Firstly, studies that were unrelated to the primary focus of the research were excluded, which accounted for 324 studies. Next, irrelevant studies, those that did not directly address the specific research questions or objectives, were also removed, totaling 174 studies. Furthermore, studies with insufficient data, those lacking the necessary information for comprehensive analysis, were excluded, amounting to 51 studies.
After this meticulous exclusion process, 194 research studies were deemed suitable for inclusion in this review. These studies provided the necessary data and relevance to contribute to the systematic review and meta-analysis of OSA and its association with cardiovascular outcomes. This detailed selection process ensured that the final pool of studies was robust, relevant, and sufficient for the intended analysis, offering a comprehensive overview of the existing research on the topic.
3. Biological Link Shows that OSA Contributes to Conditions and Mechanisms Involved in ASCVD and Stroke Development
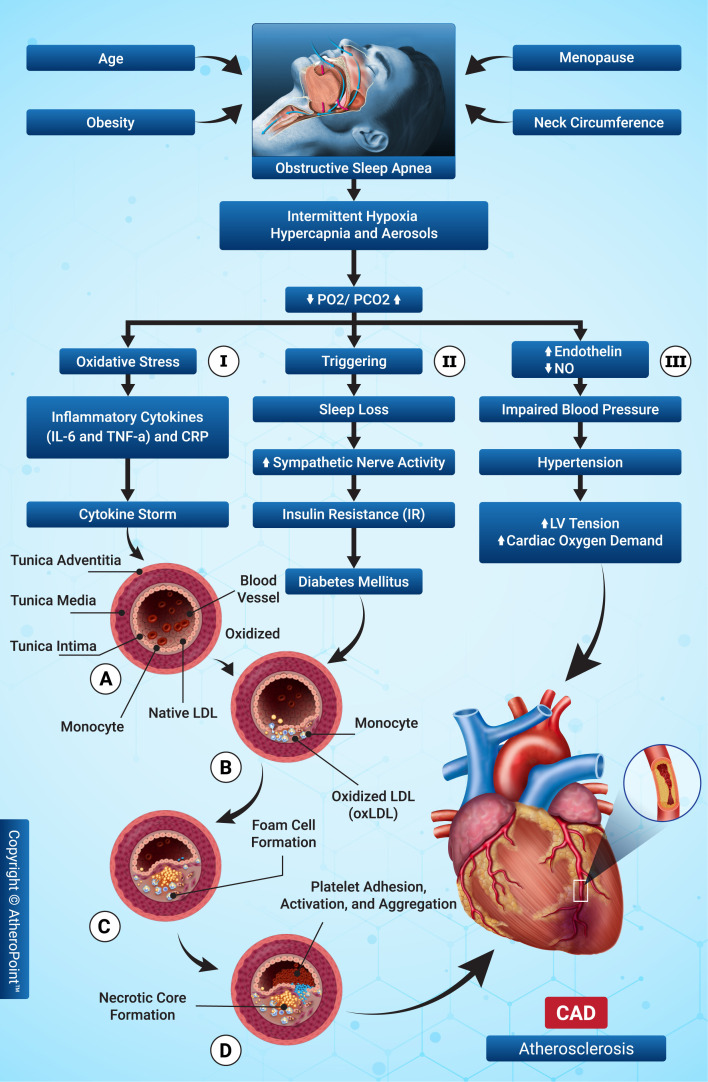
The pathophysiological link between OSA and the progression of ASCVD remains unknown [34, 35]. However, several pathogenic factors, such as macro and micro-arousals [36], intermittent hypoxia and hypercapnia [37], oxidative stress, inflammation, and vascular dysfunction, have been proposed as intermediate mechanisms linking OSA and ASCVD [38, 39]. Although discussed separately here, these mechanisms are connected and appear sequentially in patients with OSA. The biological mechanism through which OSA leads to ASCVD is depicted in Fig. 2 through three possible routes.
Fig. 2.
The biological link between obstructive sleep apnea (OSA) and atherosclerotic cardiovascular disease (ASCVD). IL-6, interleukin-6; TNF-, tumor necrosis factor ; CRP, C-reactive protein, PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; NO, nitric oxide; LV, left ventricle; LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein; CAD, coronary artery disease.
Path I: Hypoxia and hypercapnia, combined with intermittent hypoxia, cause oxidative stress, reactive oxygen species (ROS), inflammatory cytokines, and C-reactive protein (CRP) [40]. Thus, atherosclerotic plaque development in OSA is attributed to the cytokine storm [41], which causes endothelial dysfunction and favors low-density lipoproteins (LDLs) and circulating factors entering the intima of the blood vessels [42]. Moreover, increased inflammatory cytokines and ROS induce the oxidization of LDLs (OxLDL) [43]. Savransky et al. [44] reported intermittent hypoxia can cause lipid oxidation and atherosclerotic plaques. Nuclear factor kappa-B (NF-B)-activated macrophages absorb OxLDL, resulting in foam cells [45, 46]. These foam cells create the necrotic core, forming atherosclerotic plaques that cause ASCVD [47].
Path II: Obesity, OSA, and metabolic dysregulation are linked. Further, OSA, insulin resistance (IR), and ASCVD have the same pathophysiological connection and risk factors. OSA causes behavioral, metabolic, and hormonal disturbances that promote weight gain and IR [48]. Since calories are largely used when resting, these disturbances may activate the neuroendocrine region, promoting hunger and eventual weight gain [49]. OSA increases sympathetic nerve activity (SNA), which may change glucose metabolism, accelerate glycogen breakdown in skeletal muscles, and increase hepatic glucose synthesis. SNA release may raise cholesterol, triglycerides, and IR [50, 51]. IR causes more lipoproteins, which oxidize, increasing OxLDL levels and promoting atherosclerosis and ASCVD.
Path III: Intermittent hypoxia with hypercapnia causes baroreceptor dysfunction and a vasoconstriction effect of decreased endothelin and nitric acid (NO) levels due to vascular endothelial dysfunction. These changes increase blood pressure, which increases ventricular tone due to stress, leading to heart failure [22].
However, periodic hypoxia, characterized by regular and predictable episodes of low oxygen levels, can contribute to the development of ASCVD by inducing oxidative stress and endothelial dysfunction [52]. These hypoxic events promote inflammatory responses and lipid deposition in arterial walls, accelerating the progression of atherosclerosis. Therefore, controlled studies on periodic hypoxia can provide insights into its impact on ASCVD, highlighting the importance of consistent oxygenation for cardiovascular health [53].
Table 1 (Ref. [1, 2, 23, 24, 54, 55, 56, 57, 58, 59]) lists studies linking OSA to atherosclerosis in the carotid artery. OSA patients had higher atherosclerotic plaque levels and narrower lumen diameters [1, 23, 54, 60]. OSA increases proinflammatory plasma cytokines levels, such as interleukin (IL)-2, IL-1, tumor necrosis factor (TNF)-, polymerase chain reaction (PCR), and interferon-alpha (IFN-), endothelial-dependent blood channel widening, and adhesion molecule activity [2, 55, 56]. Inflammatory markers are associated with increased risk for atherosclerosis [1, 61, 62]. A severe OSA lowers blood oxygen and raises blood pressure [59].
Table 1.
Relationships between OSA syndrome and carotid artery atherosclerotic disease.
| SN | Authors | Year | REF* | PS | REL* | Comorbidities | Progression of biomarkers | Relationship between the manifestation of OSA and CaAD |
| 1 | Hui et al. [23] | 2012 | 37 | 50 | OSA with cIMT | NR | CPAP treatment reduces plaques | CPAP treatment is useful in OSA patients |
| 2 | Nadeem et al. [24] | 2013 | 38 | 1415 | OSA with cIMT | HTN, DM | Increase in carotid diameter and carotid plaques | Increased plaque levels is symptomatic of an atherosclerotic process |
| 3 | Ciccone et al. [55] | 2014 | 56 | 80 | OSA with cIMT | HTN, pulmonary | Increased levels of plaques, CRP, IL-6, TNF, and PTX-3 | The development of atherosclerosis may be influenced by inflammatory markers |
| 4 | Zhou et al. [57] | 2017 | 58 | 18 | OSA with carotid | HTN | Increase in atherosclerotic plaques | Independent risk factor for ASCVD |
| 5 | Song et al. [54] | 2020 | 62 | 95 | OSA with cIMT | NR | Increase in atherosclerotic plaques | Reduced plaque and carotid arterial elasticity |
| 6 | Bandi et al. [58] | 2021 | 47 | NR | OSA with HF | NR | Causes arrhythmogenicity and results in HF | Leads to atherosclerosis |
| 7 | Smith et al. [2] | 2021 | 36 | 96 | OSA with carotid | HTN | Elevation in important proinflammatory cytokines | The population’s inflammatory environment is a risk factor for childhood atherosclerosis |
| 8 | Suzuki et al. [56] | 2022 | 31 | 07 | OSA with carotid | HTN, DM | REM and OSA both are linked to metabolic and cardiovascular complications | Arterial stiffness and REM are increased |
| 9 | Gunnarsson et al. [1] | 2014 | 28 | 790 | OSA with carotid | HTN | Increase in carotid plaques | Increased future ASCVD and stroke risks |
| 10 | Firincioglulari et al. [59] | 2022 | 23 | 190 | OSA with carotid | NR | Increase in carotid artery calcification | More calcification leads to increased stroke risk |
REF*, references in the respective articles; PS, patient size; REL*, relationship; OSA, obstructive sleep apnea; HTN, hypertension; cIMT, carotid intima-media thickness; DM, diabetes mellitus; HF, heart failure; CRP, C-reactive protein; NR, not reported; ASCVD, atherosclerotic cardiovascular disease; REM, rapid eye movement; CPAP, continuous positive airway pressure; CaAD, carotid artery disease; SN, serial number; IL-6, Interleukin-6; TNF, tumor necrosis factor; PTX-3, pentraxin-3.
Table 2 (Ref. [6, 7, 9, 16, 17, 63, 64, 65, 66, 67]) shows the link between OSA and coronary atherosclerotic disease. Long ST intervals (flat, isoelectric section on the electrocardiograph (ECG) between the end of the S wave and the beginning of the T wave) are connected to atherosclerosis [16, 68]. OSA was related to higher inflammatory activity in this CAD sample [63]. Moreover, left ventricle (LV) pressure and diastolic dysfunction result in left atrial hypertrophy [64, 69]. Periodic hypoxia can cause sympathetic activation, decreased parasympathetic tone, and inflammation [65, 70]. Studies have shown that obesity, hypertension, and diabetes are associated with OSA [17, 64, 66].
Table 2.
Relationship between OSA syndrome and coronary atherosclerotic artery disease.
| SN | Authors | Year | REF* | PS | REL* | Comorbidities | Progression of biomarkers | Relationship between the manifestation of OSA and CAD |
| 1 | Colish et al. [9] | 2012 | 40 | 47 | OSA with CAD | HTN | Risk of ASCVD | CPAP treatment is useful in OSA |
| 2 | Tan et al. [63] | 2014 | 36 | 93 | OSA with CAD | Obesity, HTN, DM | SA patients have increased atheroma volumes | Chronic OSA is an important potential risk factor for coronary atherosclerosis |
| 3 | Miller et al. [64] | 2015 | 74 | NR | OSA with CAD | Obesity, HTN | Risk of intermittent nocturnal hypoxemia | Atrial fibrillation |
| 4 | Valo et al. [16] | 2015 | 39 | 80 | OSA with CAD | NR | ST segment depression | Myocardial necrosis |
| 5 | Singh et al. [65] | 2022 | 28 | 100 | OSA with CAD | Obesity | Young patients with angiography show OSA serve symptoms | Correlation between obesity and OSA |
| 6 | Lu et al. [17] | 2021 | 105 | 82 | OSA with CAD | Obesity, HTN | Ventricular malfunction and remodeling | Coronary atherosclerosis |
| 7 | Tang et al. [67] | 2021 | 28 | 158 | OSA with CAD | HTN | Risk of ASCVD | Pulmonary hypertension |
| 8 | Liu et al. [66] | 2022 | 44 | 255 | OSA with CAD | Obesity, HTN | Patients in China with CAD have a significantly higher incidence of OSA | OSA is a risk factor for ASCVD |
| 9 | Wang et al. [6] | 2023 | 45 | 1927 | OSA with CAD | Hypertension | Ischemia-driven unstable angina | Acute coronary syndrome |
| 10 | Wojeck et al. [7] | 2023 | 10 | 8246 | OSA with CAD | NR | Ertugliflozin to reduce the effect of OSA | Sodium-glucose transporter 2 inhibitors are beneficial in OSA |
REF*, references in the respective articles; PS, patient size; OSA, obstructive sleep apnea; DM, diabetes mellitus; HTN, hypertension; NR, not reported; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CPAP, continuous positive airway pressure; SN, serial number; REL*: relationship; SA, sleep apnea.
4. Deep Learning for OSA Detection and ASCVD Risk Stratification
ML and DL have become more prominent in medical imaging [71, 72, 73]. The fundamentals of DL are based on deep neural networks (DNNs), a subgroup of DL that functions similarly to a human brain [74]. Recently, studies have used AI employing OSA detection in the HRV framework [75, 76, 77, 78] and ASCVD diagnosis and prognosis [28, 79, 80]. While DL is becoming popular for higher accuracy in segmentation and classification, hyperparameters need to be optimized during the training paradigm [31]. This requires several epochs, including optimal learning rate, batch size, batch normalization, and dropout layers, to avoid overfitting and achieve generalization without memorization [81]. To accomplish the foremost DL architecture, one must use multiple diagnostic sources with many distinct data sets in a big data framework [82]. However, comorbidities in patients alongside ASCVD and OSA influence non-linear dynamics between gold standard and covariates [83].
4.1 Deep Learning for OSA Detection
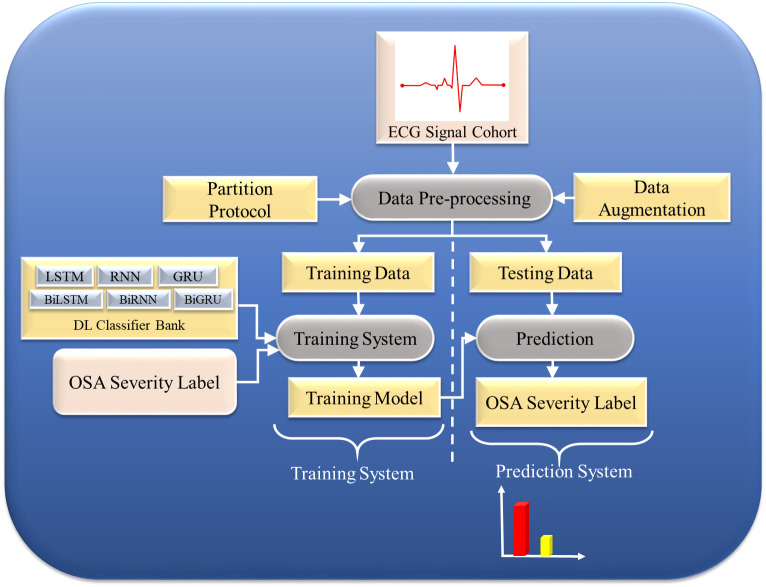
The impulses from an ECG serve as the standard physiological measurement [84]. Interestingly, there is a strong connection between breathing disorders and ECG abnormality [85]. Hence, variation in the ECG can be a strong predictor of OSA [86]. Moreover, the processing and optimization of ECG signals are cost-effective [87]. DL algorithms train the model to extract features from polysomnography (PSG) signals automatically [84]. Overnight PSG measures numerous sleep parameters, while PSG recordings should be segmented into 30-second epochs to score sleep [88]. The training set trains the models, the validation set fine-tunes the models, and the testing set evaluates the performance. Subsequently, the data extracted from PSG determine the conclusion related to OSA severity. When large amounts of high-dimensional PSG data are available, DL models regularize the data and perform more accurate predictions than ML models [89, 90]. Singh and Talwekar [91] used hybrid deep learning (HDL) with CNN to predict OSA, achieving 80% accuracy. Locharla et al. [92] used DL with K-Nearest neighbors (KNN) and achieved 78% accuracy. Thus, DL systems for OSA prediction are powerful and reliable paradigms; however, work still needs to be performed to ensure the reliability and stability of the DL systems. Several studies that use AI for OSA prediction are shown in Appendix Table 4 (Refs. [20, 21, 33, 85, 89, 90, 93, 94, 95, 96, 97, 98]), Appendix Table 4. Fig. 3 shows a typical DL-based model for OSA prediction.
Fig. 3.
Deep learning (DL)-based model for OSA prediction. OSA, obstructive sleep apnea; ECG, electrocardiogram; LSTM, long short-term memory; RNN, recurrent neural network; GRU, gated recurrent unit; BiGRU, bidirectional gated recurrent unit; BiLSTM, bidirectional long short-term memory; BiRNN, bidirectional recurrent neural network.
4.2 Deep Learning for ASCVD Risk Stratification
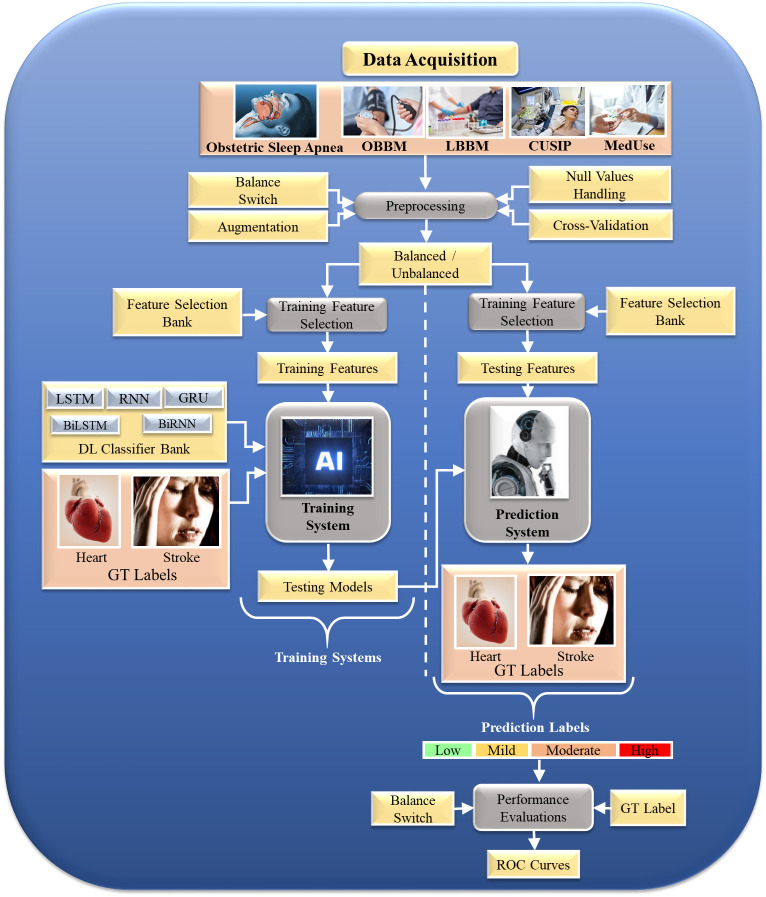
DL is an effective strategy because it can use the underlying knowledge to create automated features and offers a better training paradigm that adjusts the non-linearity among both variables (covariates) and the gold standard. Fig. 4 depicts a typical DL system. This architecture comprises (a) a training design using composite risk factors such as OSA risk labels, office base bio markers (OBBMs), lab base bio markers (LBBMs), carotid ultrasound image phenotypes (CUSIPs), medication utilization (MedUSE), and (b) clinical risk labels representative of ground truth (GT), such as heart failure ASCVD and stroke [99]. This GT may indicate CAD, similar to a cardiac computed tomography (CT) score. Indeed, CT can be scored using DL. Suri et al. [100] have described CT-based grading. Intravascular ultrasound (IVUS) can also be used as a CT to represent CAD lesions [101, 102]. A non-linear training-based approach has been employed in heart disease risk stratification [103, 104].
Fig. 4.
OSA-based multiclass DL model to predict ASCVD/stroke severity. OSA, obstructive sleep apnea; DL, deep learning; ASCVD, atherosclerotic cardiovascular disease; OBBM, office base bio markers; LBBM, lab base bio markers; CUSIP, committee on uniform securities identification procedures; MedUse, medication use; LSTM, long short-term memory; RNN, recurrent neural network; GRU, gated recurrent unit; BiLSTM, bidirectional long short-term memory; BiRNN, bidirectional recurrent neural network; GT, ground truth; AI, artificial intelligence; ROC, receiver operating characteristic.
The recurrent neural network (RNN) and long short-term memory (LSTM) models can be used to evaluate sequential data, such as ECG, text [105], speech [106], and handwriting [107]. Further, these models contain a set of continuous data patterns. Previous RNNs could not learn long-term dependencies, resulting in a bridge problem connecting old and new data [108]. This problem further promoted the vanishing gradient problem, in which error signals vanished after backpropagation, leading to model failure [106]. LSTM has input, internal, and output gates: The input gate determines how much data will be forgotten; the internal gate determines the level of current state data; the output gate state is used to derive the next hidden state [109]. These models could memorize key data and understand long-term dependencies following the backpropagation of useful information. Fig. 5 depicts LSTM architecture.
Fig. 5.
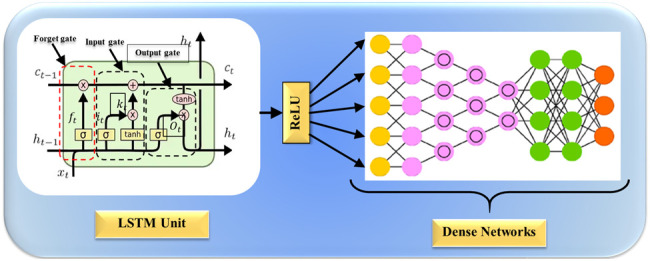
Long short-term memory (LSTM) architecture for ASCVD risk stratification. ASCVD, atherosclerotic cardiovascular disease; ReLU, rectified linear unit.
4.3 Deep Learning for Plaque Wall Segmentation and CUSIP Measurements: A Surrogate Biomarker
We have hypothesized that OSA leads to ASCVD disease via the morphological changes in the vascular network. CUSIPs refer to image-based carotid artery phenotypes [59, 110], and this training program is adaptable to non-linear adaptation [103, 104, 111]. Fig. 6 [112] represents the B-mode carotid ultrasound scan and its corresponding coronary atherosclerotic disease. The DL system can measure the atherosclerotic plaque area [113, 114]. The DL system computes the CUSIPs and helps to detect plaque aggregation in OSA patients [115]. Therefore, GT is required for DL-based ASCVD risk classification.
Fig. 6.
The carotid artery examination using intravascular ultrasound (IVUS). (a) The carotid artery is a potential surrogate marker for the coronary artery, as shown by an IVUS-based vascular cross-sectional scan. (b) B-mode carotid longitudinal imaging system using linear ultrasound [112].
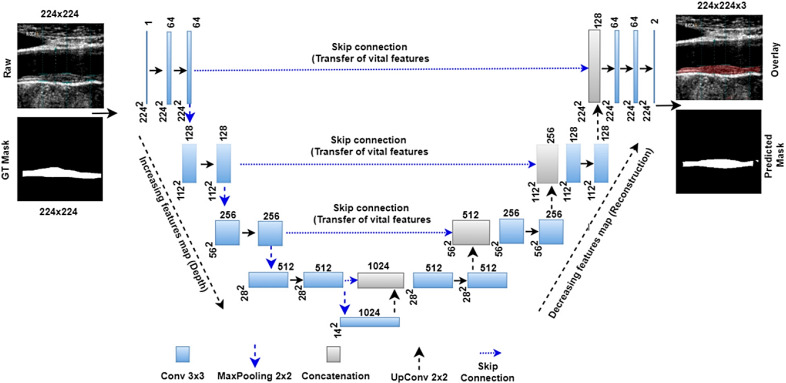
Jain et al. [116] proposed a universal neural network (UNet) model to detect atherosclerotic plaques. The model uses four layers of deep learning (DL) and a pair of encoders and decoders. The model is shown in Fig. 7 (Ref. [116]). A sample is transmitted and received by the encoder. A two-dimensional-convolution rectified linear unit (ReLU) and MaxPooling may all be found in each UNet encoder layer. Each successive decoding stage employs up-convolution (two dimensions, 2D), depth-concatenation, and 2D convolution as part of image reconstruction.
Fig. 7.
UNet model for segmentation of the atherosclerotic plaque wall [116]. UNet, U-Net (a type of convolutional neural network architecture for image segmentation); GT, ground truth.
5. Challenges and Recommendations in OSA Detection and ASCVD Risk Stratification
The DL system must address several key challenges to ensure the safety and efficacy of the medical devices used in ASCVD risk stratification for OSA patients: the comparison between carotid and retinal imaging, mitigating bias, ensuring explainability and trust in AI, optimizing ergonomics and cost-effectiveness, model pruning, cloud-based deployment, and the integration of carotid artery Doppler examinations.
5.1 A Special Note on Carotid Imaging vs. Retinal Imaging as Biomarkers for CVD Risk
The genetic makeup of the carotid artery: Previous studies have successfully established that the genetic makeup of the carotid artery is the same as the coronary artery [117]. Indeed, the coronary calcium volumes detected by IVUS are related to the automated carotid intima-media thickness (cIMT), which ensures the genetic nature of atherosclerosis disease. In another study, Araki et al. [118] used a machine learning classifier that used cIMT as the gold standard for risk stratification of coronary atherosclerosis disease, establishing the link between coronary plaques and subclinical atherosclerosis in the carotid artery, determining the genetic makeup of the atherosclerosis disease. The same pattern was established in other studies by Araki et al. [118], Banchhor et al. [101] and Narula et al. [119] . Lastly, it is easy to access the carotid artery from the innominate artery, i.e., where the innominate artery is connected to the aortic arch, which supplies blood to the brain and head [120], as the left common carotid artery branches off from the arch itself, and the right common carotid originates from the brachiocephalic trunk [101]. This anatomical connection is crucial in understanding the impact of aortic diseases, such as atherosclerosis, on cerebral blood flow and the potential risk of stroke, thereby highlighting the interdependency between the aorta and carotid arteries in vascular health shown in Fig. 8 [121]. A similar relationship exists between the coronary artery originating from the aortic arch, which supplies blood to the heart. The base of the aorta corresponds to the left and right coronary arteries (LCAs and RCAs) that supply blood to the heart, meaning the LCAs and RCAs are the first branches of the aorta. Thus, the two sets of arteries, coronary and carotid, originate from the aorta with the same genetic makeup [121, 122]. Since the genetic makeup of carotid and coronary arteries is similar, this study used the carotid arteries as a surrogate biomarker for coronary artery disease [101, 123].
Fig. 8.
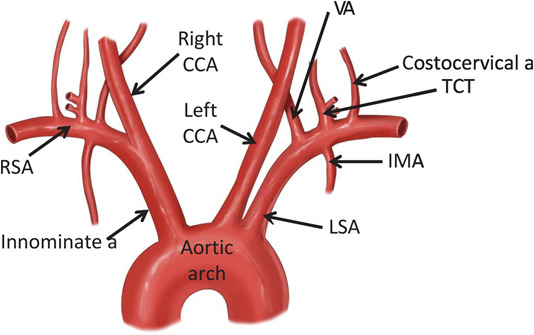
The link between the coronary and carotid arteries [121]. RSA, right subclavian artery; CCA, common carotid artery; VA, vertebral artery; TCT, total circulating tumour cell; MA, mammary artery; LSA, left subclavian artery.
A recent trend has emerged that uses the carotid artery as a biomarker instead of retinal scans to evaluate CVD risk stratification; this small note explains why the carotid artery is preferred over retinal scans. While this comparison is outside the scope of this review, we discuss the benefits the carotid artery offers. (i) The instrumentation for carotid artery imaging is ergonomic and user-friendly, meaning the sonographer, cardiologist, or radiologist can all perform carotid imaging without much training. The common carotid artery is easy to image using a linear transducer in an arterial cross-section mode and longitudinal modes [124, 125]. (ii) The economics of using carotid imaging over retinal imaging are favorable since the cost for carotid artery imaging is lower (economic) than retinal imaging devices. Meanwhile, the device used for retinal imaging is mentioned in the nutrition paper (diabetic retinopathy). Even though a normal camera can be used, the image quality is poor, meaning a stronger retinal imaging device is required, which is costly [126]. Recently, it was shown that when combined with AI, carotid imaging is very economical; however, surgeries can be prevented using carotid imaging [127]. (iii) Simplicity of processing images from carotid artery imaging: The image processing applied to carotid ultrasound is much simpler. This is because the lumen diameter is 10 mm, and the outer wall diameter is 12 mm [73, 101, 117, 128, 129]; meanwhile, the media-adventitial borders are 12 mm apart. Thus, the plaque burden is the difference between the outer and inner borders. (iv) East identification of media adventitia (MA) borders: The MA borders are easy to determine as there is a clear transition from the media region to the adventitia region, which indicates the MA borders of the carotid artery. Similarly, the lumen-intima (LI) borders are those between the lumen region (black region) and the media region (brighter region). Thus, the LI borders can also be easily estimated. The region between the LI and MA borders is the IMT region and represents the surrogate biomarker for coronary artery disease [130]. (v) Video imaging of the blood flow: Video imaging can also be taken when employing carotid artery imaging, which allows for stiffness computation, a very informative analysis for symptomatic vs. asymptomatic computations [102, 131, 132]. (vi) Deep learning paradigm: the feature extraction during the DL that represents closer to the coronary artery disease is the carotid artery.
This DL will likely provide a stronger design for CVD risk prediction, as proven before using a ML system design. Since the plaque burden in the carotid artery is a stronger biomarker for coronary heart disease (CHD), then there is a stronger chance that DL is more effective in carotid artery imaging (CAI) compared to retinal artery imaging (RAI). Carotid atherosclerosis plays a substantial role in cardiovascular morbidity and mortality. Given the multifaceted impact of carotid atherosclerosis, there has been increasing interest in harnessing AI and radiomics as complementary tools for the quantitative analysis of medical imaging data. This integrated approach holds promise in refining medical imaging data analysis and optimizing the utilization of radiologists’ expertise since AI allows radiologists to focus on more pertinent responsibilities by automating time-consuming tasks. Simultaneously, the capacity of AI in radiomics to extract nuanced patterns from raw data enhances the exploration of carotid atherosclerosis, advancing efforts in terms of (1) early detection and diagnosis, (2) risk stratification and predictive modeling, (3) improving workflow efficiency, and (4) contributing to advancements in research. This review provides an overview of general concepts related to radiomics and AI and their application in carotid-vulnerable plaques. It also offers insights into various research studies on this topic across different imaging techniques [133].
5.2 The Role of Bias in DL System Designs
Previous computer-aided diagnosis techniques lacked bias evaluations [83]; however, the importance of evaluating bias in AI models has increased significantly [134, 135]. Bias prevention can be handled using large sample sizes, proper clinical testing, introducing comorbidities, using big data configurations, unseen data analysis, and scientific validation of the training model design [100]. Identifying the AI risk of bias (RoB) [125, 136] and appropriately modifying diagnoses and treatments are key steps in patient risk stratification. Imbalanced data, common in medical datasets, can skew model performance towards over-represented classes. In the context of CVD prediction, we propose that strategies such as oversampling minority classes or undersampling majority classes can ensure the model learns equally from all categories of data. Synthetic data generation techniques such as the Synthetic Minority Oversampling Technique (SMOTE) or Adaptive Synthetic Oversampling Technique (ADASYN) [137] can also be applied in future implementations to balance class distributions without losing information [138]. Using diverse, multi-modal datasets that capture a wide range of patient demographics, symptoms, and conditions helps ensure that the model can generalize across various populations and is less likely to overfit specific groups. Furthermore, using large-scale datasets from different sources also reduces the risk of introducing bias from a single source [139].
5.3 Enhancing Model Explainability and Trust in AI
Understanding AI’s “black box” is one of the most critical challenges in its adoption. Moreover, providing clear explanations of AI model outcomes helps healthcare practitioners interpret and trust these results. Indeed, using tools such as Local Interpretable Model-agnostic Explanations (LIME) and SHapley Additive exPlanations (SHAP), which offer explainability for AI predictions, provides physicians with greater confidence in their models [73, 140]. Similarly, visualization techniques such as Gradient-weighted Class Activation Mapping (GradCAM), GradCAM+, and GradCAM++ can highlight lesions in carotid scans, aiding in interpretation [141]. These methods, which make the technology more transparent and user-friendly, can enhance the acceptance of AI in healthcare.
Explainability also makes AI systems more adaptable and cost-effective [142]. GradCAM and its variants are particularly useful for convolutional neural network (CNN)-based models such as carotid artery and retinal scans, which are commonly used in medical image analysis. These methods produce visual explanations by highlighting the areas in an image that contribute most to a prediction, thereby allowing clinicians to observe the specific features, such as artery blockages or other abnormalities, that influenced the model’s decision. This provides clinicians with a greater ability to validate or challenge AI-generated results [143, 144, 145].
LIME and SHAP provide valuable interpretability for non-image data, such as clinical, demographic, or symptom information. LIME creates local interpretable models by perturbing input features and observing changes in the output, helping clinicians understand how specific factors—such as apnea-hypopnea index (AHI) or oxygen desaturation levels in OSA patients—impact CVD or stroke risk predictions. SHAP complements this by providing a global interpretation, assigning consistent and additive importance values to each feature, and ensuring clinicians can identify the most critical factors in a patient’s risk profile [102]. Together, these tools improve the trustworthiness and clinical utility of AI models in healthcare.
Another way to improve trust is by integrating explainability tools such as GradCAM, LIME, and SHAP into clinical decision support systems. By providing real-time, transparent insights into the mechanisms through which models reach their decisions, clinicians can validate AI recommendations alongside their expertise, fostering a collaborative decision-making process [146]. Layer-wise Relevance Propagation (LRP) can further break down model predictions and assign relevance scores to individual neurons or pixels, especially in medical imaging models. This pixel-level breakdown explains how each input feature (such as a pixel in a medical scan) contributed to the final prediction, ensuring that decisions are not solely based on superficial or misleading information.
Finally, improving trust in DL models requires extensive testing and validation on diverse and representative datasets. Continuous evaluation of the performance of the models across different demographic groups, medical conditions, and geographic regions is essential to ensure the models perform robustly in various clinical settings. Future work should address potential biases during validation and improve the generalization ability of the models through fine-tuning and retraining.
5.4 The Role of Pruning-based DL Systems
As the Internet and cloud-based systems evolve, edge devices are gaining importance. Indeed, edge devices are particularly powerful when applying trained AI models for future predictions or disease risk stratifications. However, large data models are not deployable on edge devices, meaning compressed models are needed. Genetic algorithms (GA), particle swarm optimization (PSO), differential evolution (DE), and wolf optimization (WO) can be used to prune image-based DL models. Moreover, a fully connected network (FCN) or segmentation network (SegNet) can be used to compress the models [147].
5.5 Cloud-based Workflow in DL Models for ASCVD Risk Stratification for OSA Patients
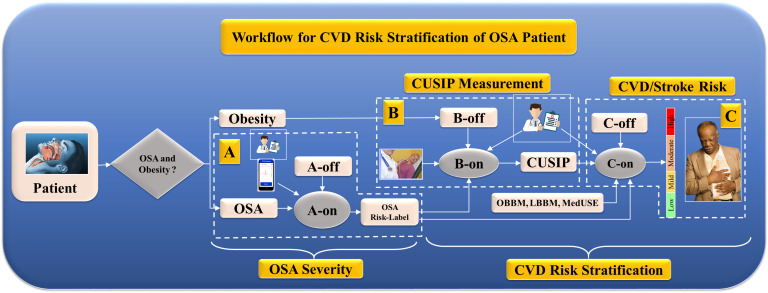
As the Internet has become more technologically advanced, cloud-based technologies have evolved. Subsequently, we anticipate using cloud-based DL models to process OSA and ASCVD risk stratification [31, 148]. Fig. 9 depicts the role of DL in OSA detection and ASCVD risk stratification via a pipeline that contains A, B, and C cascaded systems. System A is used for OSA severity prediction on the test patient via A-on, given the A-off EEG-based OSA-trained model. System B is used for CUSIP segmentation via a DL-based UNet system, a B-on system, and a B-offline trained model. Finally, system C applies a DL-based ASCVD risk stratification using C-on via a C-off trained ASCVD model. The C-off LSTM-trained model uses biomarkers such as office base bio markers (OBBM), lab base bio markers (LBBM), CUSIP, MedUSE, OSA risk, and the ASCVD gold standard. The overall system uses smart-based OSA detection by analyzing real-time ECG signals during sleep, while system C uses cloud-based ASCVD risk stratification. Thus, the overall system is cost-effective.
Fig. 9.
The architecture of ASCVD screening for patients with OSA and obesity. OSA, obstructive sleep apnea; CUISP, carotid ultrasound image phenotype; A-off, offline DL-based OSA training model; A-on, online DL-based OSA severity system; B-off, offline DL-based carotid training model; B-on, online DL-based carotid wall segmentation and quantification system; C-off, offline training-based ASCVD risk model; C-on, online DL-based ASCVD risk assessment; ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; DL, deep learning; OBBM, office base bio markers; LBBM, lab base bio markers; MedUSE, medication utilization; A, section A is OSA severity detection; B, section B is CUSIP measurement; C, section C is CVD/Stroke measurement.
5.6 Impact of Radiologist Experience on the Accuracy of Carotid Artery Doppler Examinations
An essential factor influencing the accuracy of carotid artery scanning is the experience level of the radiologists performing these procedures [149]. Experienced radiologists, often with several years of specialized training, can more precisely and efficiently image the common carotid artery, carotid bulb, and internal carotid artery [150]. Moreover, the expertise of the radiologist allows for minute pathological changes to be identified that may indicate atherosclerosis [151]. Comparatively, junior radiologists, who may still be refining their skills, could require more time to perform these examinations and additional training, particularly for imaging the more complex segments [152]. This difference in proficiency could potentially impact the diagnostic outcomes and should be considered when interpreting the results of studies involving carotid artery Doppler examinations [153]. Senior radiologists typically supervise procedures performed by junior radiologists to ensure consistency and accuracy in the imaging results.
5.7 Recommendations
ASCVD risk stratification in OSA patients has several recommendations: (i) Three hypotheses: (a) OSA leads to atherosclerosis formation promoting ASCVD, (b) DL systems are well suited for complex non-linear behavior undergoing morphological changes, and (c) combining OSA risk as a covariate with cardiovascular risk factors can improve ASCVD risk stratification; and recommendations are: (i) evaluation and validation: DL systems must undergo a clinical evaluation and scientific validation for a robust OSA detection and ASCVD risk stratification; (ii) hyperparametrization: DL systems must be hyperparameterized in both phases: (a) OSA detection and (b) ASCVD risk stratification; (iii) bias: bias in DL models can be best reduced by balancing the risk classes (control, low-risk, and high-risk); (iv) edge devices: DL systems can be ported to edge devices if these systems are pruned or compressed; (v) carotid surrogate imaging: DL systems based on surrogate carotid imaging can be low-cost without reducing ASCVD risk stratification accuracy.
6. Discussion
6.1 Principal Findings
This study emphasizes four major objectives:
(i) Establishment of the link between OSA and heart failure;
(ii) Role of DL for CVD risk stratification in OSA patients;
(iii) Role of surrogate biomarkers for CVD risk;
(iv) Fusion of OSA risk factors with other risk factors for composite CVD risk.
(i) Link between OSA and heart failure: OSA is intricately linked to numerous cardiovascular complications, including heart failure. The relationship between OSA, ASCVD, and stroke is complex and involves various pathogenic mechanisms. Studies have shown that OSA contributes to conditions such as intermittent hypoxia, hypercapnia, oxidative stress, systemic inflammation, and endothelial dysfunction, all of which are critical in the development of ASCVD and stroke. OSA-induced intermittent hypoxia leads to oxidative stress and systemic inflammation, which promote the development of atherosclerotic plaques. These plaques cause cardiovascular complications by inducing endothelial dysfunction and increasing the deposition of LDLs in arterial walls [44, 154]. Additionally, research has demonstrated that OSA patients have higher levels of inflammatory markers and greater carotid artery atherosclerosis compared to non-OSA individuals [63]. OSA has also been identified as an independent risk factor for stroke, with mechanisms such as hemodynamic instability, increased sympathetic activity, and impaired cerebral autoregulation playing significant roles [7].
(ii) Role of DL for carotid imaging: DL techniques have shown remarkable capabilities in medical imaging analyses, providing accurate and efficient predictions for various health conditions [155, 156, 157]. Thus, applying DL to carotid imaging in OSA patients offers a promising approach to stratifying ASCVD and stroke risks. DL algorithms, particularly CNN, have effectively identified and quantified atherosclerotic plaques in carotid imaging, offering detailed insights into the severity and progression of ASCVD [81, 147, 158, 159]. DL models trained on large datasets of carotid images can accurately predict cardiovascular events by analyzing plaque characteristics such as size, composition, and morphology [81, 141, 160].
(iii) Role of surrogate biomarker for CVD risk: These predictions are crucial for risk stratification in OSA patients, who are at higher risk for ASCVD and stroke. Integrating DL-based carotid imaging analysis with traditional cardiovascular risk factors enhances the precision of risk prediction models, providing more personalized and effective management plans for OSA patients [125, 161, 162].
(iv) Fusion of OSA risk factors with other risk factors for composite CVD risk: Incorporating OSA risk into cardiovascular risk models can significantly improve the accuracy of predicting adverse cardiovascular outcomes, similar to erectile dysfunction [160] or Parkinson’s disease [163, 164]. Traditional risk models for CVD and stroke typically consider factors such as age, sex, hypertension, diabetes, cholesterol levels, and smoking status [165, 166]. Therefore, adding OSA risk to these models acknowledges the substantial impact of sleep-disordered breathing on cardiovascular health. Studies have shown that OSA independently predicts cardiovascular events and its inclusion in risk models can improve the stratification of patients into different risk categories, allowing for more targeted and effective interventions [167, 168].
Validating these enhanced models through clinical trials and longitudinal studies can provide robust evidence for the utility of including OSA risk in cardiovascular risk stratification, ultimately leading to better outcomes for patients with OSA [64].
6.2 Benchmarking
Table 3 (Ref. [169, 170, 171, 172, 173, 174, 175, 176, 177]) provides a comprehensive benchmarking analysis of various studies that predict ASCVD risk in OSA patients using AI technologies. Table 3 includes 15 attributes: serial number, studies, year, references, comorbidities, body mass index (BMI), ethnicity, ECG, waist circumference, polysomnography (PSG), electromyography, oxygen saturation, AI type, Food and Drug Administration (FDA) discussion, clinical setting, and risk of bias. Key observations from Table 3 indicate that only two studies, Cao et al. [169], and Brennan and Kirby [170], specifically predicted ASCVD in OSA patients using DL. The remaining studies focused on predicting OSA alone. Among these studies, seven had hypertension as a comorbidity, nine utilized DL technologies, and two employed ML methods. Mostafa et al. [171] used DL with a decision tree (DT) classifier to predict OSA in hypertensive patients, achieving an accuracy of 85%. Munjral et al. [26] also used DL but with a random forest (RF) classifier, achieving 87% accuracy. Cao et al. [169] predicted both ASCVD and hypertension using DL with a convolutional neural network (CNN), reaching 90% accuracy. Cao et al. [169] combined DM with ASCVD and hypertension in their predictions using DL with RF, achieving 88% accuracy. Hong et al. [172] included DM and hypertension and used DL with KNN, achieving 82% accuracy. Qian et al. [173] used ML with a DT classifier to predict OSA in hypertensive patients and achieved 84% accuracy. Brennan and Kirby [170] achieved 89% accuracy using DL with RF, covering multiple comorbidities, including DM, ASCVD, and hypertension. Ferreira-Santos et al. [174] used ML with eXtreme gradient boosting (XGB) to predict OSA in patients with DM and hypertension, achieving 86% accuracy. Singh and Talwekar [91] used HDL with CNN for OSA predictions, achieving 80% accuracy. Locharla et al. [92] used DL with KNN, achieving 78% accuracy. The most recent study by V. Kumari et al. [175] used DL with CNN to predict ASCVD, achieving the highest accuracy of 91%. This presented study aimed to predict both OSA and ASCVD, including comprehensive physiological parameters, but lacked the risk of bias discussion.
Table 3.
Benchmarking data for ASCVD risk in OSA patients.
| K0 | K1 | K2 | K3 | K4 | K5 | K6 | K7 | K8 | K9 | K10 | K11 | K12 | K13 | K14 | K15 | K16 | K17 |
| 1 | Mostafa et al. [171] | 2019 | 93 | HTN | ✘ | ✘ | ✘ | ✘ | ✘ | DL | ✘ | DT | 85% | ||||
| 2 | Pépin et al. [176] | 2020 | 85 | OSA | ✘ | ✘ | ✘ | ✘ | ✘ | ✘ | DL | ✘ | RF | 87% | |||
| 3 | Loh et al. [177] | 2020 | 106 | ASCVD, HTN | ✘ | ✘ | DL | ✘ | ✘ | CNN | 90% | ||||||
| 4 | Cao et al. [169] | 2020 | 72 | DM, ASCVD, HTN | ✘ | ✘ | DL | ✘ | ✘ | ✘ | RF | 88% | |||||
| 5 | Hong et al. [172] | 2020 | 231 | DM, HTN | ✘ | ✘ | DL | ✘ | - | ✘ | KNN | 82% | |||||
| 6 | Qian et al. [173] | 2021 | 155 | HTN | ✘ | ✘ | ✘ | ML | ✘ | ✘ | DT | 84% | |||||
| 7 | Brennan et al. [170] | 2022 | 63 | DM, ASCVD, HTN | DL | ✘ | RF | 89% | |||||||||
| 8 | Ferreira-Santos et al. [174] | 2022 | 68 | DM, HTN | ✘ | ML | ✘ | ✘ | XGB | 86% | |||||||
| 9 | V. Kumari et al. [175] | 2023 | 275 | ASCVD | ✘ | ✘ | ✘ | ✘ | ✘ | DL | ✘ | ✘ | ✘ | CNN | 91% | ||
| 10 | Maindarkar et al. (proposed) | 2024 | 144 | OSA, ASCVD | ✘ | ✘ |
K0, serial number; K1, studies; K2, year; K3, references; K4, comorbidities; K5, body mass index; K6, ethnicity; K7, electrocardiograph; K8, waist circumference; K9, polysomnography; K10, electromyograph; K11, oxygen saturation; K12, AI type; K13, FDA discussion; K14, clinical setting; K15, risk of bias; K16, classifier; K17, accuracy of the model; DM, diabetes mellitus; ASCVD, cardiovascular disease; HTN, hypertension; DL, deep learning; ML, machine learning; OSA, obstructive sleep apnea; DT, decision tree; RF, random forest; CNN, convolutional neural network; KNN, K-Nearest neighbors; XGB, XGBoost (Extreme Gradient Boosting); , included; ✘, exculded.
Notably, only six studies [169, 172, 173, 174, 175, 177] discussed FDA regulations, which are crucial for product design and clinical application. The risk of bias was not discussed in any study except the proposed one, highlighting a gap in addressing potential biases in the research methodologies.
6.3 Strengths, Weakness, and Extensions
The primary strength of this review lies in its introduction of ASCVD risk stratification, which was specifically tailored for patients with OSA. This review highlights the complex biological and morphological interactions between OSA and ASCVD, forming the basis of our first hypothesis. We propose a DL solution for the dual tasks of OSA detection and ASCVD risk prediction. Our system is a cascaded framework that integrates the computation of OSA risk labels, CUSIP segmentation, and ASCVD risk stratification using LSTM models.
Despite the simplicity of the proposed system, there are important considerations for its optimization. One key aspect is minimizing the risk of bias, which is critical for ensuring the reliability and validity of the DL models. Additionally, the system needs to be generalized to account for various comorbidities often coexisting with OSA, thereby improving its robustness and applicability across diverse patient populations. This necessitates rigorous testing and validation across different datasets and clinical scenarios.
To further enhance the performance and accuracy of the system, we suggest extending the design to incorporate ensemble-based DL systems. Ensemble methods, which combine multiple models to improve predictive performance, can help address the limitations of individual models and provide more reliable predictions. By leveraging ensemble techniques, the system can achieve higher accuracy in OSA detection and ASCVD risk stratification, ultimately leading to better clinical decision-making and patient outcomes.
7. Conclusions
This review presented three key hypotheses that form the foundation of its analysis. First, it explored the biological link between OSA and ASCVD, highlighting the complex interplay of factors contributing to both conditions. Second, it proposed that incorporating OSA risk into existing models could significantly improve the stratification of ASCVD risk, suggesting that understanding and quantifying OSA can provide valuable insights into cardiovascular health. Third, it examined the capability of DL to manage the intricate relationships involved due to its sophisticated layers and superior feature extraction methods.
A clear and detailed connection was established between OSA and atherosclerotic disease, particularly in critical areas such as the carotid arteries, coronary arteries, and aorta. This review highlighted the efficacy of DL models in detecting OSA, utilizing this detection as a critical biomarker. This biomarker was combined with other office-based and laboratory-based biomarkers, carotid ultrasound imaging phenotypes, and statin usage data to create a comprehensive ASCVD risk stratification model. This multi-faceted approach aimed to provide a more accurate and personalized risk assessment for patients with OSA.
This review also delved into several significant challenges that need to be addressed to enhance the application of AI in this context. These challenges include AI bias, which can skew results and reduce the reliability of predictions; AI explainability, which is crucial for gaining clinical trust and understanding the decision-making process of DL models; AI pruning, which involves reducing the complexity of models to make them more efficient without sacrificing accuracy. Additionally, this review proposed a cloud-based cascaded system designed to provide a personalized approach to ASCVD risk stratification, leveraging the scalability and accessibility of cloud computing to implement these advanced AI methods effectively.
Acknowledgment
Not applicable.
Abbreviations
ACC, American College of Cardiology; ARDS, acute respiratory distress syndrome; ASCVD, atherosclerotic cardiovascular disease; ANS, autonomic nervous system; AUC, area-under-the-curve; AI, artificial intelligence; BMI, body mass index; CAD, coronary artery disease; CAS, coronary artery syndrome; CHD, coronary heart disease; CT, computed tomography; CUSIP, carotid ultrasound image phenotype; CV, cross-validation; CVD, cardiovascular disease; CVE, cardiovascular events; CNN, convolution neural network; DL, deep learning; DM, diabetes mellitus; EEGS, event-equivalent gold standard; EMG, electromyography; EC, endothelial cell; GT, ground truth; HTN, hypertension; HDL, hybrid deep learning; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; LBBM, lab base bio markers; MRI, magnetic resonance imaging; NR, not reported; NPV, negative predictive value; NB, naive bayes; NO, nitric oxide; nOH, neurogenic orthostatic hypotension; Non-ML, non-machine learning; OBBM, office base bio markers; OH, orthostatic hypotension; OxLDL, oxidation of low-density lipoprotein; OSA, obstructive sleep apnea; PE, performance evaluation; PPV, positive predictive value; PCA, principal component analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PTC, plaque tissue characterization; RA, rheumatoid arthritis; PR, the period measured in milliseconds; RF, random forest; ROS, reactive oxides stress; RoB, risk of bias; ROC, receiver operating-characteristics; RNN, recurrent neural network; SCORE, systematic coronary risk evaluation; SMOTE, synthetic minority over-sampling technique; SVM, support vector machine; SAA, sleep apnea association; TPA, total plaque area; TC, tissue characterization; US, ultrasound.
Appendix
See Appendix Table 4.
Table 4.
DL-based OSA studies use ECG alongside risk factors and GT as input modalities.
| SN | Studies | Year | REF | DS | MOD | IC | Risk factors | GT | ||||||
| R1 | R2 | R3 | R4 | R5 | R6 | R7 | ||||||||
| 1 | Anitha et al. [85] | 2021 | 31 | 285 | LBBM, OBBM | ECG | ✘ | ✘ | ✘ | ✘ | ✘ | OSA, non-OSA | ||
| 2 | Kim et al. [93] | 2021 | 45 | 279 | LBBM, OBBM | ECG | ✘ | ✘ | ✘ | ✘ | OSA, non-OSA | |||
| 3 | Ma et al. [94] | 2021 | 71 | 2277 | LBBM, OBBM | ECG | OSA, non-OSA | |||||||
| 4 | Panindre et al. [95] | 2021 | 169 | 30 | LBBM, OBBM | ECG | ✘ | OSA, non-OSA | ||||||
| 5 | Brink-Kjaer et al. [33] | 2022 | 77 | 2500 | LBBM, OBBM | ECG | ✘ | ✘ | ✘ | ✘ | ✘ | ✘ | SA, life expectancy | |
| 6 | Almutairi et al. [90] | 2021 | 71 | 70 | LBBM, OBBM | ECG | ✘ | ✘ | ✘ | ✘ | ✘ | OSA, non-OSA | ||
| 7 | Tsai et al. [96] | 2022 | 69 | 10,391 | LBBM, OBBM | ECG | ✘ | ✘ | ✘ | OSA, non-OSA | ||||
| 8 | Ramesh et al. [89] | 2021 | 80 | 1500 | LBBM, OBBM | ECG | - | ✘ | ✘ | OSA, non-OSA | ||||
| 9 | Gourishetti et al. [20] | 2022 | 39 | 6814 | LBBM, OBBM | ECG | OSA, ASCVD | |||||||
| 10 | Samadi et al. [97] | 2022 | 33 | 18 | LBBM, OBBM | ECG | OSA, non-OSA | |||||||
| 11 | Tasmi et al. [21] | 2022 | 43 | 136 | LBBM, OBBM | ECG | SA, mortality | |||||||
| 12 | H. Liu et al. [98] | 2023 | 36 | 70 | LBBM, OBBM | ECG | ✘ | ✘ | SA, mortality | |||||
REF, references in the respective articles; DS, data size; IC, input covariates; MOD, modality; LBBM, lab base bio markers; OBBM, office base bio markers; ECG, electrocardiograph; GT, ground truth; R1, body mass index; R2, hypertension; R3, electrocardiogram; R4, waist circumference; R5, polysomnography; R6, electromyography; R7, oxygen saturation; DL, deep learning; OSA, obstructive sleep apnea; SN, serial number; SA, sleep apnea; ASCVD, atherosclerotic cardiovascular disease.
Footnotes
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author Contributions
MM, LS: Design of the manuscript, proofreading many iterations, researching PubMed and other research sites for article search; JSS: Design of manuscript, validation, proof reading; AJ, NNK, AP, MM, GF, JSS: Resources, imaging contribution and proofreading of the manuscript, design of the manuscript; MM, AJ, ET, EI, MMF: Design of the OSA and CVD component of the manuscript, proofreading many iterations, researching PubMed and other research sites for article search, design of the manuscript; JSS, NNK, GF, MKK: Proofreading and guidance of cardiology components of the manuscript, design of the manuscript; JSS, LS, MM, NNK: The vision of cardiac risk assessment and proofreading the manuscript, final approval of the manuscript, design of the manuscript; MKK, LS, MM: Design and support of radiology components such as CT and carotid ultrasound, design of the manuscript; GF, EI, AJ, MMF: Proofreading and guidance of cardiology imaging components of the manuscript, design of the manuscript; EI, AJ, MMF, ET: Proofreading and guidance of cardiology and OSA components, design of the manuscript; MM, LS, JSS: Design and solid proofreading of the manuscript, especially the imaging component, revising it critically for important intellectual content, and final approval of the manuscript; MKK, JSS: OSA and proofreading of the manuscript, design of the manuscript; JSS: Principal investigator-design, proofreading of the manuscript and management. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest. Jasjit S. Suri is with AtheroPoint™ LLC (Roseville, CA, USA), which does cardiovascular and stroke imaging. Luca Saba and Jasjit S. Suri are serving as Guest Editor of this journal. We declare that Luca Saba and Jasjit S. Suri had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Takatoshi Kasai.
References
- [1].Gunnarsson SI, Peppard PE, Korcarz CE, Barnet JH, Aeschlimann SE, Hagen EW, et al. Obstructive sleep apnea is asso-ciated with future subclinical carotid artery disease: thirteen-year follow-up from the Wisconsin sleep cohort. Arteriosclerosis, Thrombosis, and Vascular Biology . 2014;34:2338–2342. doi: 10.1161/ATVBAHA.114.303965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smith DF, Schuler CL, Hossain MM, Huang G, McConnell K, Urbina EM, et al. Early Atherosclerotic Inflammatory Pathways in Children with Obstructive Sleep Apnea. The Journal of Pediatrics . 2021;239:168–174. doi: 10.1016/j.jpeds.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation . 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- [4].Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. Journal of the American College of Cardiology . 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Respiratory Medicine . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang X, Fan J, Guo R, Hao W, Gong W, Yan Y, et al. Association of obstructive sleep apnoea with cardiovascular events in women and men with acute coronary syndrome. The European Respiratory Journal . 2023;61:2201110. doi: 10.1183/13993003.01110-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wojeck BS, Inzucchi SE, Neeland IJ, Mancuso JP, Frederich R, Masiukiewicz U, et al. Ertugliflozin and incident obstruc-tive sleep apnea: an analysis from the VERTIS CV trial. Sleep & Breathing = Schlaf & Atmung . 2023;27:669–672. doi: 10.1007/s11325-022-02594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leong WB, Arora T, Jenkinson D, Thomas A, Punamiya V, Banerjee D, et al. The prevalence and severity of obstructive sleep apnea in severe obesity: the impact of ethnicity. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2013;9:853–858. doi: 10.5664/jcsm.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest . 2012;141:674–681. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- [10].Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, et al. Hypertension and cardiac arrhythmias: executive summary of a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE) Cardiovascular Pharmacotherapy . 2017;3:235–250. doi: 10.1093/ehjcvp/pvx019. European Heart Journal. [DOI] [PubMed] [Google Scholar]
- [11].Fuat A, Adlen E, Monane M, Coll R, Groves S, Little E, et al. A polygenic risk score added to a QRISK® 2 cardiovascular disease risk calculator demonstrated robust clinical acceptance and clinical utility in the primary care setting. European Journal of Preventive Cardiology . 2024;31:716–722. doi: 10.1093/eurjpc/zwae004. [DOI] [PubMed] [Google Scholar]
- [12].Moridian P, Shoeibi A, Khodatars M, Jafari M, Pachori RB, Khadem A, et al. Automatic diagnosis of sleep apnea from biomedical signals using artificial intelligence techniques: Methods, challenges, and future works. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery . 2022;12:e1478. doi: 10.1002/widm.1478. [DOI] [Google Scholar]
- [13].de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, et al. Multimodality Strategy for Cardio-vascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation . 2017;135:2119–2132. doi: 10.1161/CIRCULATIONAHA.117.027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kilty H L, Prentice D. Early identification of cardiovascular risk factors in adolescents and follow-up intervention strategies. Car-diovascular Risk Factors. Ed. AY Gasparyan . 2012:17–60. doi: 10.5772/33089. [DOI] [Google Scholar]
- [15].Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ (Clinical Research Ed.) . 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Valo M, Wons A, Moeller A, Teupe C. Markers of Myocardial Ischemia in Patients With Coronary Artery Disease and Obstruc-tive Sleep Apnea: Effect of Continuous Positive Airway Pressure Therapy. Clinical Cardiology . 2015;38:462–468. doi: 10.1002/clc.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu M, Wang Z, Zhan X, Wei Y. Obstructive sleep apnea increases the risk of cardiovascular damage: a systematic review and meta-analysis of imaging studies. Systematic Reviews . 2021;10:212. doi: 10.1186/s13643-021-01759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chaw H T, Kamolphiwong S, Wongsritrang K. Sleep apnea detection using deep learning. Tehnički Glasnik . 2019;13:261–266. doi: 10.31803/tg-20191104191722. [DOI] [Google Scholar]
- [19].Vattamthanam S, Mrudula GB, Kumar CS. Sleep apnea classification using deep neural network. In 2020 IEEE International Conference on Distributed Computing, VLSI, Electrical Circuits and Robotics (DISCOVER) . 2020:133–136. doi: 10.1109/DISCOVER50404.2020.9278045. [DOI] [Google Scholar]
- [20].Gourishetti SC, Taylor R, Isaiah A. Stratifying the Risk of Cardiovascular Disease in Obstructive Sleep Apnea Using Machine Learning. The Laryngoscope . 2022;132:234–241. doi: 10.1002/lary.29852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tasmi ST, Raihan M MS, Shams AB. Obstructive Sleep Apnea (OSA) and COVID-19: Mortality Prediction of COVID-19-Infected Patients with OSA Using Machine Learning Approaches. COVID . 2022;2:877–894. doi: 10.3390/covid2070064. [DOI] [Google Scholar]
- [22].Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiologica Scandinavica . 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- [23].Hui DS, Shang Q, Ko FW, Ng SS, Szeto CC, Ngai J, et al. A prospective cohort study of the long-term effects of CPAP on carotid artery intima-media thickness in obstructive sleep apnea syndrome. Respiratory Research . 2012;13:22. doi: 10.1186/1465-9921-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nadeem R, Harvey M, Singh M, Khan AA, Albustani M, Baessler A, et al. Patients with obstructive sleep apnea display increased carotid intima media: a meta-analysis. International Journal of Vascular Medicine . 2013;2013:839582. doi: 10.1155/2013/839582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hesamian MH, Jia W, He X, Kennedy P. Deep Learning Techniques for Medical Image Segmentation: Achievements and Chal-lenges. Journal of Digital Imaging . 2019;32:582–596. doi: 10.1007/s10278-019-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Munjral S, Maindarkar M, Ahluwalia P, Puvvula A, Jamthikar A, Jujaray T, et al. Cardiovascular Risk Stratification in Diabetic Retinopathy via Atherosclerotic Pathway in COVID-19/Non-COVID-19 Frameworks Using Artificial Intelligence Paradigm: A Narrative Review. Diagnostics (Basel, Switzerland) . 2022;12:1234. doi: 10.3390/diagnostics12051234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Khanna NN, Maindarkar M, Saxena A, Ahluwalia P, Paul S, Srivastava SK, et al. Cardiovascular/Stroke Risk Assess-ment in Patients with Erectile Dysfunction-A Role of Carotid Wall Arterial Imaging and Plaque Tissue Characterization Using Artificial Intelligence Paradigm: A Narrative Review. Diagnostics (Basel, Switzerland) . 2022;12:1249. doi: 10.3390/diagnostics12051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Makimoto H, Kohro T. Adopting artificial intelligence in cardiovas-cular medicine: A scoping review. Hypertension Research . 2024;47:685–699. doi: 10.1038/s41440-023-01469-7. [DOI] [PubMed] [Google Scholar]
- [29].Amato M, Montorsi P, Ravani A, Oldani E, Galli S, Ravagnani PM, et al. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultra-sound findings. European Heart Journal . 2007;28:2094–2101. doi: 10.1093/eurheartj/ehm244. [DOI] [PubMed] [Google Scholar]
- [30].Khanna NN, Maindarkar M, Puvvula A, Paul S, Bhagawati M, Ahluwalia P, et al. Vascular Implications of COVID-19: Role of Radiological Imaging, Artificial Intelligence, and Tissue Characterization: A Special Report. Journal of Cardiovascular Develop-ment and Disease . 2022;9:268. doi: 10.3390/jcdd9080268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Suri JS, Agarwal S, Chabert GL, Carriero A, Paschè A, Danna PSC, et al. COVLIAS 2.0-cXAI: Cloud-Based Explaina-ble Deep Learning System for COVID-19 Lesion Localization in Computed Tomography Scans. Diagnostics (Basel, Switzerland) . 2022;12:1482. doi: 10.3390/diagnostics12061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kuanr M, Mohapatra P, Mittal S, Maindarkar M, Fauda MM, Saba L, et al. Recommender System for the Efficient Treatment of COVID-19 Using a Convolutional Neural Network Model and Image Similarity. Diagnostics (Basel, Switzerland) . 2022;12:2700. doi: 10.3390/diagnostics12112700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brink-Kjaer A, Leary EB, Sun H, Westover MB, Stone KL, Peppard PE, et al. Age estimation from sleep studies using deep learning predicts life expectancy. NPJ Digital Medicine . 2022;5:103. doi: 10.1038/s41746-022-00630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Butt M, Dwivedi G, Khair O, Lip GYH. Obstructive sleep apnea and cardiovascular disease. International Journal of Cardiology . 2010;139:7–16. doi: 10.1016/j.ijcard.2009.05.021. [DOI] [PubMed] [Google Scholar]
- [35].Arias MA, Sánchez AM. Obstructive sleep apnea and its relationship to cardiac arrhythmias. Journal of Cardiovascular Electro-physiology . 2007;18:1006–1014. doi: 10.1111/j.1540-8167.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- [36].Nami M, Mehrabi S, Derman S. Employing Neural Network Methods to Label Sleep EEG Micro-Arousals in Obstructive Sleep Apnea Syndrome. Journal of Advanced Medical Sciences Applied Technologies . 2017;3:221–226. doi: 10.32598/jamsat.3.4.221. [DOI] [Google Scholar]
- [37].Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckland, N.Z.) . 2016;4:99–108. doi: 10.2147/HP.S103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Regev D, Etzion S, Haddad H, Gopas J, Goldbart A. Obstructive Sleep Apnea Syndrome In Vitro Model: Controlled Intermittent Hypoxia Stimulation of Human Stem Cells-Derived Cardiomyocytes. International Journal of Molecular Sciences . 2022;23:10272. doi: 10.3390/ijms231810272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Allen AJH, Peres BU, Liu Y, Jen R, Shah A, Laher I, et al. Circulating markers of oxidative stress and risk of incident cardiovascular events in obstructive sleep apnea. Sleep and Biological Rhythms . 2022;20:533–540. doi: 10.1007/s41105-022-00399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wohlrab P, Johann Danhofer M, Schaubmayr W, Tiboldi A, Krenn K, Markstaller K, et al. Oxygen conditions oscillating between hypoxia and hyperoxia induce different effects in the pulmonary endothelium compared to constant oxygen conditions. Physio-logical Reports . 2021;9:e14590. doi: 10.14814/phy2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ma L, Zhang J, Liu Y. Roles and Mechanisms of Obstructive Sleep Apnea-Hypopnea Syndrome and Chronic Intermittent Hy-poxia in Atherosclerosis: Evidence and Prospective. Oxidative Medicine and Cellular Longevity . 2016;2016:8215082. doi: 10.1155/2016/8215082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, et al. Mechanisms used to restore ventilation after par-tial upper airway collapse during sleep in humans. Thorax . 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matsuura E, Hughes GRV, Khamashta MA. Oxidation of LDL and its clinical implication. Autoimmunity Reviews . 2008;7:558–566. doi: 10.1016/j.autrev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [44].Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, et al. Chronic intermittent hypoxia induces ather-osclerosis. American Journal of Respiratory and Critical Care Medicine . 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Imamura T, Poulsen O, Haddad GG. Intermittent hypoxia induces murine macrophage foam cell formation by IKK-β-dependent NF-κB pathway activation. Journal of Applied Physiology (Bethesda, Md . 2016;121:670–677. doi: 10.1152/japplphysiol.00307.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bekkering S, Quintin J, Joosten LAB, van der Meer JWM, Netea MG, Riksen NP. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arteriosclerosis, Thrombosis, and Vascular Biology . 2014;34:1731–1738. doi: 10.1161/ATVBAHA.114.303887. [DOI] [PubMed] [Google Scholar]
- [47].Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, et al. The role of lipids and lipoproteins in ath-erosclerosis. Endotext . 2019 [Google Scholar]
- [48].Song SO, He K, Narla RR, Kang HG, Ryu HU, Boyko EJ. Metabolic Consequences of Obstructive Sleep Apnea Especially Pertaining to Diabetes Mellitus and Insulin Sensitivity. Diabetes & Metabolism Journal . 2019;43:144–155. doi: 10.4093/dmj.2018.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shechter A, Grandner MA, St-Onge MP. The Role of Sleep in the Control of Food Intake. American Journal of Lifestyle Medi-cine . 2014;8:371–374. doi: 10.1177/1559827614545315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kjeldsen SE, Rostrup M, Moan A, Mundal HH, Gjesdal K, Eide IK. The sympathetic nervous system may modulate the meta-bolic cardiovascular syndrome in essential hypertension. Journal of Cardiovascular Pharmacology . 1992;20:S32–S39. [PubMed] [Google Scholar]
- [51].Dimsdale JE, Coy T, Ziegler MG, Ancoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep . 1995;18:377–381. [PubMed] [Google Scholar]
- [52].Driessen S, Francque SM, Anker SD, Castro Cabezas M, Grobbee DE, Tushuizen ME, et al. Metabolic dysfunc-tion-associated steatotic liver disease and the heart. Hepatology (Baltimore, Md.) . 2023 doi: 10.1097/HEP.0000000000000735. [DOI] [PubMed] [Google Scholar]
- [53].Tomas L. Lund University, Faculty of Medicine Doctoral Dis-sertation Series . 2019. Immunometabolic and Cellular Traits in Cardiovascular Disease; p. 94. [Google Scholar]
- [54].Song F, Zou J, Song Z, Xu H, Qian Y, Zhu H, et al. Association of Adipocytokines With Carotid Intima Media Thick-ness and Arterial Stiffness in Obstructive Sleep Apnea Patients. Frontiers in Endocrinology . 2020;11:177. doi: 10.3389/fendo.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules (Basel, Switzerland) . 2014;19:1651–1662. doi: 10.3390/molecules19021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Suzuki M, Shimamoto K, Tatsumi F, Tsuji T, Satoya N, Inoue Y, et al. Long-term outcomes regarding arterial stiffness and carotid artery atherosclerosis in female patients with rapid eye movement obstructive sleep apnea. The Journal of International Medical Research . 2022;50:3000605221121941. doi: 10.1177/03000605221121941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The Association Between Obstructive Sleep Apnea and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Angiology . 2017;68:575–583. doi: 10.1177/0003319716665985. [DOI] [PubMed] [Google Scholar]
- [58].Bandi PS, Panigrahy PK, Hajeebu S, Ngembus NJ, Heindl SE. Pathophysiological Mechanisms to Review Association of Atrial Fibrillation in Heart Failure With Obstructive Sleep Apnea. Cureus . 2021;13:e16086. doi: 10.7759/cureus.16086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Firincioglulari M, Aksoy S, Orhan K, Rasmussen F. Comparison of Intracranial and Extracranial Carotid Artery Calcifications between Obstructive Sleep Apnea Patients and Healthy Individuals: A Combined Cone-Beam Computed Tomography and Poly-somnographic Study. Radiology Research and Practice . 2022:1625779. doi: 10.1155/2022/1625779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension (Dallas, Tex.: 1979) . 2009;53:64–69. doi: 10.1161/HYPERTENSIONAHA.108.119420. [DOI] [PubMed] [Google Scholar]
- [61].Friedlander AH, Friedlander IK, Yueh R, Littner MR. The prevalence of carotid atheromas seen on panoramic radiographs of patients with obstructive sleep apnea and their relation to risk factors for atherosclerosis. Journal of Oral and Maxillofacial Surgery: Offi-cial Journal of the American Association of Oral and Maxillofacial Surgeons . 1999;57:516–516. doi: 10.1016/s0278-2391(99)90065-4. [DOI] [PubMed] [Google Scholar]
- [62].Nielsen S, Nyvad J, Christensen KL, Poulsen PL, Laugesen E, Grove EL, et al. Obstructive sleep apnea, coronary calcification and arterial stiffness in pa-tients with diabetic kidney disease. Atherosclerosis . 2024;394:117170. doi: 10.1016/j.atherosclerosis.2023.06.076. [DOI] [PubMed] [Google Scholar]
- [63].Tan A, Hau W, Ho HH, Ghaem Maralani H, Loo G, Khoo SM, et al. OSA and coronary plaque characteristics. Chest . 2014;145:322–330. doi: 10.1378/chest.13-1163. [DOI] [PubMed] [Google Scholar]
- [64].Miller JD, Aronis KN, Chrispin J, Patil KD, Marine JE, Martin SS, et al. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. Journal of the American College of Cardiology . 2015;66:2899–2906. doi: 10.1016/j.jacc.2015.10.047. [DOI] [PubMed] [Google Scholar]
- [65].Singh P, Chopra M, Vardhan V. Detection of obstructive sleep apnea in young patients suffering from coronary artery disease by performing portable polysomnography studies. Medical Journal, Armed Forces India . 2022;78:394–399. doi: 10.1016/j.mjafi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liu Y, Wang M, Shi J. Influence of obstructive sleep apnoea on coronary artery disease in a Chinese population. The Journal of International Medical Research . 2022;50:3000605221115389. doi: 10.1177/03000605221115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tang M, Wang Y, Wang M, Tong R, Shi T. Risk for Cardiovascular Disease and One-Year Mortality in Patients With Chronic Obstructive Pulmonary Disease and Obstructive Sleep Apnea Syndrome Overlap Syndrome. Frontiers in Pharmacology . 2021;12:767982. doi: 10.3389/fphar.2021.767982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lüthje L, Andreas S. Obstructive sleep apnea and coronary artery disease. Sleep Medicine Reviews . 2008;12:19–31. doi: 10.1016/j.smrv.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [69].Cohen O, Sánchez-de-la-Torre M, Al-Taie Z, Khan S, Kundel V, Kovacic JC, et al. Heterogeneous Effects of Continuous Positive Airway Pressure in Non-Sleepy Obstructive Sleep Apnea on Cardiovascular Disease Outcomes: Post Hoc Machine Learning Analysis of the ISAACC Trial (ECSACT Study) Annals of the American Thoracic Society . 2024;21:1074–1084. doi: 10.1513/AnnalsATS.202309-799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Al-Maini M, Maindarkar M, Kitas GD, Khanna NN, Misra DP, Johri AM, et al. Artificial intelligence-based preventive, personalized and precision medicine for cardiovascular disease/stroke risk assessment in rheumatoid arthritis patients: a narrative review. Rheumatology International . 2023;43:1965–1982. doi: 10.1007/s00296-023-05415-1. [DOI] [PubMed] [Google Scholar]
- [71].Winston PH. Artificial intelligence . Addison-Wesley Longman Publishing Co., Inc.: USA; 1992. [Google Scholar]
- [72].Ramesh AN, Kambhampati C, Monson JRT, Drew PJ. Artificial intelligence in medicine. Annals of the Royal College of Sur-geons of England . 2004;86:334–338. doi: 10.1308/147870804290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Biswas M, Kuppili V, Saba L, Edla DR, Suri HS, Cuadrado-Godia E, et al. State-of-the-art review on deep learning in medical imaging. Frontiers in Bioscience (Landmark Edition) . 2019;24:392–426. doi: 10.2741/4725. [DOI] [PubMed] [Google Scholar]
- [74].Jain PK, Sharma N, Kalra MK, Viskovic K, Saba L, et al. Four Types of Multiclass Frameworks for Pneumo-nia Classification and Its Validation in X-ray Scans Using Seven Types of Deep Learning Artificial Intelligence Models. Diagnostics (Ba-sel, Switzerland) . 2022;12:652. doi: 10.3390/diagnostics12030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Khandoker AH, Palaniswami M, Karmakar CK. Support vector machines for automated recognition of obstructive sleep apnea syndrome from ECG recordings. IEEE Transactions on Information Technology in Biomedicine: a Publication of the IEEE Engineering in Medicine and Biology Society . 2009;13:37–48. doi: 10.1109/TITB.2008.2004495. [DOI] [PubMed] [Google Scholar]
- [76].Sun LM, Chiu HW, Chuang CY, Liu L. A prediction model based on an artificial intelligence system for moderate to severe ob-structive sleep apnea. Sleep & Breathing = Schlaf & Atmung . 2011;15:317–323. doi: 10.1007/s11325-010-0384-x. [DOI] [PubMed] [Google Scholar]
- [77].Li K, Pan W, Li Y, Jiang Q, Liu G. A method to detect sleep apnea based on deep neural network and hidden Markov model using single-lead ECG signal. Neurocomputing . 2018;294:94–101. doi: 10.1016/j.neucom.2018.03.011. [DOI] [Google Scholar]
- [78].Hafezi M, Montazeri N, Zhu K, Alshaer H, Yadollahi A, Taati B. Sleep Apnea Severity Estimation from Respiratory Related Movements Using Deep Learning. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE En-gineering in Medicine and Biology Society. Annual International Conference . 2019:1601–1604. doi: 10.1109/EMBC.2019.8857524. IEEE En-gineering in Medicine and Biology Society. [DOI] [PubMed] [Google Scholar]
- [79].Jamthikar AD, Gupta D, Johri AM, Mantella LE, Saba L, Kolluri R, et al. Low-Cost Office-Based Cardiovascular Risk Stratification Using Machine Learning and Focused Carotid Ultrasound in an Asian-Indian Cohort. Journal of Medical Systems . 2020;44:208. doi: 10.1007/s10916-020-01675-7. [DOI] [PubMed] [Google Scholar]
- [80].Aggarwal Y, Das J, Mazumder PM, Kumar R, Sinha RK. Heart rate variability features from nonlinear cardiac dynamics in iden-tification of diabetes using artificial neural network and support vector machine. Biocybernetics and Biomedical Engineering . 2020;40:1002–1009. doi: 10.1016/j.bbe.2020.05.001. [DOI] [Google Scholar]
- [81].Skandha SS, Gupta SK, Saba L, Koppula VK, Johri AM, Khanna NN, et al. 3-D optimized classification and characteri-zation artificial intelligence paradigm for cardiovascular/stroke risk stratification using carotid ultrasound-based delineated plaque: Athero-matic™ 2.0. Computers in Biology and Medicine . 2020;125:103958. doi: 10.1016/j.compbiomed.2020.103958. [DOI] [PubMed] [Google Scholar]
- [82].Fourcade A, Khonsari RH. Deep learning in medical image analysis: A third eye for doctors. Journal of Stomatology, Oral and Maxillofacial Surgery . 2019;120:279–288. doi: 10.1016/j.jormas.2019.06.002. [DOI] [PubMed] [Google Scholar]
- [83].Suri JS, Agarwal S, Gupta S, Puvvula A, Viskovic K, Suri N, et al. Systematic Review of Artificial Intelligence in Acute Respiratory Distress Syndrome for COVID-19 Lung Patients: A Biomedical Imaging Perspective. IEEE Journal of Biomedical and Health Informatics . 2021;25:4128–4139. doi: 10.1109/JBHI.2021.3103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tsinalis O, Matthews PM, Guo Y. Automatic Sleep Stage Scoring Using Time-Frequency Analysis and Stacked Sparse Autoen-coders. Annals of Biomedical Engineering . 2016;44:1587–1597. doi: 10.1007/s10439-015-1444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Anitha J, Hemanth DJ. An automatic screening approach for obstructive sleep apnea from photoplethysmograph using machine learning techniques. TELKOMNIKA (Telecommunication Computing Electronics and Control) . 2021;19:1260–1272. doi: 10.12928/telkomnika.v19i4.19371. [DOI] [Google Scholar]
- [86].Faust O, Acharya UR, Ng EYK, Fujita H. A review of ECG-based diagnosis support systems for obstructive sleep apnea. Jour-nal of Mechanics in Medicine Biology . 2016;16:1640004. doi: 10.1142/S0219519416400042. [DOI] [Google Scholar]
- [87].Mishra J, Tiwari M. IoT-enabled ECG-based heart disease prediction using three-layer deep learning and meta-heuristic approach. Signal, Image and Video Processing . 2024;18:361–367. doi: 10.1007/s11760-023-02743-4. [DOI] [Google Scholar]
- [88].Schulz H. Rethinking sleep analysis: Comment on the AASM manual for the scoring of sleep and associated events. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine . 2008;4:99–103. [PMC free article] [PubMed] [Google Scholar]
- [89].Ramesh J, Keeran N, Sagahyroon A, Aloul F. Towards Validating the Effectiveness of Obstructive Sleep Apnea Classification from Electronic Health Records Using Machine Learning. Healthcare (Basel, Switzerland) . 2021;9:1450. doi: 10.3390/healthcare9111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Almutairi H, Hassan GM, Datta A. Classification of Obstructive Sleep Apnoea from single-lead ECG signals using convolutional neural and Long Short Term Memory networks. Biomedical Signal Processing and Control . 2021;69:102906. doi: 10.1016/j.bspc.2021.102906. [DOI] [Google Scholar]
- [91].Singh N, Talwekar RH. Journal of Physics: Conference Series . Vol. 2273. IOP Publishing; 1742. Comparison of machine learning and deep learning classifier to detect sleep apnea using single-channel ECG and HRV: A Systematic Literature Review; p. 012015. [DOI] [Google Scholar]
- [92].Locharla GR, Sheela VK, Lakshmi S, Tiwari T. Review of deep learning based methods for sleep apnea detection. Hindawi . 2021;12:123. doi: 10.53730/ijhs.v6nS2.6556. [DOI] [Google Scholar]
- [93].Kim YJ, Jeon JS, Cho SE, Kim KG, Kang SG. Prediction Models for Obstructive Sleep Apnea in Korean Adults Using Ma-chine Learning Techniques. Diagnostics (Basel, Switzerland) . 2021;11:612. doi: 10.3390/diagnostics11040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ma EY, Kim JW, Lee Y, Cho SW, Kim H, Kim JK. Combined unsupervised-supervised machine learning for phenotyping complex diseases with its application to obstructive sleep apnea. Scientific Reports . 2021;11:4457. doi: 10.1038/s41598-021-84003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Panindre P, Gandhi V, Kumar S. In 2021 11th International Conference on Cloud Computing, Data Science & Engineering (Confluence) (pp . IEEE; 2021. Artificial Intelligence-based Remote Diagnosis of Sleep Apnea using Instantaneous Heart Rates; pp. 169–174. [DOI] [Google Scholar]
- [96].Tsai CY, Liu WT, Lin YT, Lin SY, Houghton R, Hsu WH, et al. Machine learning approaches for screening the risk of obstructive sleep apnea in the Taiwan population based on body profile. Informatics for Health & Social Care . 2022;47:373–388. doi: 10.1080/17538157.2021.2007930. [DOI] [PubMed] [Google Scholar]
- [97].Samadi B, Samadi S, Samadi M, Samadi S, Samadi M, Mohammadi M. Systematic Review of Detecting Sleep Apnea Using Artificial Intelligence: An Insight to Convolutional Neural Network Method. Archives of Neuroscience . 2024;11. doi: 10.5812/ans-144058. [DOI] [Google Scholar]
- [98].Liu H, Cui S, Zhao X, Cong F. Detection of obstructive sleep apnea from single-channel ECG signals using a CNN-transformer architecture. Biomedical Signal Processing and Control . 2023;82:104581. doi: 10.1016/j.bspc.2023.104581. [DOI] [Google Scholar]
- [99].Suri JS, Puvvula A, Majhail M, Biswas M, Jamthikar AD, Saba L, et al. Integration of cardiovascular risk assessment with COVID-19 using artificial intelligence. Reviews in Cardiovascular Medicine . 2020;21:541–560. doi: 10.31083/j.rcm.2020.04.236. [DOI] [PubMed] [Google Scholar]
- [100].Suri JS, Bhagawati M, Paul S, Protogeron A, Sfikakis PP, Kitas GD, et al. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Computers in Biology and Medicine . 2022;142:105204. doi: 10.1016/j.compbiomed.2021.105204. [DOI] [PubMed] [Google Scholar]
- [101].Banchhor SK, Araki T, Londhe ND, Ikeda N, Radeva P, Elbaz A, et al. Five multiresolution-based calcium volume measurement techniques from coronary IVUS videos: A comparative approach. Computer Methods and Programs in Biomedicine . 2016;134:237–258. doi: 10.1016/j.cmpb.2016.07.009. [DOI] [PubMed] [Google Scholar]
- [102].Nannini G, Saitta S, Mariani L, Maragna R, Baggiano A, Mushtaq S, et al. An au-tomated and time-efficient framework for simulation of coronary blood flow under steady and pulsatile condi-tions. Computer Methods and Programs in Biomedicine . 2024;257:108415. doi: 10.1016/j.cmpb.2024.108415. [DOI] [PubMed] [Google Scholar]
- [103].Ferrari M, Urtis M, Giuliani L, Lionetti A, Bortolotto C, Prati F, et al. 2024 IEEE International Symposium on Medical Measurements and Applica-tions (MeMeA).IEEE. 2024. CATE (Coronary Artery Tortuosity Evaluator): A Semi-Automatic Tool for Quantitative Assessment of Coronary Artery Tortuosity from CT Angiography; pp. 1–6. [Google Scholar]
- [104].Nannini G, Saitta S, Baggiano A, Maragna R, Mushtaq S, Pontone G, et al. A fully automated deep learning approach for coronary artery segmentation and comprehensive characterization. APL Bioengineering . 2024;8:016103. doi: 10.1063/5.0181281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Goehring T, Keshavarzi M, Carlyon RP, Moore BCJ. Using recurrent neural networks to improve the perception of speech in non-stationary noise by people with cochlear implants. The Journal of the Acoustical Society of America . 2019;146:705. doi: 10.1121/1.5119226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Coto-Jiménez M. Improving Post-Filtering of Artificial Speech Using Pre-Trained LSTM Neural Networks. Biomimetics (Basel, Switzerland) . 2019;4:39. doi: 10.3390/biomimetics4020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Graves A, Liwicki M, Fernández S, Bertolami R, Bunke H, Schmidhuber J. A novel connectionist system for unconstrained handwriting recognition. IEEE Transactions on Pattern Analysis and Machine Intelligence . 2009;31:855–868. doi: 10.1109/TPAMI.2008.137. [DOI] [PubMed] [Google Scholar]
- [108].Drzazga J, Cyganek B. An LSTM Network for Apnea and Hypopnea Episodes Detection in Respiratory Signals. Sensors (Basel, Switzerland) . 2021;21:5858. doi: 10.3390/s21175858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Abd El Aal HA, Taie SA, El-Bendary N. An optimized RNN-LSTM approach for parkinson’s disease early detection using speech features. Bulletin of Electrical Engineering and Informatics . 2021;10:2503–2512. doi: 10.11591/eei.v10i5.3128. [DOI] [Google Scholar]
- [110].Ikeda N, Gupta A, Dey N, Bose S, Shafique S, Arak T, et al. Improved correlation between carotid and coronary ather-osclerosis SYNTAX score using automated ultrasound carotid bulb plaque IMT measurement. Ultrasound in Medicine & Biology . 2015;41:1247–1262. doi: 10.1016/j.ultrasmedbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- [111].Bhagawati M, Paul S, Mantella L, Johri AM, Gupta S, Laird JR, et al. Cardiovascular Disease Risk Stratification Using Hybrid Deep Learning Paradigm: First of Its Kind on Canadian Trial Data. Diagnostics . 2024;14:1894. doi: 10.3390/diagnostics14171894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Munjral S, Ahluwalia P, Jamthikar AD, Puvvula A, Saba L, Faa G, et al. Nutrition, atherosclerosis, arterial imaging, cardiovascular risk stratification, and manifestations in COVID-19 framework: a narrative review. Frontiers in Bioscience (Landmark Edi-tion) . 2021;26:1312–1339. doi: 10.52586/5026. [DOI] [PubMed] [Google Scholar]
- [113].Sanches JM, Laine AF, Suri JS. Ultrasound imaging . Springer; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- [114].Molinari F, Liboni W, Giustetto P, Badalamenti S, Suri JS. Automatic computer-based tracings (ACT) in longitudinal 2-D ul-trasound images using different scanners. Journal of Mechanics in Medicine and Biology . 2009;9:481–505. doi: 10.1142/S0219519409003115. [DOI] [Google Scholar]
- [115].Pewowaruk RJ, Tedla Y, Korcarz CE, Tattersall MC, Stein JH, Chesler NC, et al. Carotid Artery Stiffening With Aging: Structural Versus Load-Dependent Mechanisms in MESA (the Multi-Ethnic Study of Atherosclerosis) Hypertension (Dallas, Tex.: 1979) . 2022;79:150–158. doi: 10.1161/HYPERTENSIONAHA.121.18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jain PK, Sharma N, Saba L, Paraskevas KI, Kalra MK, Johri A, et al. Unseen Artificial Intelligence-Deep Learning Paradigm for Segmentation of Low Atherosclerotic Plaque in Carotid Ultrasound: A Multicenter Cardiovascular Study. Diagnostics (Basel, Switzerland) . 2021;11:2257. doi: 10.3390/diagnostics11122257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Araki T, Banchhor SK, Londhe ND, Ikeda N, Radeva P, Shukla D, et al. Reliable and Accurate Calcium Volume Meas-urement in Coronary Artery Using Intravascular Ultrasound Videos. Journal of Medical Systems . 2016;40:51. doi: 10.1007/s10916-015-0407-z. [DOI] [PubMed] [Google Scholar]
- [118].Araki T, Ikeda N, Dey N, Chakraborty S, Saba L, Kumar D, et al. A comparative approach of four different image registration techniques for quantitative assessment of coronary artery calcium lesions using intravascular ultrasound. Computer Methods and Programs in Biomedicine . 2015;118:158–172. doi: 10.1016/j.cmpb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- [119].Narula J, Stuckey TD, Nakazawa G, Ahmadi A, Matsumura M, Petersen K, et al. Prospective deep learning–based quantitative assessment of coronary plaque by computed tomography angiography compared with intravascular ultrasound: the REVEALPLAQUE study. European Heart Journal-Cardiovascular Imaging . 2024;25:1287–1295. doi: 10.1093/ehjci/jeae115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].El-Nashar H, Sabry M, Tseng YT, Francis N, Latif N, Parker KH, et al. Multiscale structure and function of the aortic valve apparatus. Physiological Reviews . 2024;104:1487–1532. doi: 10.1152/physrev.00038.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gaipova S, Zoirxo’jayev S, Khalilov S. THORACIC AND ABDOMINAL AORTA: MAIN BRANCHES, BLOOD SUPPLY TO INTERNAL ORGANS. Центральноазиатский журнал междисциплинарных исследований и исследований в области управления . 2024;1:105–109. doi: 10.5281/zenodo.10976590. [DOI] [Google Scholar]
- [122].Saba L, Agarwal N, Cau R, Gerosa C, Sanfilippo R, Porcu M, et al. Review of imaging biomarkers for the vulnerable carotid plaque. JVS-vascular Science . 2021;2:149–158. doi: 10.1016/j.jvssci.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].He J, Gao Y, Yang C, Guo Y, Liu L, Lu S, et al. Navigating the landscape: Prospects and hurdles in targeting vascular smooth muscle cells for atherosclerosis diagnosis and therapy. Journal of Controlled Release . 2024;366:261–281. doi: 10.1016/j.jconrel.2023.12.047. [DOI] [PubMed] [Google Scholar]
- [124].Saba L, Antignani PL, Gupta A, Cau R, Paraskevas KI, Poredos P, et al. International Union of Angiology (IUA) con-sensus paper on imaging strategies in atherosclerotic carotid artery imaging: From basic strategies to advanced approaches. Atherosclerosis . 2022;354:23–40. doi: 10.1016/j.atherosclerosis.2022.06.1014. [DOI] [PubMed] [Google Scholar]
- [125].Suri JS, Bhagawati M, Paul S, Protogerou AD, Sfikakis PP, Kitas GD, et al. A Powerful Paradigm for Cardiovascular Risk Stratification Using Multiclass, Multi-Label, and Ensemble-Based Machine Learning Paradigms: A Narrative Review. Diagnostics (Basel, Switzerland) . 2022;12:722. doi: 10.3390/diagnostics12030722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Munjral S, Ahluwalia P, Jamthikar AD, Puvvula A, Saba L, Faa G, et al. Nutrition, atherosclerosis, arterial imaging, cardiovascular risk stratification, and manifestations in COVID-19 framework: a narrative review. Frontiers in Bioscience (Landmark Edi-tion) . 2021;26:1312–1339. doi: 10.52586/5026. [DOI] [PubMed] [Google Scholar]
- [127].Acharya UR, Vinitha Sree S, Mookiah MRK, Yantri R, Molinari F, Zieleźnik W, et al. Diagnosis of Hashimoto’s thy-roiditis in ultrasound using tissue characterization and pixel classification. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine . 2013;227:788–798. doi: 10.1177/0954411913483637. [DOI] [PubMed] [Google Scholar]
- [128].Patel B, Patel P. Relationship Between Serum Homocysteine and Lipid Profile, in Type II Diabetes Mellitus Patients: A Com-parative Study. Journal of Cardiovascular Disease Research . 2022;13. [Google Scholar]
- [129].Lal BK, Jreij G, Chrencik M, Clarke WM, Chamorro Á, Metzger DC, et al. Carotid plaque characteristics in the CREST-2 trial. JVS-Vascular Insights . 2024;2:100134. [Google Scholar]
- [130].Jader RF, Kareem SW, Awla HQ. Ensemble Deep Learning Technique for Detecting MRI Brain Tu-mor. Applied Computational Intelligence and Soft Computing . 2024;2024:6615468. [Google Scholar]
- [131].Aschenbach R, Steiner T, Kerl MJ, Zangos S, Basche S, Vogl TJ. Endovascular embolisation therapy in men with erectile im-potence due to veno-occlusive dysfunction. European Journal of Radiology . 2013;82:504–507. doi: 10.1016/j.ejrad.2012.10.030. [DOI] [PubMed] [Google Scholar]
- [132].Araki T, Ikeda N, Shukla D, Londhe ND, Shrivastava VK, Banchhor SK, et al. A new method for IVUS-based coro-nary artery disease risk stratification: A link between coronary & carotid ultrasound plaque burdens. Computer Methods and Programs in Biomedicine . 2016;124:161–179. doi: 10.1016/j.cmpb.2015.10.022. [DOI] [PubMed] [Google Scholar]
- [133].Scicolone R, Vacca S, Pisu F, Benson JC, Nardi V, Lanzino G, et al. Radiomics and artificial intelligence: General no-tions and applications in the carotid vulnerable plaque. European Journal of Radiology . 2024;176:111497. doi: 10.1016/j.ejrad.2024.111497. [DOI] [PubMed] [Google Scholar]
- [134].Vlachopoulos C, Aznaouridis K, Ioakeimidis N, Rokkas K, Vasiliadou C, Alexopoulos N, et al. Unfavourable endothe-lial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. European Heart Journal . 2006;27:2640–2648. doi: 10.1093/eurheartj/ehl341. [DOI] [PubMed] [Google Scholar]
- [135].Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, Montorsi P, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. European Urology . 2014;65:968–978. doi: 10.1016/j.eururo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- [136].Suri J S, Agarwal S, Jena B, Saxena S, El-Baz A, Agarwal V, et al. Five Strategies for Bias Estimation in Artificial In-telligence-based Hybrid Deep Learning for Acute Respiratory Distress Syndrome COVID-19 Lung Infected Patients using AP(ai)Bias 2.0: A Systematic Review. IEEE Transactions on Instrumentation and Measurement . 2022 doi: 10.1109/TIM.2022.3174270. [DOI] [Google Scholar]
- [137].Teji JS, Jain S, Gupta SK, Suri JS. NeoAI 1.0: Machine learning-based paradigm for prediction of neonatal and infant risk of death. Computers in Biology and Medicine . 2022;147:105639. doi: 10.1016/j.compbiomed.2022.105639. [DOI] [PubMed] [Google Scholar]
- [138].Gupta A, Kesavabhotla K, Baradaran H, Kamel H, Pandya A, Giambrone AE, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke . 2015;46:91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Biswas M, Kuppili V, Saba L, Edla DR, Suri HS, Sharma A, et al. Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: a tool for stroke risk. Medical & Biological Engineering & Computing . 2019;57:543–564. doi: 10.1007/s11517-018-1897-x. [DOI] [PubMed] [Google Scholar]
- [140].Jena B, Saxena S, Nayak GK, Balestrieri A, Gupta N, Khanna NN, et al. Brain Tumor Characterization Using Radiogenomics in Artificial Intelligence Framework. Cancers . 2022;14:4052. doi: 10.3390/cancers14164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Remzan N, Tahiry K, Farchi A. Advancing brain tumor classification accuracy through deep learning: harnessing radimagenet pre-trained convolutional neural networks, ensemble learning, and machine learning clas-sifiers on MRI brain images. Multimedia Tools and Applications . 2024;83:82719–82747. doi: 10.1007/s11042-024-18780-1. [DOI] [Google Scholar]
- [142].Khanna NN, Maindarkar MA, Viswanathan V, Fernandes JFE, Paul S, Bhagawati M, et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare (Basel, Switzerland) . 2022;10:2493. doi: 10.3390/healthcare10122493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Tandel GS, Tiwari A, Kakde OG, Gupta N, Saba L, Suri JS. Role of Ensemble Deep Learning for Brain Tumor Classification in Multiple Magnetic Resonance Imaging Sequence Data. Diagnostics (Basel, Switzerland) . 2023;13:481. doi: 10.3390/diagnostics13030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gupta S, Dubey AK, Singh R, Kalra MK, Abraham A, Kumari V, et al. Four Transformer-Based Deep Learning Clas-sifiers Embedded with an Attention U-Net-Based Lung Segmenter and Layer-Wise Relevance Propagation-Based Heatmaps for COVID-19 X-ray Scans. Diagnostics (Basel, Switzerland) . 2024;14:1534. doi: 10.3390/diagnostics14141534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Saba L, Maindarkar M, Johri AM, Mantella L, Laird JR, Khanna NN, et al. UltraAIGenomics: Artificial Intelli-gence-Based Cardiovascular Disease Risk Assessment by Fusion of Ultrasound-Based Radiomics and Genomics Features for Preventive, Personalized and Precision Medicine: A Narrative Review. Reviews in Cardiovascular Medicine . 2024;25:184. doi: 10.31083/j.rcm2505184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Alves MA, Castro GZ, Oliveira BAS, Ferreira LA, Ramírez JA, Silva R, et al. Explaining machine learning based diag-nosis of COVID-19 from routine blood tests with decision trees and criteria graphs. Computers in Biology and Medicine . 2021;132:104335. doi: 10.1016/j.compbiomed.2021.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Agarwal M, Agarwal S, Saba L, Chabert GL, Gupta S, Carriero A, et al. Eight pruning deep learning models for low storage and high-speed COVID-19 computed tomography lung segmentation and heatmap-based lesion localization: A multicenter study using COVLIAS 2.0. Computers in Biology and Medicine . 2022;146:105571. doi: 10.1016/j.compbiomed.2022.105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jain PK, Tadepalli KV, Roy S, Sharma N. Exploring deep learning for carotid artery plaque segmenta-tion: atherosclerosis to cardiovascular risk biomarkers. Multimedia Tools and Applications . 2024;83:42765–42797. [Google Scholar]
- [149].Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis-Society of Radiologists in Ultrasound Consensus Conference. Radiology . 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- [150].Romero-Sanchez G, Dabiri M, Mossa-Basha M. Primary Large Vessel Vasculitis: Takayasu Arteritis and Giant Cell Arteritis. Neuroimaging Clinics . 2024;34:53–65. doi: 10.1016/j.nic.2023.07.002. [DOI] [PubMed] [Google Scholar]
- [151].Clemente G, Quaranta C, Basso MG, Pintus C, Rizzo G, Vullo C, et al. Chest Pain: Wellens Syndrome Due to Sponta-neous Dissection of the Left Anterior Descending Coronary Artery - A Case Report and Literature Review. Reviews in Cardiovascular Medicine . 2024;25:70. doi: 10.31083/j.rcm2502070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kalli VDR. Creating an AI-powered platform for neurosurgery alongside a usability examination: Progressing towards mini-mally invasive robotics. Journal of Artificial Intelligence General Science (JAIGS) ISSN: 3006-4023 . 2024;3:363–375. doi: 10.60087/jaigs.v3i1.125. [DOI] [Google Scholar]
- [153].Petzinna SM, Burg LC, Bauer CJ, Karakostas P, Terheyden JH, Behning C, et al. Transorbital ultrasound in the diagno-sis of giant cell arteritis. Rheumatology (Oxford, England) . 2024;63:2379–2386. doi: 10.1093/rheumatology/keae287. [DOI] [PubMed] [Google Scholar]
- [154].Arena R, Ozemek C, Laddu D, Campbell T, Rouleau CR, Standley R, et al. Applying Precision Medicine to Healthy Living for the Prevention and Treatment of Cardiovascular Disease. Current Problems in Cardiology . 2018;43:448–483. doi: 10.1016/j.cpcardiol.2018.06.001. [DOI] [PubMed] [Google Scholar]
- [155].Singhal S, Sharma A, Verma PK, Kumar M, Verma S, Kaur M, et al. Energy Efficient Load Balancing Algorithm for Cloud Computing Using Rock Hyrax Optimization. IEEE Access . 2024 [Google Scholar]
- [156].Saimassay G, Begenov M, Sadyk U, Baimukashev R, Maratov A, Omarov B. Enhanced U-Net Architecture for Lung Segmentation on Computed Tomography and X-Ray Images. International Journal of Advanced Computer Science & Applications . 2024;15:921–930. [Google Scholar]
- [157].Pawar L, Patil S, Dhotre S, Sonawane S. 2024 8th International Artificial Intelligence and Data Processing Symposium (IDAP) IEEE; 2024. A Comparative Study of Artificial Intelligence and eXplainable AI Techniques for Pulmonary Disease Detection and Its Severity Classification; pp. 1–7. [Google Scholar]
- [158].Shin HW, Cho K, Rhee CS, Hong IH, Cho SH, Kim SW, et al. Urine 5-Eicosatetraenoic Acids as Diagnostic Markers for Obstructive Sleep Apnea. Antioxidants (Basel, Switzerland) . 2021;10:1242. doi: 10.3390/antiox10081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tandel GS, Balestrieri A, Jujaray T, Khanna NN, Saba L, Suri JS. Multiclass magnetic resonance imaging brain tumor classifi-cation using artificial intelligence paradigm. Computers in Biology and Medicine . 2020;122:103804. doi: 10.1016/j.compbiomed.2020.103804. [DOI] [PubMed] [Google Scholar]
- [160].Saba L, Maindarkar M, Johri AM, Mantella L, Laird JR, Khanna NN, et al. UltraAIGenomics: Artificial Intelligence-Based Cardiovascular Disease Risk Assessment by Fu-sion of Ultrasound-Based Radiomics and Genomics Features for Preventive, Personalized and Precision Medicine: A Narrative Review. Reviews in Cardiovascular Medicine . 2024;25:184. doi: 10.31083/j.rcm2505184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Konstantonis G, Singh KV, Sfikakis PP, Jamthikar AD, Kitas GD, Gupta SK, et al. Cardiovascular disease detection using machine learning and carotid/femoral arterial imaging frameworks in rheumatoid arthritis patients. Rheumatology International . 2022;42:215–239. doi: 10.1007/s00296-021-05062-4. [DOI] [PubMed] [Google Scholar]
- [162].Bhagawati M, Paul S, Agarwal S, Protogeron A, Sfikakis PP, Kitas GD, et al. Cardiovascular disease/stroke risk strati-fication in deep learning framework: a review. Cardiovascular Diagnosis and Therapy . 2023;13:557–598. doi: 10.21037/cdt-22-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Paul S, Maindarkar M, Saxena S, Saba L, Turk M, Kalra M, et al. Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’s Disease: A Narrative Review. Diagnostics (Basel, Switzerland) . 2022;12:166. doi: 10.3390/diagnostics12010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Suri JS, Paul S, Maindarkar MA, Puvvula A, Saxena S, Saba L, et al. Cardiovascular/Stroke Risk Stratification in Park-inson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review. Metabolites . 2022;12:312. doi: 10.3390/metabo12040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Viswanathan V, Jamthikar AD, Gupta D, Puvvula A, Khanna NN, Saba L, et al. Integration of estimated glomerular filtration rate biomarker in image-based cardiovascular disease/stroke risk calculator: a south Asian-Indian diabetes cohort with moderate chronic kidney disease. International Angiology: a Journal of the International Union of Angiology . 2020;39:290–306. doi: 10.23736/S0392-9590.20.04338-2. [DOI] [PubMed] [Google Scholar]
- [166].Jamthikar AD, Gupta D, Puvvula A, Johri AM, Khanna NN, Saba L, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis using carotid ultrasound B-mode imaging. Rheumatology International . 2020;40:1921–1939. doi: 10.1007/s00296-020-04691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Tietjens JR, Claman D, Kezirian EJ, De Marco T, Mirzayan A, Sadroonri B, et al. Obstructive Sleep Apnea in Cardio-vascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. Journal of the American Heart Association . 2019;8:e010440. doi: 10.1161/JAHA.118.010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zinchuk A, Yaggi HK. Phenotypic Subtypes of OSA: A Challenge and Opportunity for Precision Medicine. Chest . 2020;157:403–420. doi: 10.1016/j.chest.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cao W, Luo J, Xiao Y. A Review of Current Tools Used for Evaluating the Severity of Obstructive Sleep Apnea. Nature and Science of Sleep . 2020;12:1023–1031. doi: 10.2147/NSS.S275252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Brennan HL, Kirby SD. Barriers of artificial intelligence implementation in the diagnosis of obstructive sleep apnea. Journal of Otolaryngology - Head & Neck Surgery = Le Journal D’oto-rhino-laryngologie et De Chirurgie Cervico-faciale . 2022;51:16. doi: 10.1186/s40463-022-00566-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Mostafa SS, Mendonça F, Ravelo-García AG, Morgado-Dias F. A Systematic Review of Detecting Sleep Apnea Using Deep Learning. Sensors (Basel, Switzerland) . 2019;19:4934. doi: 10.3390/s19224934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Hong S, Zhou Y, Shang J, Xiao C, Sun J. Opportunities and challenges of deep learning methods for electrocardiogram data: A systematic review. Computers in Biology and Medicine . 2020;122:103801. doi: 10.1016/j.compbiomed.2020.103801. [DOI] [PubMed] [Google Scholar]
- [173].Qian K, Janott C, Schmitt M, Zhang Z, Heiser C, Hemmert W, et al. Can Machine Learning Assist Locating the Excita-tion of Snore Sound? A Review. IEEE Journal of Biomedical and Health Informatics . 2021;25:1233–1246. doi: 10.1109/JBHI.2020.3012666. [DOI] [PubMed] [Google Scholar]
- [174].Ferreira-Santos D, Amorim P, Silva Martins T, Monteiro-Soares M, Pereira Rodrigues P. Enabling Early Obstructive Sleep Apnea Diagnosis With Machine Learning: Systematic Review. Journal of Medical Internet Research . 2022;24:e39452. doi: 10.2196/39452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kumari V, Kumar N, Kumar K S, Kumar A, Skandha SS, Saxena S, et al. Deep Learning Paradigm and Its Bias for Coronary Artery Wall Segmentation in Intravascular Ultrasound Scans: A Closer Look. Journal of Cardiovascular Development and Dis-ease . 2023;10:485. doi: 10.3390/jcdd10120485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Pépin JL, Bailly S, Tamisier R. Big Data in sleep apnoea: Opportunities and challenges. Respirology (Carlton, Vic.) . 2020;25:486–494. doi: 10.1111/resp.13669. [DOI] [PubMed] [Google Scholar]
- [177].Loh HW, Ooi CP, Vicnesh J, Oh SL, Faust O, Gertych A, et al. Automated detection of sleep stages using deep learning techniques: a systematic review of the last decade (2010–2020) Applied Sciences . 2020;10:2010–2020. doi: 10.3390/app10248963. [DOI] [Google Scholar]