Abstract
1. The subunit structure of rabbit subcomponent C1q was examined in a previous publication (Reid et al., 1972). The present paper describes some aspects of the structure of the polypeptide chains derived from the molecule. 2. The three polypeptide chains, produced by performic oxidation, of rabbit subcomponent C1q were isolated by ion-exchange chromatography in 8m-urea on DEAE-cellulose. 3. Each chain was found to contain 15–18% glycine and significant amounts of the amino acids hydroxyproline and hydroxylysine. 4. By means of collagenase digestion it was shown that all three chains of rabbit subcomponent C1q contain collagen-like sequences of amino acids which constitute about 40% of each chain. 5. By use of carboxypeptidase A it was established, indirectly, that the collagen-like sequences, in one of the chains, are probably located near, or at, the N-terminal end of the chain. 6. Collagenase digestion and heating at 52°C (but not at 49°C) caused rapid loss of native rabbit subcomponent C1q haemolytic activity.
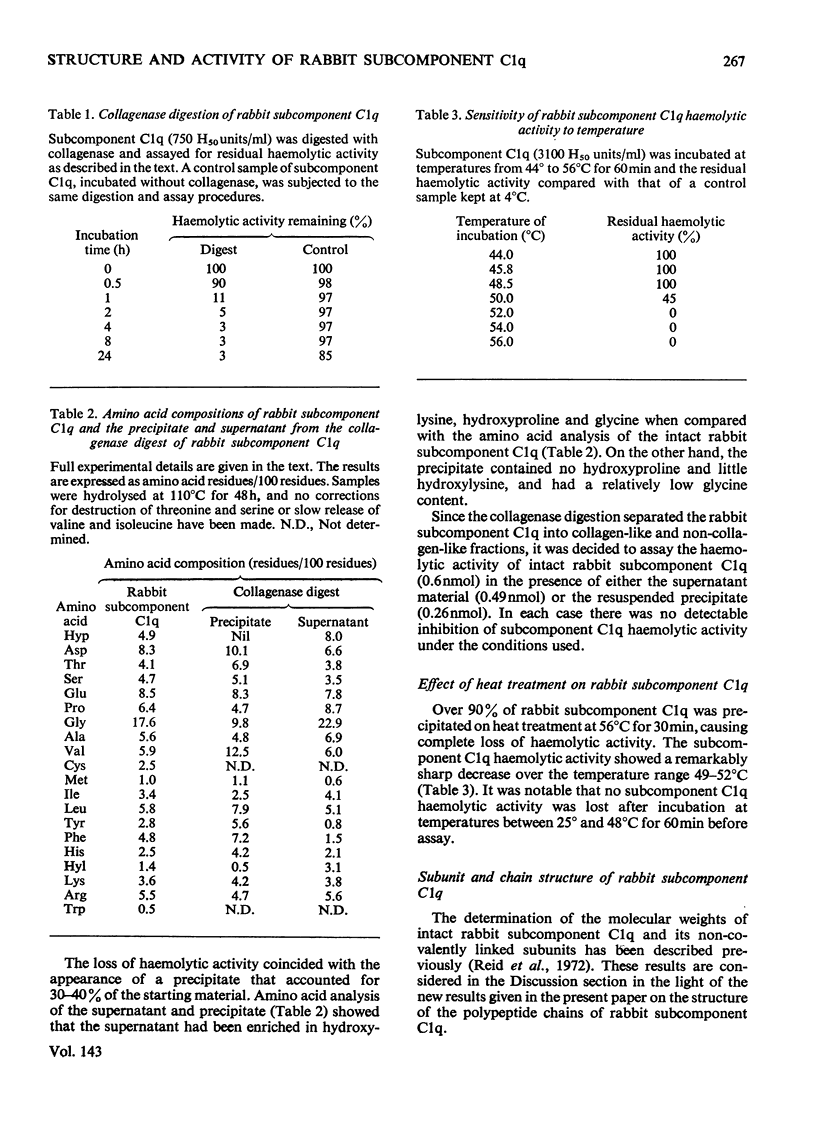
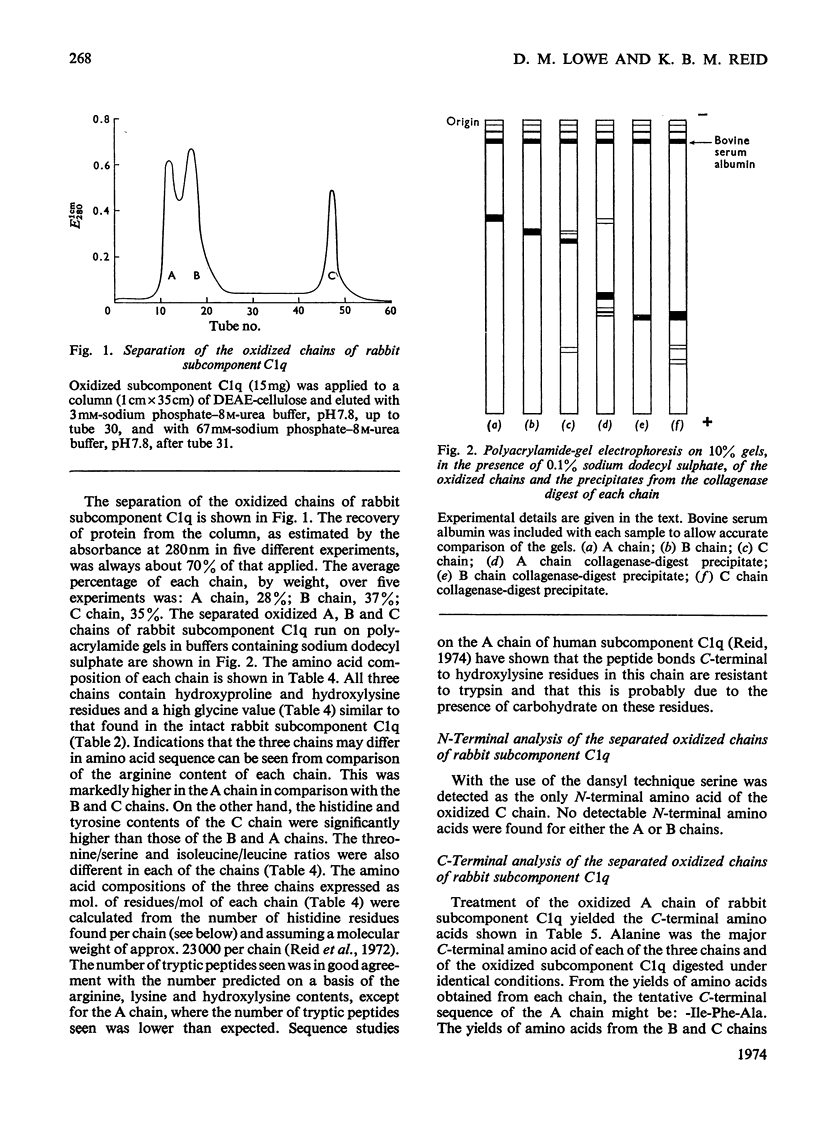
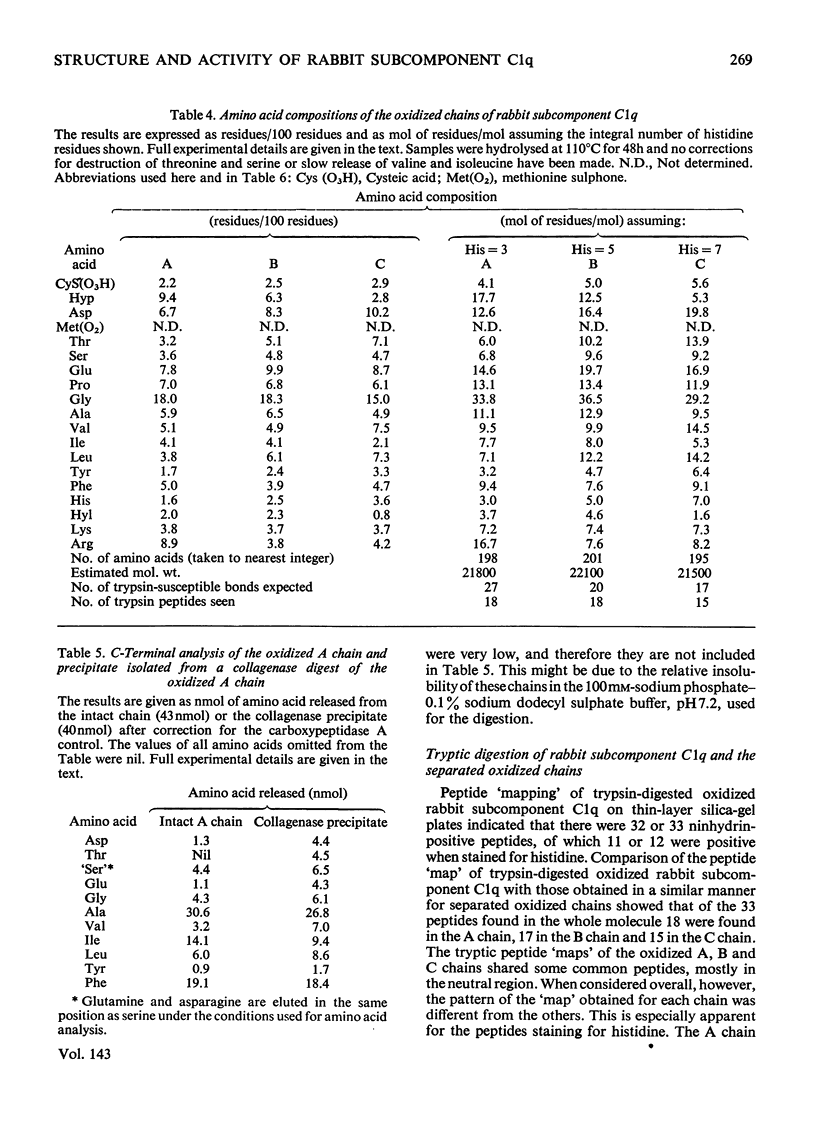
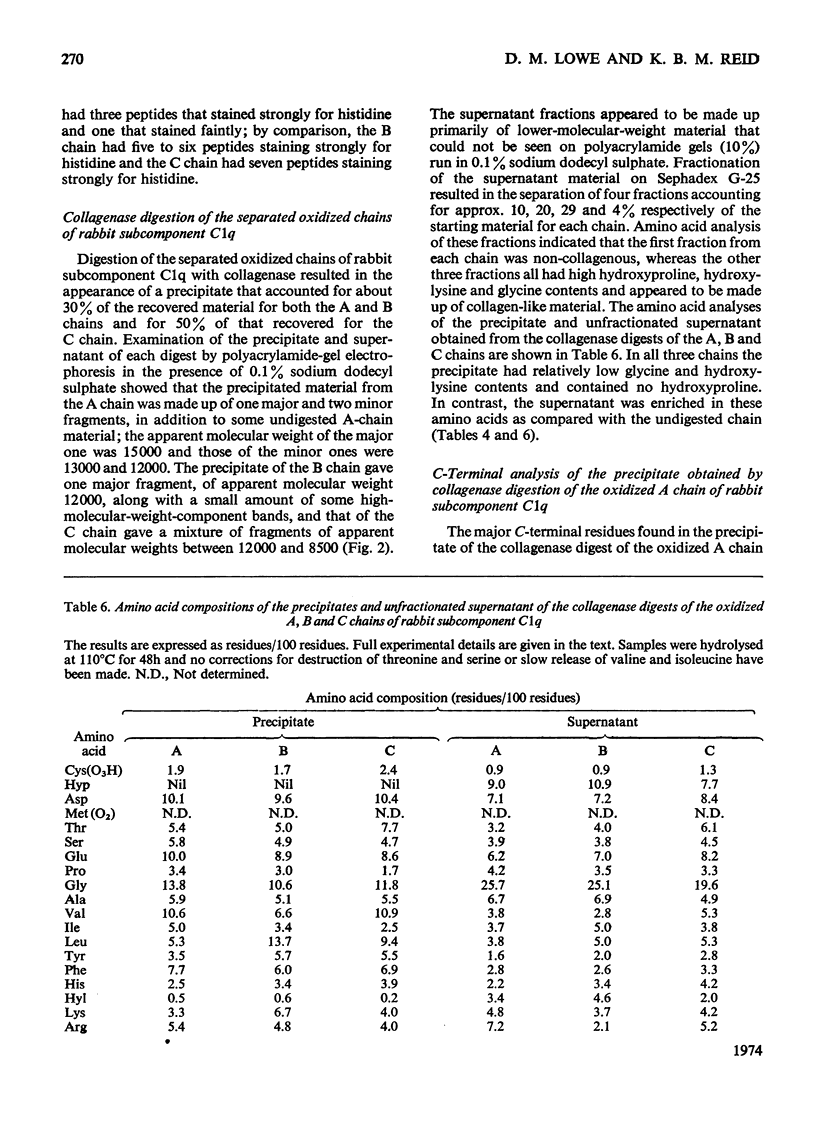
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augener W., Grey H. M., Cooper N. R., Müller-Eberhard H. J. The reaction of monomeric and aggregated immunoglobulins with C1. Immunochemistry. 1971 Nov;8(11):1011–1020. doi: 10.1016/0019-2791(71)90489-7. [DOI] [PubMed] [Google Scholar]
- Calcott M. A., Müller-Eberhard H. J. C1q protein of human complement. Biochemistry. 1972 Aug 29;11(18):3443–3450. doi: 10.1021/bi00768a018. [DOI] [PubMed] [Google Scholar]
- Dopheide T. A., Moore S., Stein W. H. The carboxyl-terminal sequence of porcine pepsin. J Biol Chem. 1967 Apr 25;242(8):1833–1837. [PubMed] [Google Scholar]
- Hauschka P. V., Harrington W. F. Collagen structure in solution. 3. Effec of ross-links on thermal stability and refolding kinetics. Biochemistry. 1970 Sep 15;9(19):3734–3745. doi: 10.1021/bi00821a012. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., KUNKEL H. G. Isolation of a thermolabile serum protein which precipitates gamma-globulin aggregates and participates in immune hemolysis. Proc Soc Exp Biol Med. 1961 Feb;106:291–295. doi: 10.3181/00379727-106-26313. [DOI] [PubMed] [Google Scholar]
- O'Daly J. A., Cebra J. J. Chemical and physicochemical studies of the component polypeptide chains of rabbit secretory immunoglobulin A. Biochemistry. 1971 Oct 12;10(21):3843–3850. doi: 10.1021/bi00797a007. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Electrophoresis of peptides on thin layers of silica gel. Anal Biochem. 1968 Oct 24;25(1):583–587. doi: 10.1016/0003-2697(68)90137-1. [DOI] [PubMed] [Google Scholar]
- Sassano F. G., Colten H. R., Borsos T., Rapp H. J. Resolution of the first component of guinea pig complement into three subunits, Clq, Clr and Cls, and their hybridization with human Cl subunits. Immunochemistry. 1972 Apr;9(4):405–412. doi: 10.1016/0019-2791(72)90310-2. [DOI] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Volanakis J. E., Stroud R. M. Rabbit C1q: purification, functional and structural studies. J Immunol Methods. 1972 Nov;2(1):25–34. doi: 10.1016/0022-1759(72)90014-2. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M., Niedermeier W., Butler W. T. Chemical studies of Clq; a modulator of immunoglobulin biology. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1388–1394. doi: 10.1016/s0006-291x(71)80028-1. [DOI] [PubMed] [Google Scholar]


