Abstract
Rationale:
Previous neuroimaging studies of cognition involving nicotinic acetylcholine receptor (nAChR) agonist administration have repeatedly found enhanced task-induced deactivation of regions of the default mode network (DMN), a group of brain systems that is more active at rest and mediates task-independent thought processes. This effect may be related to pro-cognitive nAChR agonist effects.
Objectives:
The present study sought to test whether nAChR modulation of the DMN is bi-directional, i.e., whether a nAChR antagonist would reduce task-induced deactivation.
Methods:
Eighteen healthy non-smokers underwent functional Magnetic Resonance Imaging while performing a letter N-back task. Scans were performed after nicotine administration (7 mg/24 h, transdermally), after administration of the nAChR antagonist mecamylamine (7.5 mg, p.o.), and after double placebo, in counterbalanced sequence. BOLD signal was analyzed within ventromedial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC) regions of interest - central hubs of the DMN in which consistent nAChR agonist-induced changes had previously been identified.
Results:
Nicotine enhanced hit rate in both the 0-back and 2-back condition, while mecamylamine slowed reaction time in the 2-back condition. Mecamylamine reduced task-induced deactivation of vmPFC and PCC. Nicotine had no significant effects on the BOLD signal.
Conclusions:
The finding that nAChR tone reduction by mecamylamine weakened task-induced DMN deactivation indicates that a constant tone of nAChR activation helps regulate DMN activity in healthy individuals. This suggests that low nAChR tone may play a causal role in DMN dysregulation seen in conditions such as Mild Cognitive Impairment or Alzheimer’s disease.
Keywords: Mecamylamine, nicotine, nicotinic acetylcholine receptor, cognition, N-back, fMRI, default mode network, deactivation
1. Introduction
Much evidence has built up suggesting that nicotine and other nicotinic acetylcholine receptor (nAChR) agonists can enhance cognitive performance, as reported with particular consistency in tests of attention (Hahn 2015; Heishman et al. 2010; Newhouse et al. 2004; Stolerman et al. 1995). Several disease states marked by cognitive deficits involve nAChR hypofunction, most prominently Alzheimer’s disease, Mild Cognitive Impairment, and schizophrenia (Adams and Stevens 2007; Contestabile 2011; Kendziorra et al. 2011; Nakaizumi et al. 2018; Newhouse et al. 2001; Perry et al. 2000; Petrovsky et al. 2010; Sabri et al. 2018). The potential therapeutic utility of nAChR agonists as cognitive enhancers in these conditions has motivated efforts to gain a better understanding of their effects on systems-level brain function.
Neuroimaging studies have observed changes in neuronal responses to cognitive tasks after acute nicotine administration. Employing functional Magnetic Resonance Imaging (fMRI), Hahn et al. (2007) demonstrated for the first time that nicotine potentiated task-induced deactivation of the default mode network (DMN), a group of brain systems that are more active in the absence of directed cognitive processes, mediate various task-independent thought processes (such as mind-wandering), and whose deactivation facilitates attention to external signals (Buckner et al. 2008; Buckner and DiNicola 2019; Gusnard and Raichle 2001; Mason et al. 2007; Raichle et al. 2001; Shulman et al. 1997; Sonuga-Barke and Castellanos 2007). The effects of nicotine on the DMN were robustly correlated with nicotine’s beneficial effects on attention task performance, even when controlling for individual differences in nicotine blood levels (Hahn et al. 2007).
Since this discovery, enhanced task-induced DMN deactivation by nicotine has been replicated across a wide range of cognitive tasks, both in non-smokers and in minimally deprived or withdrawn smokers (Beaver et al. 2011; Ettinger et al. 2009; Froeliger et al. 2012; Hahn et al. 2009; Tanabe et al. 2011). The same pattern was seen with varenicline (Loughead et al. 2010), and with the α7 nAChR partial agonist DMXB-A in people with schizophrenia (Tregellas et al. 2011). The interpretation has been that nicotine facilitates external information processing by aiding the downregulation of task-independent thought processes. This would be consistent with rodent studies suggesting that nAChR stimulation induces a mode shift toward improved readiness to respond to external stimuli (Hasselmo and Sarter 2011).
A recent meta-analysis aggregated neuroimaging results of cognitive studies involving nAChR agonist administration to identify consistent functional brain changes that may be related to pro-cognitive drug effects (Sutherland et al. 2015). Enhanced deactivation in the ventromedial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC), central hubs of the DMN, alongside increased activation of lateral frontoparietal regions, were the most consistent changes identified in both smokers and non-smokers. vmPFC and PCC regions were neuroanatomically consistent, while regions of increased activity with nAChR agonist administration were more variable across studies and cognitive tasks (Newhouse et al. 2011; Sutherland et al. 2015).
Abnormal DMN regulation has been described in conditions such as MCI, AD, and schizophrenia (e.g., Lustig et al. 2003; Metzak et al. 2012; Whitfield-Gabrieli and Ford 2012). Given the effects of nAChR agonists on the DMN described above, there is a possibility that low nAChR tone, which marks these conditions, may be an underlying cause. A state of low nAChR tone can be modeled in healthy subjects by administering a nAChR antagonist.
The non-competitive nAChR antagonist mecamylamine has been reported to impair cognitive performance in intact rodents (Mirza and Stolerman 2000, Stewart et al. 2001, Rezvani et al. 2002, Leblond et al. 2002) and healthy humans (Alvarez-Jimenez et al. 2017; Newhouse et al. 1992; Pickworth et al. 1997; Stolerman et al. 1973). This finding indicates that a constant tone of nAChR activation supports cognitive functioning in the healthy individual. Knowledge about the neural mechanisms of mecamylamine-induced cognitive impairment may unveil a role of low nAChR tone in DMN dysregulation and associated cognitive impairment seen in the above disorders.
The aim of the present fMRI study was to test the hypothesis that mecamylamine would weaken task-induced DMN deactivation, producing effects opposite to nicotine. To this end, we tested the effects of nicotine (7 mg/24 h, transdermally) and mecamylamine (7.5 mg, p.o.) on cognitive task performance and task-induced DMN deactivation in healthy non-smokers. A secondary aim was to test whether effects of nAChR tone on DMN activity depend on baseline DMN activity. To this end, we manipulated task load in an N-back paradigm as previous studies indicated that greater DMN deactivation can be expected in the 2-back than 0-back condition (e.g., Ceko et al. 2015; Esposito et al. 2006).
2. Methods
Participants
Twenty-one healthy non-smokers (9 females) were enrolled in the study. Of these, three participants were withdrawn before completion, and their data were excluded from analysis. Two of these participants experienced side effects from the nicotine patch (nausea and vomiting), and one participant could not stay awake in the scanner. Thus, 18 participants completed the study.
Completers were 26–55 years of age (mean ± SD, 36.7 ± 11.4 years) and had completed 11–18 years of education (mean ± SD, 15.2 ± 2.0 years). Subjects had not consumed any nicotine-containing products more than 20 times in their lifetime and not at all within the last two years. Non-smokers were selected as the study population to avoid potential confounds related to chronic nicotine exposure and associated neuroadaptive changes and nicotine withdrawal.
Subjects were recruited from the general population through internet and newspaper advertising, flyers, and referrals and gave written informed consent for a protocol approved by the National Institute on Drug Abuse-Intramural Research Program (NIDA-IRP) and University of Maryland, Baltimore (UMB) Institutional Review Boards. Subjects were screened for major medical illnesses, claustrophobia, history of neurological or psychiatric disorders, drug and alcohol abuse, and pregnancy. A urine sample was assessed for common drugs of abuse.
Drugs
Nicotine patches were Nicoderm CQ patches (GlaxoSmithKline, Moon Township, PA) releasing 7 mg of nicotine in 24 h, the lowest dose available in the US. Placebo patches were size-matched adhesive bandages.
GMP-grade mecamylamine HCI powder (Poli Industria Chimica S.p.A., Milan, Italy) was packaged into capsules at a dose of 7.5 mg/capsule by a compounding pharmacist, who also produced matching placebo capsules filled with methylcellulose. Mecamylamine is FDA-approved for the control of hypertension, which is usually achieved at an average total daily dosage of 25 mg, in three divided doses. It is well absorbed from the gastrointestinal tract and crosses the blood-brain barrier. For its use in the present study, an IND was obtained.
Study design and procedures
In a within-subject design, each participant was tested on three separate days, separated by at least two intermediate days to ensure complete drug washout. On each test day, a skin patch was applied, and the participant swallowed a capsule. On one day, both the patch and the capsule were a placebo (placebo session). On another day, the patch was a nicotine patch (7 mg/24 h) and the capsule was a placebo (nicotine session), and on another, the patch was a placebo and the capsule contained 7.5 mg of mecamylamine HCI (mecamylamine session). The three conditions were tested in a sequence that was double-blind and counterbalanced across participants.
The study involved five total visits: one consent and screening visit, one training visit, and the three test sessions. Screening included a medical history and physical exam, an electrocardiogram, blood and urine labs, a vision test, and tests for drug use, smoking, and pregnancy. During the training visit, participants were given task instructions and performed a full-length version of the cognitive tasks to be performed in the MRI scanner, to minimize practice effects between test sessions.
Each of the three test sessions took approximately 7 h. Participants were asked to refrain from caffeine use on test days, and from alcohol use on test and the immediate preceding days. Upon arrival in the morning, participants were tested for recent alcohol use or smoking (as additional verification of non-smoking status) via alcohol and CO breathalyzer, and a urine sample was tested for pregnancy and drug use, all of which had to be negative for the session to proceed. Resting blood pressure and heart rate measurements were taken, and participants completed a drug side effect checklist, rating possible adverse effects of nicotine and mecamylamine (restlessness, weakness/fatigue, dizziness, headache, dry mouth, nausea, abdominal pain, sweating, palpitations, jitteriness, sleepiness, blurred vision, constipation, anxiety, difficulty urinating) as none (1), mild (2), moderate (3), or severe (4).
Next, the study patch was administered. Vital signs and the side effect checklist were obtained hourly thereafter. During the drug absorption period, participants were permitted to read, watch movies, or use the internet. Three hours after patch administration, participants swallowed the study capsule. Six hours after patch application (3 h after capsule administration), the fMRI scan began. This timing was based on available pharmacokinetic data. Mecamylamine plasma concentrations reach tmax on average 3 h following oral administration, and its elimination half-life is approximately 10 h (Singh et al. 2006; Young et al. 2001). Nicotine plasma concentrations asymptote on average 5 h post-patch administration, and steady-state blood levels are more likely after 6 h of absorption than at any earlier time point (Fant et al. 2000; Gupta et al. 1993; Palmer et al. 1992). Thus, the long absorption period ensured stable plasma concentrations across the entire testing period. It also allowed acute tolerance to develop to potential adverse effects of nicotine (Perkins et al. 1994) prior to testing.
MRI scans began with a 6-min resting scan, followed by a ~45-min visuospatial attention task (data not reported here) and an anatomical scan. Five blocks of a Letter N-back task (described below) were then performed, separated by 1 min rest periods. The N-back task started approximately 7 h after patch application and 4 h after capsule administration.
After the scan, the side effect checklist was completed and vital signs were taken one last time, and a 5-ml blood sample was obtained from a forearm vein for analysis of nicotine and mecamylamine concentrations. The blood draw was performed approximately 5.5 h after mecamylamine/placebo dosing, in the continued presence of the nicotine/placebo skin patch.
Letter N-back task
The N-back task allows parametric manipulation of processing load by varying the number of items to be held in working memory. Participants viewed a sequence of uppercase or lowercase consonants (~3° visual angle), each presented for 500 ms in the middle of the screen, black against white background. Each letter was followed by a 1500-ms fixation cross. A button press response was required when the currently displayed letter equaled that displayed N letters ago, regardless of case. A 0-back and a 2-back condition was employed. In the 0-back condition, a response was required whenever the letter “d” or “D” was shown. In the 2-back condition, the second f in the sequence “g + F + b + f + T” would require a response, as an example. Graded levels of DMN deactivation have been reported when comparing different N-back load conditions (e.g., Ceko et al. 2015; Esposito et al. 2006).
The task was performed in five 306-s scan runs (~30 min total task duration). Each run was comprised of eight blocks, presented in randomized sequence: two rest blocks, during which only a central fixation cross was presented, two blocks of the 2-back condition, two blocks of the 0-back condition, and another two blocks of the 0-back condition employing a slower trial presentation rate (one stimulus every 4 s instead of every 2 s). The longer no-event periods in-between trials were expected to invite task-independent thought processes and DMN activity intrusions, thus providing another gradation of baseline DMN activity for the study of drug effects. The slower presentation rate was not tested in the 2-back condition because increased memory storage demands of a longer retention interval would confound effects. Baseline DMN activity did not actually differ between slow and fast blocks of the 0-back condition; thus, only fast blocks, which matched the presentation rate of the 2-back condition, were analyzed for drug effects.
Each block started with the presentation of the fixation cross for 2 s, followed by a 4-s instruction screen (“look at cross”, “press for d”, or “2-back”). During rest blocks, only the fixation cross was then presented for 32 s. During 0-back and 2-back blocks, sixteen consecutive letter stimuli were displayed; each block had 4 targets.
Magnetic Resonance Imaging
Scanning was performed on a 3 Tesla Siemens Tim Trio scanner (Erlangen, Germany). Whole-brain EPI images were acquired for measurement of T2*-weighted blood oxygen-level dependent (BOLD) effects [4-mm oblique (30°) axial slices; 64×64 matrix; FOV=22×22 cm; TE=27 ms; FA=78°; TR=2.0 s]. An oblique (30%) axial T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1-mm3 voxels, TR=1.9 s, TE=3.51 ms, FA=9°).
Data were processed using AFNI (Cox 1996). Motion correction was performed by registering each volume to a base volume. Frames with >0.5 mm displacement or >0.5° rotation relative to the preceding TR were censored out. The time series was analyzed by voxel-wise multiple regression. Four 32-s boxcar regressors, corresponding to 0-back and 2-back blocks, and to slow 0-back blocks, were convolved with a model hemodynamic response function. The six motion parameter curves were included as regressors of no interest. All correlations between any of the regressors of interest and any of the motion regressors were near zero (all ps>0.8). For each subject, the voxel-wise average amplitude of signal change produced by 0-back and 2-back blocks relative to rest blocks was determined. These maps were re-sampled to a 1-μL resolution, converted to a standard coordinate system (Talairach and Tournoux 1988), and spatially blurred using a Gaussian 5-mm rms isotropic kernel.
Drug effects on task-induced DMN deactivation were analyzed within regions of interest (ROIs) defined by an independent study, to avoid bias through “double dipping” (Kriegeskorte et al. 2009). Seventeen-mm-diameter spheres were centered on vmPFC (center-of-mass x = −8, y = 46, z = 0 mm) and PCC (x = −2, y = −56, z = 14 mm) ROIs, central hubs of the DMN identified in the meta-analysis by Sutherland et al. (2015) as DMN regions in which consistent nAChR agonist-induced changes occurred in both smokers and non-smokers. Activity was averaged within each ROI for each drug condition. A control ROI in the supplementary motor area (SMA; x = 0, y = 18, z = 52 mm; 17 mm diameter), which was sensitive to task load but has never been shown to be modulated by nAChR agonist administration, was analyzed to test specificity of mecamylamine effects. Figure 1 shows all ROIs, overlaid onto a task map derived by voxel-wise paired t-test comparing activity in the 0-back and 2-back conditions. Voxel-wise p<0.005 combined with a 3930 μL minimum cluster-size yielded overall p<0.05 based on Monte Carlo simulation.
Fig. 1.
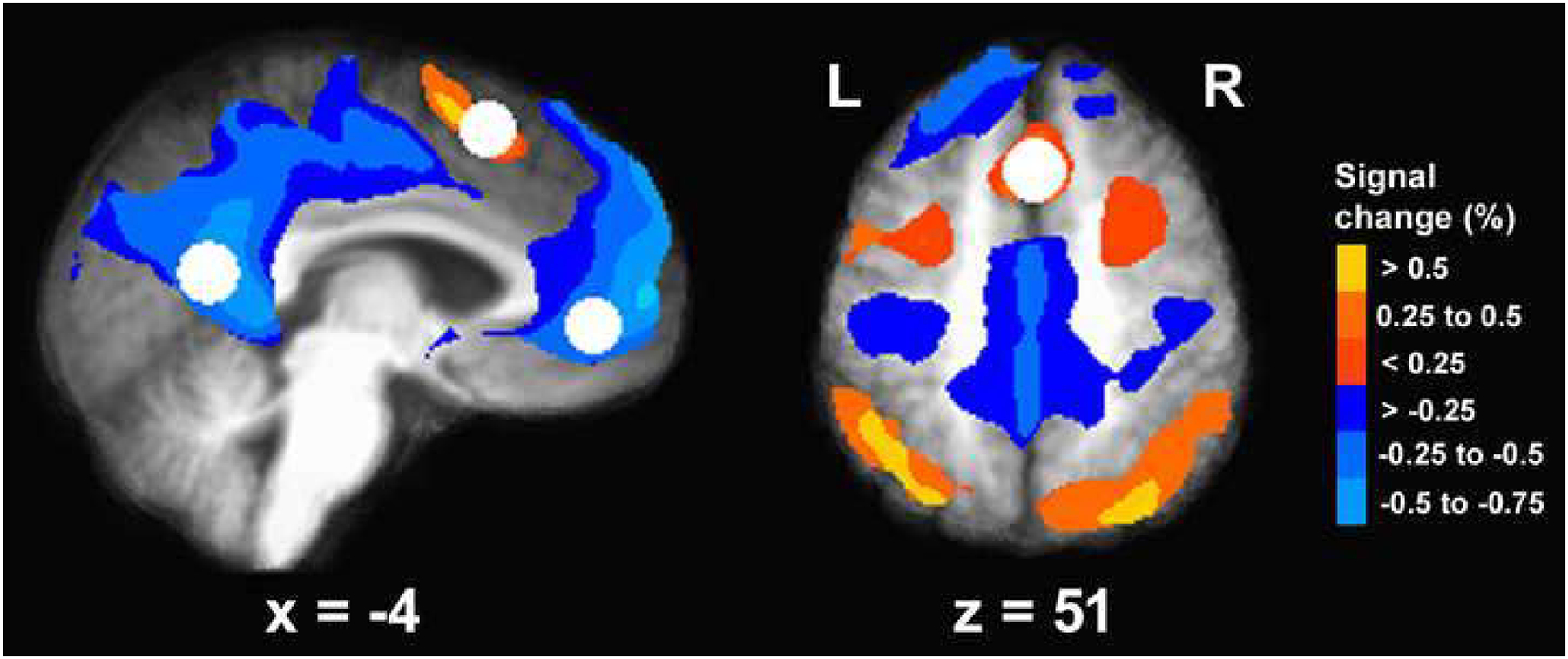
Regions of interest (white spheres) overlaid onto a task map reflecting regions of significant signal difference between the 0-back and the 2-back condition. Regions displaying greater activation with greater task load are drawn in warm colors. Region displaying greater deactivation with greater task load are drawn in cold colors. The activation map is overlaid onto anatomical scans in Talairach space, averaged over all 18 participants.
Blood analyses
Immediately after the blood draw at the end of each test session, the sample was centrifuged to separate plasma from red blood cells. Plasma samples were frozen at −80 °C until analysis upon study completion. Plasma samples were assayed concurrently in 0.5 mL plasma specimens for nicotine and mecamylamine concentrations via solid phase extraction and liquid chromatography coupled to tandem mass spectrometry (LC-MSMS). Two mL 0.1% formic acid were added to plasma specimens and the supernatant, after centrifugation at 4,000xg 5 min 4°C, was submitted to solid phase extraction using Strata-XC cartridges (Phenomenex, San Jose, CA). Conditioning was performed with methanol and water and washing with 0.1 M acetic acid and methanol. The final elution was accomplished with 3% NH4OH in MeOH. Samples were reconstituted in 100 μL of mobile phase and 20 μL injected into the LC-MSMS. Deuterated analogs of the target analytes were employed as internal standards.
LC-MSMS analysis was performed with Shimadzu liquid chromatography system (Shimadzu Corporation, Columbia, MD) interfaced to a 3200 QTrap (AB Sciex, Foster City, CA) with a Turbo V ESI source. The Shimadzu system consisted of LC-20AD binary pump, DGU-20A3 degasser, SIL-20AD autosampler and CTO-10AC column oven. The chromatographic separation was achieved with a Synergi Polar-RP 100A, 100×2 mm, 4 pm, with a 4×2 mm identically packed guard column (Phenomenex, Torrance, CA). Gradient elution was with mobile phase A (1 mM Ammonium Formate pH 3.3 with 0.1% Formic Acid) and mobile phase B (Acetonitrile) at a flow rate of 0.3 mL/min. The initial mixture (94 A: 6 B) was maintained for 3 min, mobile phase B was increased to 60% at 5 min and held for 3 min. The mixture returned to the initial conditions at 10 min, followed by 2 min equilibration. Total run time was 12 min. Mass spectrometric data were acquired in positive electrospray ionization mode with the following source parameters: IonSpray voltage 3,000 V; temperature 450°C; curtain gas 35; ion source gas1 50 and ion source gas2 50. Data were recorded in multiple reaction monitoring mode (MRM). Transitions monitored were 163.2>132.2 (quantifier) and 163.2>84.2 (qualifier) for nicotine, and 168.2>81.2 (quantifier) and 168.2>137.2 (qualifier) for mecamylamine. Linearity range with 1/x2 weighting was from 1 to 500 ng/mL. The lower limit of quantification and the limit of detection was 1 ng/mL for both compounds.
Data analysis
Effects of nicotine and effects of mecamylamine were analyzed in separate ANOVAs, with the same placebo baseline entering both analyses, because effects of each drug were of interest independently of the other.
Vital signs (systolic and diastolic blood pressure and heart rate, each measured after three minutes of sitting and after three minutes of standing) and the subjective state scales from the side effect checklist were analyzed by 2-factor ANOVA with drug (nicotine vs. placebo, or mecamylamine vs. placebo) and time as within-subject factors. These analyses included only the last four measurement time points (4, 5, and 6 hours after patch application, and post-scan) at which absorption of nicotine and mecamylamine can be expected to have taken place (if administered).
N-back task performance was measured by the percentage all targets that were correctly identified (Hit rate), and by the RT of correct responses. Commission errors, i.e. responses to non-target trials, were very rare overall (0.7 ± 0.1 %, averaged across conditions); thus, this measure was not further analyzed. Hit rate and RT and were analyzed by 2-factor ANOVA for repeated measures with drug (nicotine vs. placebo, or mecamylamine vs. placebo) and load condition (0-back vs. 2-back) as within-subject factors.
Average BOLD activity within the vmPFC and PCC ROIs was analyzed by 3-factor ANOVA for repeated measures with drug (nicotine vs. placebo, or mecamylamine vs. placebo), load condition, and region (vmPFC vs. PCC) as within-subject factors. Average BOLD activity within the SMA control region was analyzed by 2-factor ANOVA with drug and load condition as within-subject factors.
3. Results
3.1. Blood concentrations of nicotine and mecamylamine
We were unable to collect a blood sample from one participant in the nicotine and placebo sessions. Plasma concentrations of nicotine averaged 6.7 ± 2.3 (SD) ng/ml in the nicotine session (range 1.4 – 9.1 ng/ml), comparable to plasma concentrations observed in past studies testing smokers or non-smokers with a nicotine patch of the same dose (Gorsline et al. 1993; Hahn et al. 2020a; Hahn et al. 2020b). No nicotine was detectable in the placebo or mecamylamine session. Plasma concentrations of mecamylamine averaged 19.7 ± 5.8 ng/ml in the mecamylamine session (range 8.7 – 29.8 ng/ml). No mecamylamine was detectable in the placebo or nicotine session.
3.2. Vital signs
Mecamylamine reduced systolic blood pressure by approximately 4 mmHg on average, both when measured after three minutes of sitting [main effect of mecamylamine F(1,17)=8.43, p=0.010] and after three minutes of standing [F(1,17)=6.52, p=0.021]. No other main effects or drug x time interactions were significant on vital signs for either drug.
3.3. Subjective side effects
Participants reported feeling less “sleepy” in the nicotine than in the placebo session [main effect of nicotine F(1,17)=5.67, p=0.029], replicating previous findings with this dose of nicotine in non-smokers (Hahn et al. 2020a; Hahn et al. 2020b). The only other drug effect was a significant mecamylamine x time interaction on “jittery” [F(3,51)=3.40, p=0.025]. This was based on three participants reporting mild jitteriness after the scan in the mecamylamine session, while all other ratings were “none” in both the mecamylamine and the placebo session.
3.4. N-Back task performance
Nicotine effects
Figure 2 shows that the hit rate was lower and RT was slower in the 2-back than the 0-back condition, as confirmed by a significant main effect of load condition on both measures [Fs(1,17)>21, ps<0.001]. Nicotine increased the hit rate in both load conditions (Figure 2a), as supported by a significant main effect of nicotine [F(1,17)=10.5, p=0.005]. This effect did not interact with load condition [F(1,17)=1.56, p=0.23]. On RT, there was no main effect of nicotine and no interaction of nicotine with load condition [both ps>0.4].
Fig. 2.
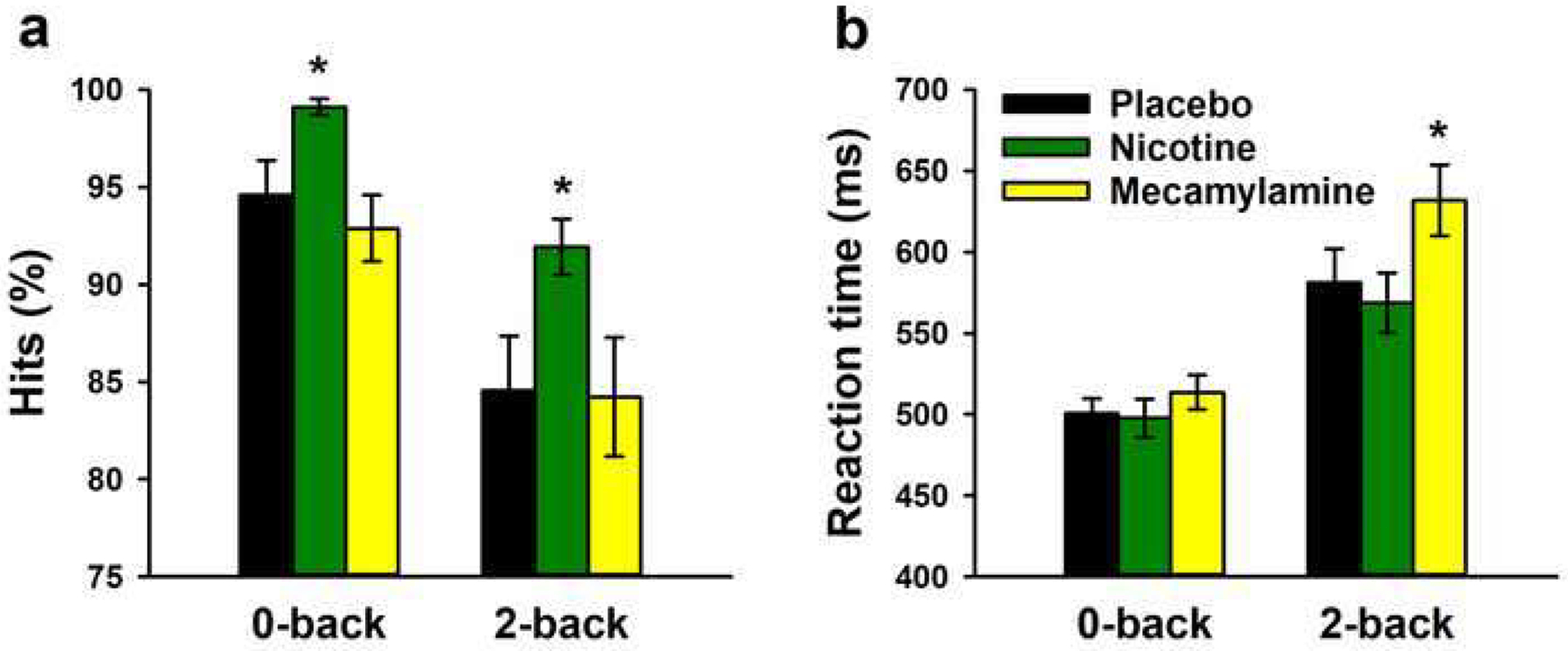
Effects of nicotine and mecamylamine on hit rate and reaction time in the letter N-back task. Averages (±SEM) are shown for each load and drug condition. * P<0.05 in paired sample t-test
Mecamylamine effects
The main effects of load condition were significant for both the hit rate and RT [Fs(1,17)>21, ps<0.001]. On the hit rate, there was no significant main effect of mecamylamine and no mecamylamine x back interaction [both ps>0.5]. However, mecamylamine slowed RT (Figure 2b), as supported by a significant main effect of mecamylamine [F(1,17)=6.71, p=0.019]. This effect was more pronounced in the 2-back condition, as supported by a significant interaction of mecamylamine with load condition [F(1,17)=7.37, p=0.015].
3.5. Task-induced vmPFC and PCC deactivation
Nicotine effects
In 3-factor ANOVA (nicotine x load condition x region), a significant main effect of load condition [F(1,17)=128.5, p<0.001] reflected greater deactivation in the 2-back than 0-back condition in both DMN regions (Figure 3a & b). There was no significant main effect of nicotine [F(1,17)=0.16, p=0.69], and none of the interactions were significant.
Fig. 3.
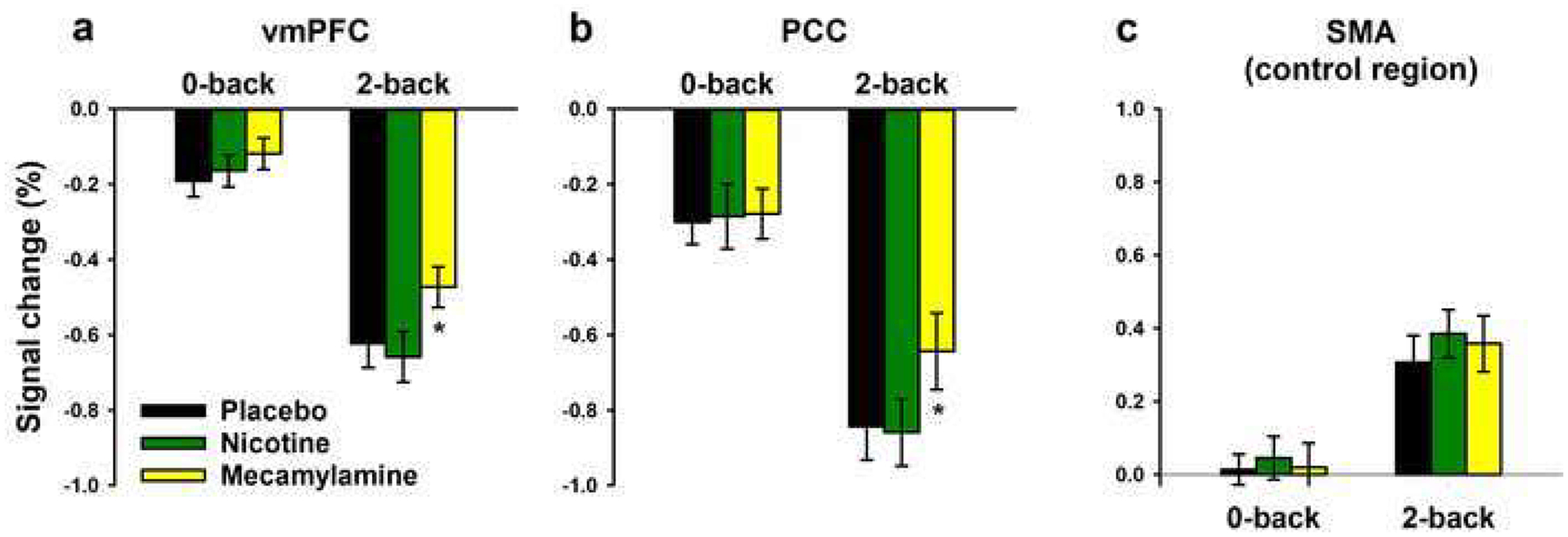
Effects of nicotine and mecamylamine on average (±SEM) task-induced BOLD activity within the ventromedial prefrontal cortex (vmPFC), posterior cingulate cortex (PCC), and supplementary motor area (SMA) ROIs for each load condition. * P<0.05 in paired sample t-test
Mecamylamine effects
In 3-factor ANOVA (mecamylamine x load condition x region), the main effect of load condition was again significant [F(1,17)=59.4, p<0.001]. There was also a significant main effect of mecamylamine [F(1,17)=10.3, p=0.005]. Figure 3a & b shows that mecamylamine attenuated task-induced deactivation in the 2-back condition in both the vmPFC and the PCC. In the 0-back condition, effects of mecamylamine were smaller and not significant. However, the interaction of mecamylamine effects with load condition was only a non-significant trend [F(1,17)=2.98, p=0.10]. None of the interactions were significant.
As a control for the possibility that effects of mecamylamine reflected non-specific effects on vascular tone or neurovascular coupling, we repeated analysis of mecamylamine effects within an ROI in the SMA, which was sensitive to task load but has never been shown to be modulated by nAChR agonist administration. As shown in Figure 3c, the SMA was more active in the 2-back than 0-back condition [main effect of load condition: F(1,17)=19.2, P<0.001], but there was no main effect of mecamylamine [F(1,17)=0.70, P=0.41] and no mecamylamine x load interaction [F(1,17)=0.24, P=0.63]. For completeness, we report that there was also no main effect of nicotine [F(1,17)=1.80, P=0.20] and no nicotine x load interaction [F(1,17)=0.38, P=0.55] in the SMA.
4. Discussion
The present study aimed at testing whether acute nAChR modulation of the DMN was bi-directional. Multiple previous studies have reported that nAChR agonist administration enhances task-induced deactivation in regions of the DMN (reviewed by Sutherland et al. 2015). However, it was unknown whether nAChR antagonism reduces task-induced deactivation, which would suggest that (a) tonic nAChR activation generally helps regulate DMN activity in healthy individuals, and (b) low nAChR tone may play a causal role in DMN dysregulation and associated cognitive impairment seen in conditions such as MCI and AD.
The present findings supported this hypothesis. The nAChR antagonist mecamylamine reduced task-induced deactivation of two central regions of the DMN, with ROIs centered on regions in which nAChR agonists consistently enhanced deactivation across previous (although not the present) studies in smokers and non-smokers (Sutherland et al. 2015). Reduced deactivation with mecamylamine was seen in both vmPFC and PCC and appeared to be baseline dependent; there was a trend for this effect to be more pronounced in the 2-back condition which induced greater cognitive task load and greater DMN deactivation than the 0-back condition. In line with this observation, the performance-impairing effects of mecamylamine were also more pronounced in the 2-back condition.
In contrast, and contrary to expectation, nicotine had no significant effects on task-induced deactivation in vmPFC and PCC, despite robust performance-enhancing effects. We can only speculate about possible reasons. First, the dose of nicotine employed was small; most previous fMRI studies of transdermal nicotine effects employed larger doses. While this may raise the question of whether previous findings of enhanced DMN deactivation with nicotine reflected effects of nAChR desensitization, the present finding that a nAChR antagonist had opposite effects on DMN deactivation denies this interpretation. Another possibility is that task-induced DMN deactivation is near optimal in healthy non-smokers. Indeed, the majority (although not all) of previous fMRI studies with nicotine were performed in abstinent or minimally deprived smokers (Sutherland et al. 2015), and previous research has shown that nicotine withdrawal reduces task-induced DMN deactivation (Aronson Fischell et al. 2020; Loughead et al. 2015).
Quite possibly, the absence of significant nicotine effects on DMN deactivation in the present study was due to a combination of low dosing, a near optimal baseline, and a moderate sample size. This finding does, however, suggest that cognitive-enhancing effects of nicotine, which were robust in the present study, do not depend on its effects on the DMN under all conditions. Effect on other systems likely also play a role.
An interesting observation was that the negative effects of mecamylamine on cognitive task performance were not the opposite of the beneficial effects of nicotine. In fact, there was no overlap between aspects of performance enhanced by nicotine (hit rate in both load conditions) and impaired by mecamylamine (RT in the 2-back condition). This may relate to the finding that effects of these two agents on the DMN ROIs also were not opposite and may suggest that the systems mediating cognitive effects of nAChR modulation depend on nAChR tone. Against a background of low nAChR tone as seen in deprived smokers, in certain clinical populations, or in the presence of a nAChR antagonist, the ability to down-regulate DMN functions may be a performance-limiting factor guarded by the level of tonic nAChR activation. In contrast, other systems may mediate effects of small elevations in nAChR tone from a near-optimal baseline.
A clear limitation of the present study was the limited sample size, which precluded meaningful whole-brain analyses and discouraged exploration of a wider range of ROIs not directly related to the primary study hypothesis. The sample size may also have been the reason preventing us from detecting a significant load dependency of the mecamylamine effect on DMN deactivation. However, our main conclusion that mecamylamine reduces task-induced deactivation of central regions of the DMN is substantiated by a robust main effect (p=0.005), by consistency of the effect across both ROIs, and to a degree also by the tendency for this effect to be more pronounced in the high-load condition, paralleling effects on behavioral task performance. That these effects did not reflect a non-specific mode of action on vascular tone or neurovascular coupling, perhaps secondary to reductions in blood pressure, was indicated by an absence of effect on the SMA - another task-sensitive midline region not part of the DMN.
In summary, the present study found evidence in support of the hypothesis that nAChR antagonism reduces down-regulation of DMN activity when engaging in a cognitive task (which was accompanied by RT slowing). This finding implies that a constant tone of nAChR activation guards the ability to effectively down-regulate task-independent thought processes in the healthy organism. The finding further suggests that a pathologically low nAChR tone such as in MCI or AD is likely to impede this ability, which may contribute to the cognitive deficits observed in these conditions.
Acknowledgements
This work was funded by National Institutes of Health grant R21 DA027894 to B. Hahn, and the National Institute on Drug Abuse - Intramural Research Program.
Footnotes
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Adams CE, Stevens KE (2007) Evidence for a role of nicotinic acetylcholine receptors in schizophrenia Front Biosci 12:4755–4772 [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez R et al. (2017) Pharmacokinetics and pharmacodynamics of oral mecamylamine - development of a nicotinic acetylcholine receptor antagonist cognitive challenge test using modelling and simulation J Psychopharmacol 31:192–203 doi: 10.1177/0269881116681417 [DOI] [PubMed] [Google Scholar]
- Aronson Fischell S, Ross TJ, Deng ZD, Salmeron BJ, Stein EA (2020) Transcranial Direct Current Stimulation Applied to the Dorsolateral and Ventromedial Prefrontal Cortices in Smokers Modifies Cognitive Circuits Implicated in the Nicotine Withdrawal Syndrome Biol Psychiatry Cogn Neurosci Neuroimaging 5:448–460 doi: 10.1016/j.bpsc.2019.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, Matthews PM (2011) The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG Neuropsychopharmacology 36:1792–1800 doi: 10.1038/npp.2011.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Buckner RL, DiNicola LM (2019) The brain’s default network: updated anatomy, physiology and evolving insights Nat Rev Neurosci 20:593–608 doi: 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Ceko M, Gracely JL, Fitzcharles MA, Seminowicz DA, Schweinhardt P, Bushnell MC (2015) Is a Responsive Default Mode Network Required for Successful Working Memory Task Performance? J Neurosci 35:11595–11605 doi: 10.1523/JNEUROSCI.0264-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A (2011) The history of the cholinergic hypothesis Behav Brain Res 221:334–340 doi: 10.1016/j.bbr.2009.12.044 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages Comput Biomed Res 29:162–173 [DOI] [PubMed] [Google Scholar]
- Esposito F et al. (2006) Independent component model of the default-mode brain function: Assessing the impact of active thinking Brain Res Bull 70:263–269 [DOI] [PubMed] [Google Scholar]
- Ettinger U et al. (2009) Effects of acute nicotine on brain function in healthy smokers and non-smokers: estimation of inter-individual response heterogeneity Neuroimage 45:549–561 doi: 10.1016/j.neuroimage.2008.12.029 [DOI] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP (2000) A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches Pharmacol Biochem Behav 67:479–482 [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin L, Wang L, Kozink RV, McClernon FJ (2012) Nicotine withdrawal modulates frontal brain function during an affective Stroop task Psychopharmacology (Berl) 220:707–718 doi: 10.1007/S00213-011-2522-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsline J, Gupta SK, Dye D, Rolf CN (1993) Steady-state pharmacokinetics and dose relationship of nicotine delivered from Nicoderm (Nicotine Transdermal System) J Clin Pharmacol 33:161–168 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Benowitz NL, Jacob P 3rd, Rolf CN, Gorsline J (1993) Bioavailability and absorption kinetics of nicotine following application of a transdermal system Br J Clin Pharmacol 36:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain Nat Rev Neurosci 2:685–694 [DOI] [PubMed] [Google Scholar]
- Hahn B (2015) Nicotinic receptors and attention Curr Top Behav Neurosci 23:103–135 doi: 10.1007/978-3-319-13665-3_5 [DOI] [PubMed] [Google Scholar]
- Hahn B, Olmstead CK, Yuille MB, Chiappelli JJ, Wells AK (2020a) Attention-enhancing effects of propranolol and synergistic effects with nicotine Cogn Affect Behav Neurosci 20:658–668 doi: 10.3758/si3415-020-00794-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA (2009) Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study Cereb Cortex 19:1990–2000 doi: 10.1093/cercor/bhn226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA (2007) Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network J Neurosci 27:3477–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B et al. (2020b) Evidence for positive allosteric modulation of cognitive-enhancing effects of nicotine in healthy human subjects Psychopharmacology (Berl) 237:219–230 doi: 10.1007/S00213-019-05363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M (2011) Modes and models of forebrain cholinergic neuromodulation of cognition Neuropsychopharmacology 36:52–73 doi: 10.1038/npp.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance Psychopharmacology (Berl) 210:453–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendziorra K et al. (2011) Decreased cerebral alpha4beta2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer’s disease assessed with positron emission tomography Eur J Nucl Med Mol Imaging 38:515–525 doi: 10.1007/S00259-010-1644-5 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI (2009) Circular analysis in systems neuroscience: the dangers of double dipping Nat Neurosci 12:535–540 doi: 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J et al. (2010) Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers Biol Psychiatry 67:715–721 doi: 10.1016/j.biopsych.2010.01.016 [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C (2015) Working memory-related neural activity predicts future smoking relapse Neuropsychopharmacology 40:1311–1320 doi: 10.1038/npp.2014.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C et al. (2003) Functional deactivations: change with age and dementia of the Alzheimer type Proc Natl Acad Sci U S A 100:14504–14509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007) Wandering minds: the default network and stimulus-independent thought Science 315:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS (2012) Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia Schizophr Bull 38:803–813 doi: 10.1093/schbul/sbq154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaizumi K et al. (2018) In vivo Depiction of alpha7 Nicotinic Receptor Loss for Cognitive Decline in Alzheimer’s Disease J Alzheimers Dis 61:1355–1365 doi: 10.3233/JAD-170591 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R (1992) Acute nicotinic blockade produces cognitive impairment in normal humans Psychopharmacology (Berl) 108:480–484 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Kelton M, Corwin J (2001) Nicotinic treatment of Alzheimer’s disease Biol Psychiatry 49:268–278 doi: 10.1016/s0006-3223(00)01069-6 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A (2004) Effects of nicotinic stimulation on cognitive performance Curr Opin Pharmacol 4:36–46 [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter AS, Dumas JA, Thiel CM (2011) Functional brain imaging of nicotinic effects on higher cognitive processes Biochem Pharmacol 82:943–951 doi: 10.1016/j.bcp.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KJ, Buckley MM, Faulds D (1992) Transdermal Nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation Drugs 44:498–529 [DOI] [PubMed] [Google Scholar]
- Perkins KA et al. (1994) Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans J Pharmacol Exp Ther 270:628–638 [PubMed] [Google Scholar]
- Perry E et al. (2000) Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases Eur J Pharmacol 393:215–222 [DOI] [PubMed] [Google Scholar]
- Petrovsky N et al. (2010) Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers Neuropsychopharmacology 35:1429–1439 doi: 10.1038/npp.2010.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Butschky MF, Henningfield JE (1997) Effects of mecamylamine on spontaneous EEG and performance in smokers and non-smokers Pharmacol Biochem Behav 56:181–187 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O et al. (2018) Cognitive correlates of alpha4beta2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia Brain 141:1840–1854 doi: 10.1093/brain/awy099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997) Common blood flow changes across visual tasks: II. Decreases in cerebral cortex Journal of Cognitive Neuroscience 9:648–663 [DOI] [PubMed] [Google Scholar]
- Singh A, Das DK, Kelley ME (2006) Mecamylamine (Targacept) IDrugs 9:205–217 [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX (2007) Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis Neurosci Biobehav Rev [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Goldfarb T, Fink R, Jarvik ME (1973) Influencing cigarette smoking with nicotine antagonists Psychopharmacologia 28:247–259 [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Shoaib M (1995) Nicotine psychopharmacology: addiction, cognition and neuroadaptation Med Res Rev 15:47–72 [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR (2015) Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies Biol Psychiatry 78:711–720 doi: 10.1016/j.biopsych.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, New York [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR (2011) Nicotine effects on default mode network during resting state Psychopharmacology (Berl) 216:287–295 doi: 10.1007/S00213-011-2221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR et al. (2011) Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia Biol Psychiatry 69:7–11 doi: 10.1016/j.biopsych.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology Annu Rev Clin Psychol 8:49–76 doi: 10.1146/annurev-clinpsy-032511-143049 [DOI] [PubMed] [Google Scholar]
- Young JM, Shytle RD, Sanberg PR, George TP (2001) Mecamylamine: new therapeutic uses and toxicity/risk profile Clin Ther 23:532–565 [DOI] [PubMed] [Google Scholar]


