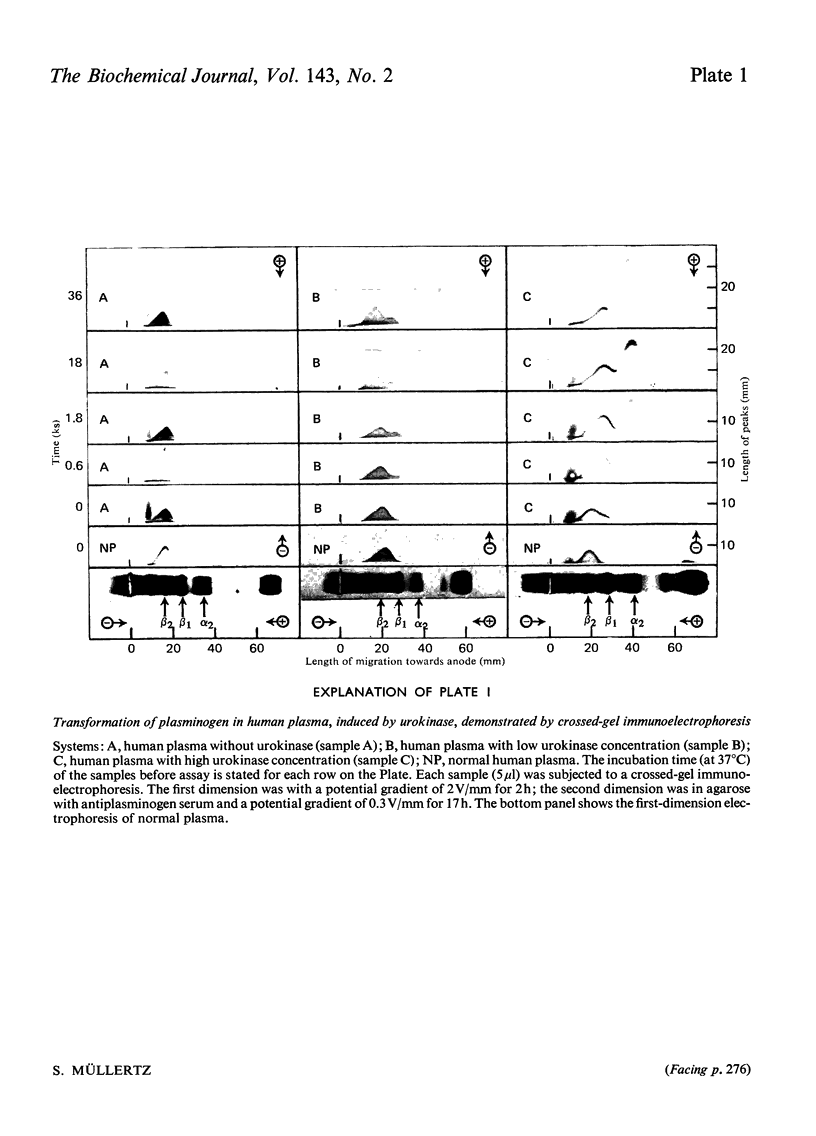
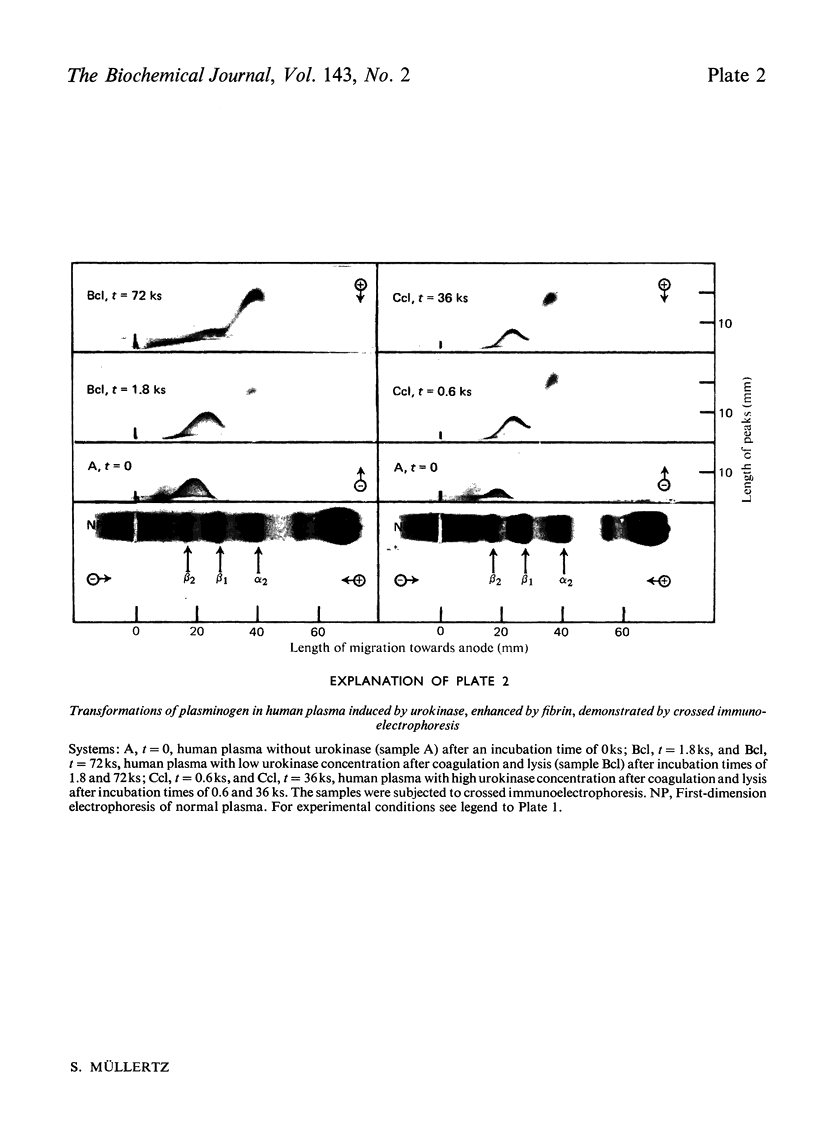
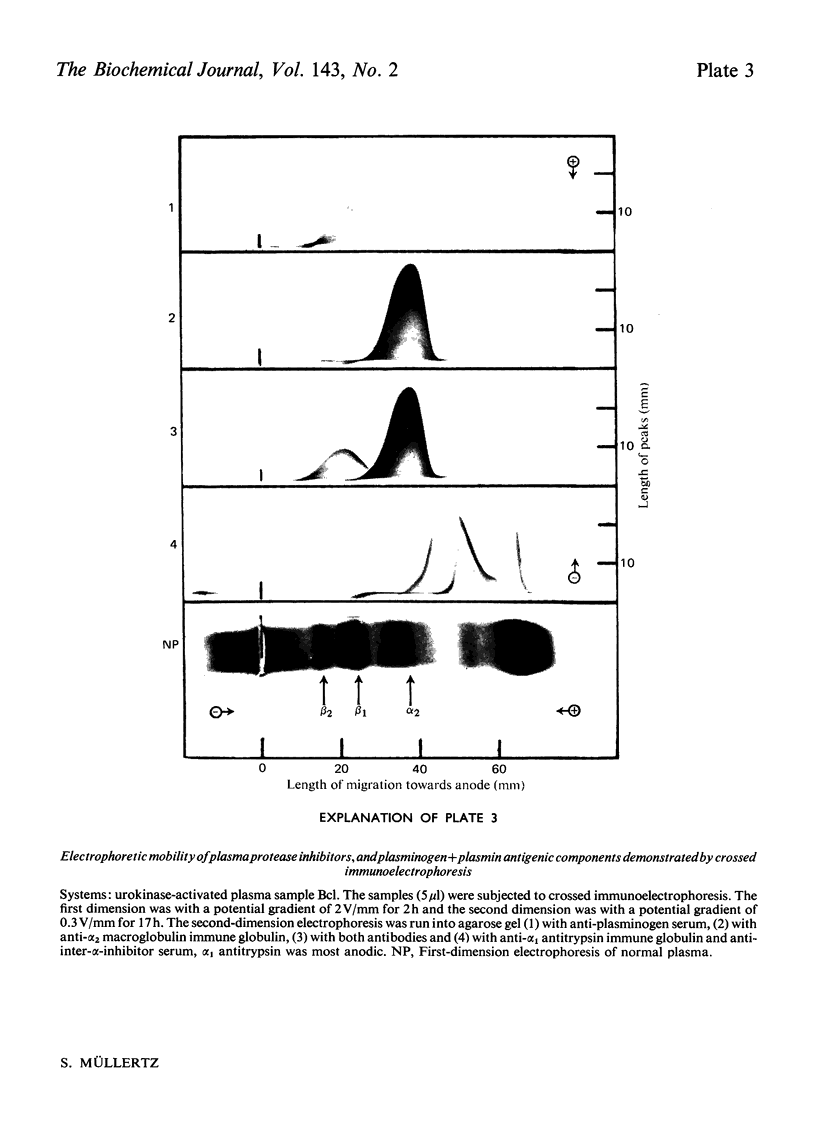
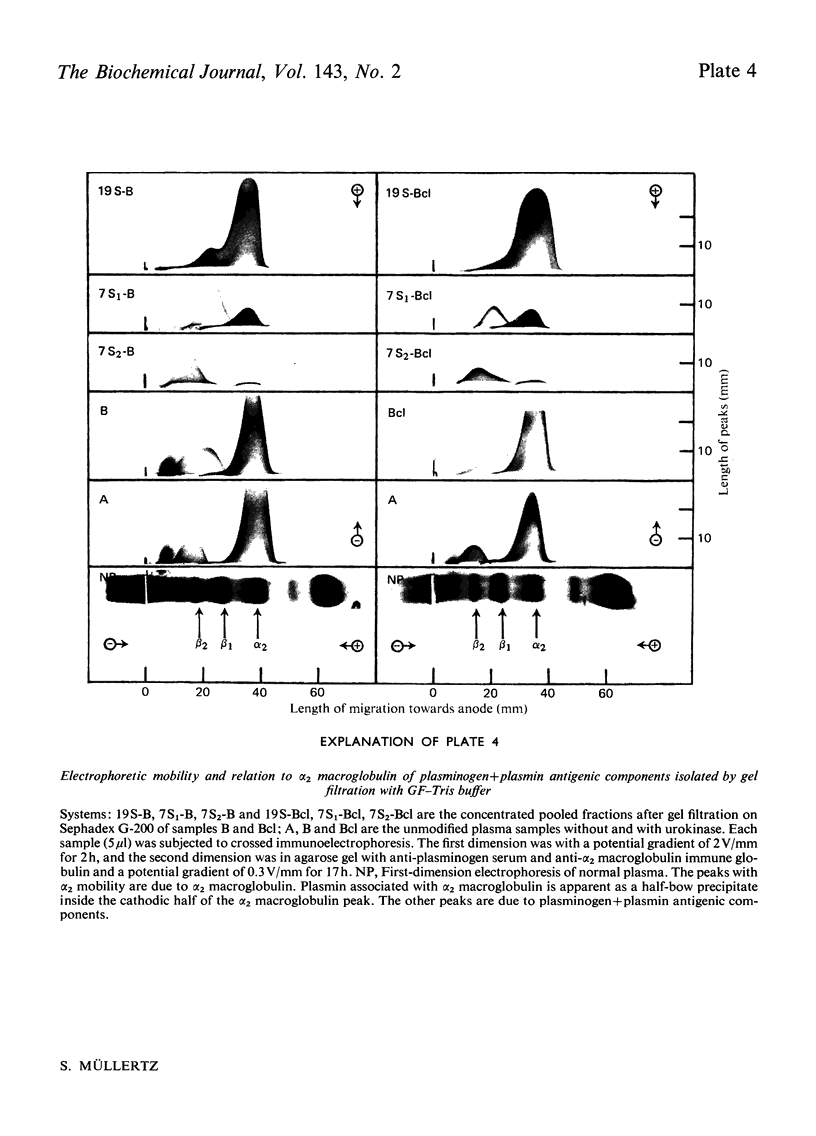
Abstract
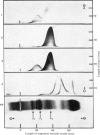
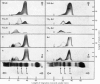
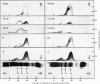
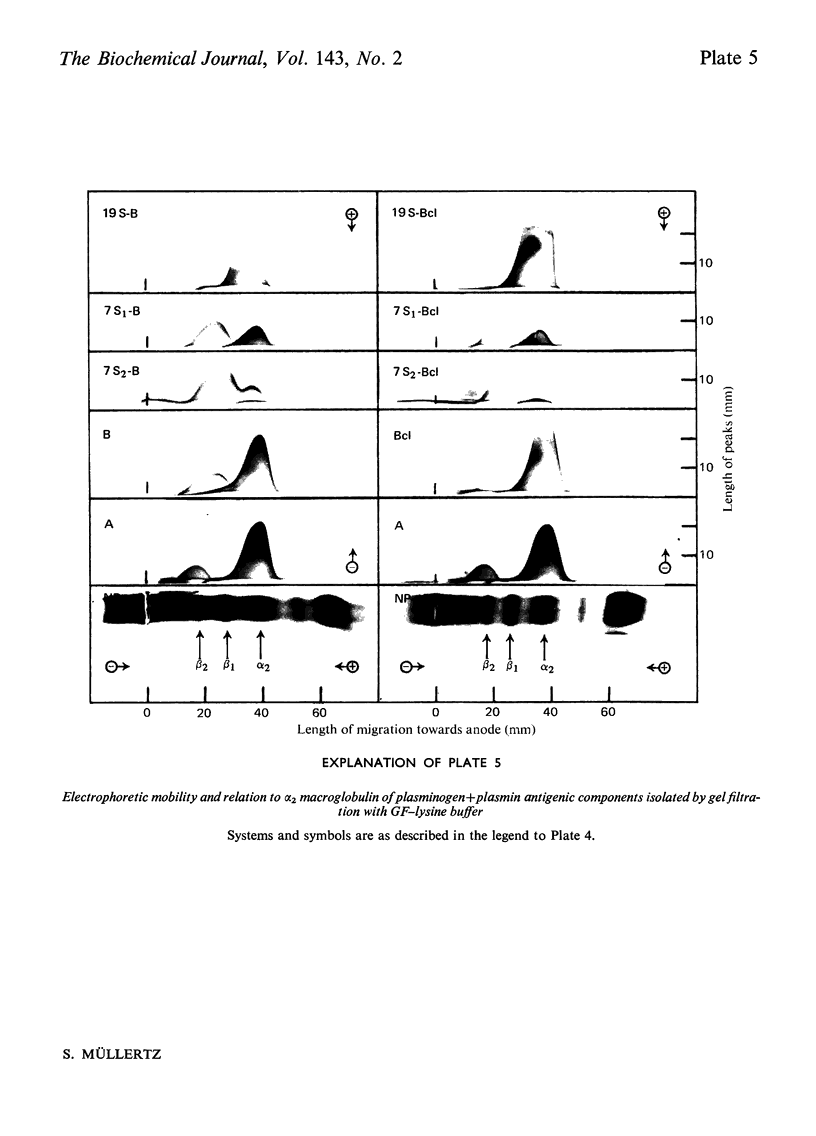
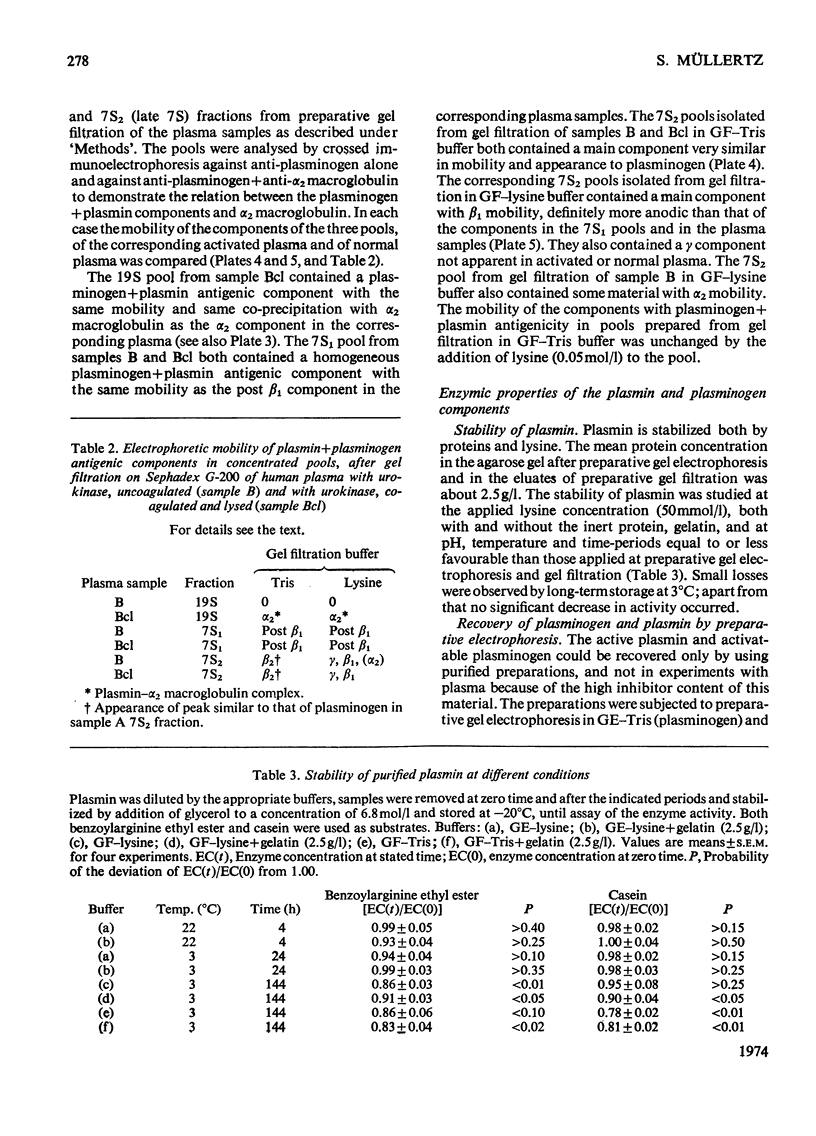
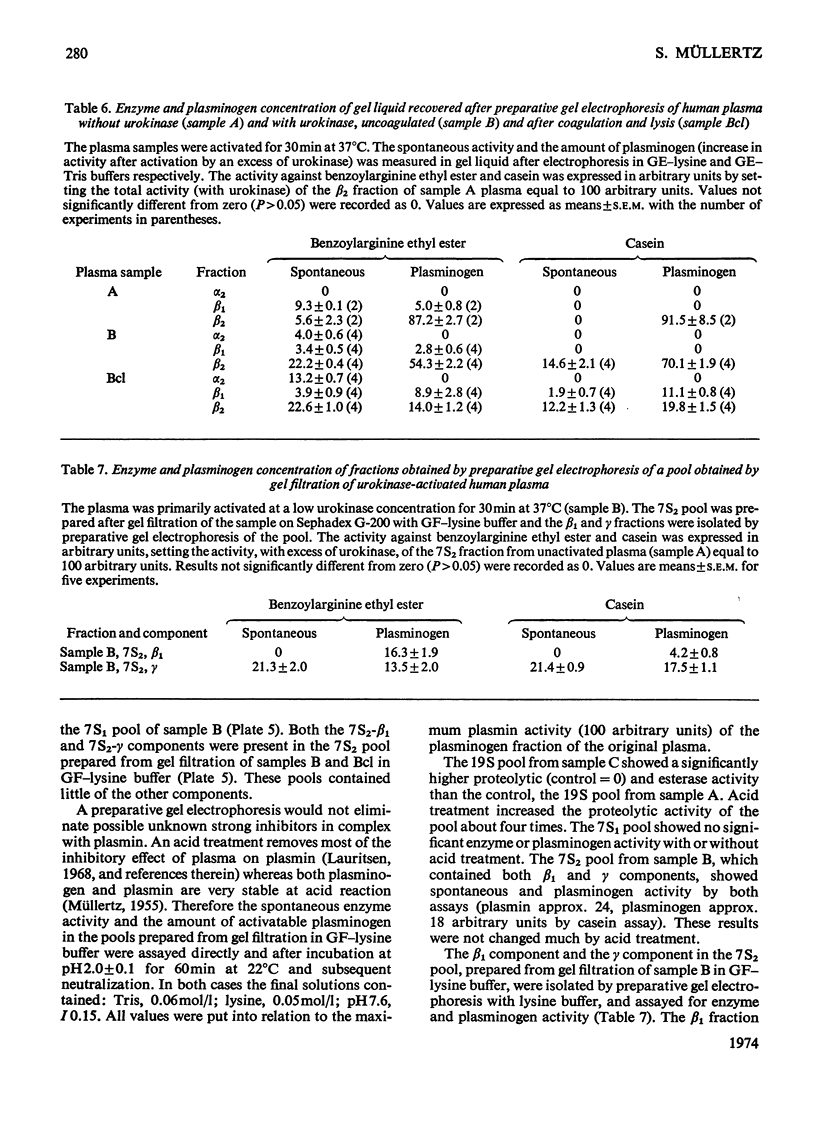
Urokinase-activated human plasma was studied by gel electrophoresis, gel filtration, crossed immunoelectrophoresis and electroimmunoassay with specific antibodies and by assay of esterase and protease activity of isolated fractions. Urokinase induced the formation of different components with plasminogen+plasmin antigenicity. At low concentrations of urokinase, a component with a KD value of 0.18 by gel filtration and post β1 mobility by gel electrophoresis was detected. The isolated component had no enzyme or plasminogen activity. In this plasma sample fibrinogen was not degraded for 10h, but when fibrin was formed, by addition of thrombin, fibrin was quickly lysed, and simultaneously a component with a KD value of 0 and α2 mobility appeared, which was probably plasmin in a complex with α2 macroglobulin. This complex showed both esterase and protease activity. After gel filtration with lysine buffer of the clotted and lysed plasma another two components were observed with about the same KD value by gel filtration as plasminogen (0.35), but β1 and γ mobilities by gel electrophoresis. They appeared to be modified plasminogen molecules, and possibly plasmin with γ mobility. Similar processes occurred without fibrin at higher urokinase concentrations. Here a relatively slow degradation of fibrinogen was correlated to the appearance of the plasmin–α2 macroglobulin complex. The fibrin surface appeared to catalyse the ultimate production of active plasmin with a subsequent preferential degradation of fibrin and the formation of a plasmin–α2 macroglobulin complex. The gel filtration and electrophoresis of the plasma protease inhibitors, α1 antitrypsin, inter-α-inhibitor, antithrombin III, and C1-esterase inhibitor indicated that any complex between plasmin and these inhibitors was completely dissociated. The β1 and post β1 components appear to lack correlates among components occurring in purified preparations of plasminogen and plasmin.
Full text
PDF


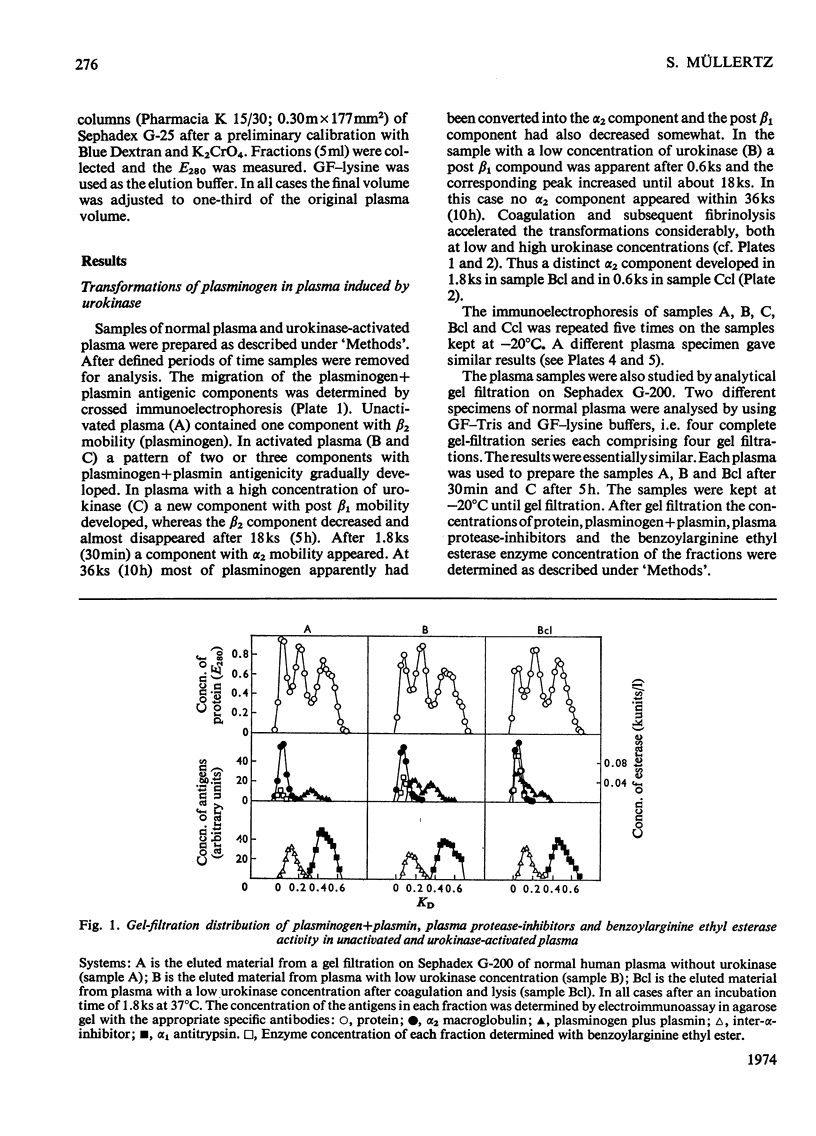





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Ganrot P. O. Crossed immunoelectrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:39–47. doi: 10.3109/00365517209102749. [DOI] [PubMed] [Google Scholar]
- Johansson B. G. Agarose gel electrophoresis. Scand J Clin Lab Invest Suppl. 1972;124:7–19. doi: 10.3109/00365517209102747. [DOI] [PubMed] [Google Scholar]
- Kok P., Astrup T. Isolation and purification of a tissue plasminogen activator and its comparison with urokinase. Biochemistry. 1969 Jan;8(1):79–86. doi: 10.1021/bi00829a013. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Lauritsen O. S. Activation of porcin plasminogen by human urokinase. Scand J Clin Lab Invest. 1968;22(3):239–246. doi: 10.3109/00365516809166495. [DOI] [PubMed] [Google Scholar]
- MULLERTZ S. Formation and properties of the activator of plasminogen and of human and bovine plasmin. Biochem J. 1955 Nov;61(3):424–434. doi: 10.1042/bj0610424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLERTZ S. The action of plasmin on fibrin and fibrinogen in blood. Acta Physiol Scand. 1953 Mar 31;28(1):29–40. doi: 10.1111/j.1748-1716.1953.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Müllertz S. Molecular forms of plasmin and protease inhibitors in human fibrinolytic post-mortem plasma. Scand J Clin Lab Invest. 1972 Dec;30(4):369–379. doi: 10.3109/00365517209080272. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D. Studies on a proteolytic enzyme in human plasma. IX. Fibrinogen and fibrin as substrates for the proteolytic enzyme of plasma. J Clin Invest. 1953 Jun;32(6):473–479. doi: 10.1172/JCI102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner S. F., Goldfine I. D., Hart A., Summaria L., Robbins K. C. Radioimmunoassay of human plasminogen and plasmin. J Lab Clin Med. 1969 Aug;74(2):265–273. [PubMed] [Google Scholar]
- Rickli E. E., Cuendet P. A. Isolation of plasmin-free human plasminogen with N-terminal glutamic acid. Biochim Biophys Acta. 1971 Nov 13;250(2):447–451. doi: 10.1016/0005-2744(71)90202-6. [DOI] [PubMed] [Google Scholar]
- Rickli E. E., Otavsky W. I. Release of an N-terminal peptide from human plasminogen during activation with urokinase. Biochim Biophys Acta. 1973 Jan 25;295(1):381–384. doi: 10.1016/0005-2795(73)90106-2. [DOI] [PubMed] [Google Scholar]
- Summaria L., Arzadon L., Bernabe P., Robbins K. C. Studies on the isolation of the multiple molecular forms of human plasminogen and plasmin by isoelectric focusing methods. J Biol Chem. 1972 Jul 25;247(14):4691–4702. [PubMed] [Google Scholar]
- Summaria L., Arzadon L., Bernabe P., Robins K. C. Characterization of the NH 2 -terminal glutamic acid and NH 2 -terminal lysine forms of human plasminogen isolated by affinity chromatography and isoelectric focusing methods. J Biol Chem. 1973 May 10;248(9):2984–2991. [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Robbins K. C. The specific mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 Oct 10;242(19):4279–4283. [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. I. On the relationship between different molecular forms of plasminogen demonstrated in plasma and found in purified preparations. Biochim Biophys Acta. 1970 Oct 20;221(1):20–30. [PubMed] [Google Scholar]
- Wallén P., Wiman B. Characterization of human plasminogen. II. Separation and partial characterization of different molecular forms of human plasminogen. Biochim Biophys Acta. 1972 Jan 26;257(1):122–134. doi: 10.1016/0005-2795(72)90261-9. [DOI] [PubMed] [Google Scholar]
- Wiman B., Wallén P. Activation of human plasminogen by an insoluble derivative of urokinase. Structural changes of plasminogen in the course of activation to plasmin and demonstration of a possible intermediate compound. Eur J Biochem. 1973 Jul 2;36(1):25–31. doi: 10.1111/j.1432-1033.1973.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Worning H., Müllertz S. pH and pancreatic enzymes in the human duodenum during digestion of a standard meal. Scand J Gastroenterol. 1966;1(4):268–283. doi: 10.1080/00365521.1966.11800642. [DOI] [PubMed] [Google Scholar]