Abstract
Objectives
To describe changes in respiratory syncytial virus (RSV) epidemiology, its associated clinical outcomes and predictors of severe acute lower respiratory tract infection (ALRTI) pre- and post-COVID-19.
Methods
In this retrospective cohort, we analysed data from electronic medical record of children <5 years who were hospitalized at Jordan University Hospital with RSV-associated ALRTI from 2018 to 2022.
Results
325 inpatients with respiratory infections were included. Rate of RSV infections decreased from 74% pre-pandemic to 30% post-pandemic. Patients diagnosed with ALRTI post-COVID had significantly higher SpO2, less chronic disease, lower temperature and respiratory rate at admission and fewer days in hospital compared with those diagnosed pre-COVID. Furthermore, patients diagnosed pre-pandemic were significantly more likely to have abnormal X-rays, used more antibiotics and antivirals, and had higher rates of severe disease than those with infection post-COVID.
Conclusion
COVID-19 and its associated social restriction measures led to changes in RSV epidemiology, characterized by a decline in rates and clinical severity in the post-pandemic period. However, further studies are needed to characterize the impact of COVID-19 on subsequent RSV seasons.
Keywords: COVID-19, respiratory syncytial virus, lower respiratory tract infections, Jordan, epidemiology
Introduction
Respiratory syncytial virus (RSV), a member of the pneumoviridae family, is a common global cause of acute lower respiratory tract infection (ALRTI). 1 The virus affects both children and vulnerable groups including the elderly and those with weakened immune systems.2,3 Although RSV symptoms can vary from mild to severe, it places a considerable burden on children, being the leading cause of hospitalization in neonates and contributing to approximately 200,000 deaths annually among children under 5 years old in low-income countries. 4 The World Health Organization (WHO) estimates that >60% of acute respiratory infections in children and >80% in infants younger than one year are due to RSV. 5 In addition, RSV is linked to over 50% of hospitalized acute bronchiolitis cases in North America and nearly 40% of severe pneumonia cases in developing countries. 2 Due to its burden, RSV infections are a pertinent public health issue. Importantly, the burden is believed to be partially associated with a lack of virus-specific vaccination. 6
RSV is characterized by distinct seasonality, with the disease burden typically peaking during colder temperatures and higher humidity levels. 7 In temperate countries, RSV is most common during the winter months, while in tropical and subtropical regions, it tends to peak during the rainy season.3,8 However, the outbreak of the COVID-19 pandemic significantly intervened with the seasonality of RSV and its burden by association. Studies indicate that in 2020, the typical autumn and winter RSV epidemics were nearly absent, with some countries experiencing an unprecedented shift in the RSV season to spring. 9 Additionally, in countries with cold and dry climates, RSV has shown an unusual pattern, with a large early epidemic followed by a smaller, delayed epidemic the following year. 10 Due to the similar route of transmission between COVID-19 and RSV, public and social health measures (e.g., masks, social distancing, hand hygiene) were believed to have an enormous impact on viral diseases during the pandemic. 11 Furthermore, widespread changes in medication-related behaviours, such as the increased use of antibiotics, have also been linked to alterations in RSV seasonality. 12
Interestingly, these shifts in RSV seasonality were linked to distinct clinical outcomes. For instance, delayed RSV outbreaks were linked to high hospitalization rates, even though the clinical presentations were mild.6,7,9–11 Therefore, in the post-pandemic era, it is crucial to understand the changes in RSV seasonality and their associated clinical outcomes, to optimize treatment strategies and anticipate control measures if similar shifts in seasonality occur in the future. This study aimed to characterize the epidemiology of RSV, its associated clinical outcomes, and the predictors of severe ALRTI, based on data extracted from a cohort of hospitalized paediatric patients at a tertiary hospital in Jordan.
Methodology
Patients
This retrospective, observational study was conducted among hospitalised patients at Jordan University Hospital, a tertiary care facility located in Amman, Jordan. The study covered the periods from 1 January 2018 to 11 March 2020 (pre COVID) and from 11 March 2020 to 31 December 2022 (post-COVID).
Children under 5 years of age who had undergone nasopharyngeal aspiration (NPA) followed by viral polymerase chain reaction (PCR) testing, and, had tested positive for RSV were included in the study. Nasopharyngeal swabs were collected in Copan universal transport and preservation media and stored at −20°C. Conventional real-time PCR (FTD Respiratory Pathogens 21 Assay Kit and EZ 1 and 2 Virus Mini Kit V2.0 by Qiagen), was used for the nucleic acid extraction. Viruses detected included: RSV; adenovirus (AdV-B, AdV-C); rhinovirus (A, B &C); enterovirus; parainfluenza viruses 1–4; rotavirus; parechovirus; bocavirus; metapneumovirus; influenza (type A, B, C, and A/B); Coronavirus SARS-CoV-2.
The reporting of this study conforms to STROBE guidelines. 13 The study obtained formal approval from the University of Jordan Institutional Review Board (Approval #2022-2023/6). Written informed consent was not required due to the retrospective design of the study and patient data were anonymized prior to analysis.
Clinical data
The following data were extracted from the patients’ electronic medical records: age; sex; prematurity (defined as gestational age <37 weeks); low birth weight (defined as birth weight <2.5 kg); 14 neonatal intensive care unit (NICU) admission; infection exposure; smoking exposure; presence of chronic disease; received flu vaccine; antibiotic use before hospitalization.
Other data (collected on admission) included: oxygen saturation (SpO2); temperature; respiratory rate (RR); presence of signs of respiratory distress (e.g., grunting, nasal flaring, retractions); results of plain radiographs (reviewed and interpreted by a radiologist blinded to patients’ clinical data). In addition, the following were noted: admission to paediatric intensive care unit (PICU); length of hospital stay (LoS); mechanical ventilation requirement; use of oxygen supplementation; duration of oxygen supplementation; antibiotic use; corticosteroid use; antiviral drug use.
Patients were classified as having severe ALRTI if they had a cough or difficulty in breathing in addition to one or more of the following: grunting; nasal flaring; retractions; SpO2 <90%; ICU admission; severe tachypnoea (i.e., respiratory rate >60/min); death. 15
Statistical analysis
Statistical analysis was performed using SPSS software (version 26.0 for Windows®; IBM Corp, Armonk, NY, USA). A P-value <0.5 was considered to indicate statistical significance. Data were described in terms of median and range, or absolute numbers and percentages as appropriate. Comparison of categorical variables between groups were performed using χ2 tests.
Comparison of continuous variables between groups was performed using Mann Whitney U test for independent samples or Kruskal-Wallis test, as appropriate. Predictors of severe ALRTI were assessed using a multivariate binary logistic regression model. Results were presented as odds ratio (OR) and 95% confidence intervals (CIs).
Results
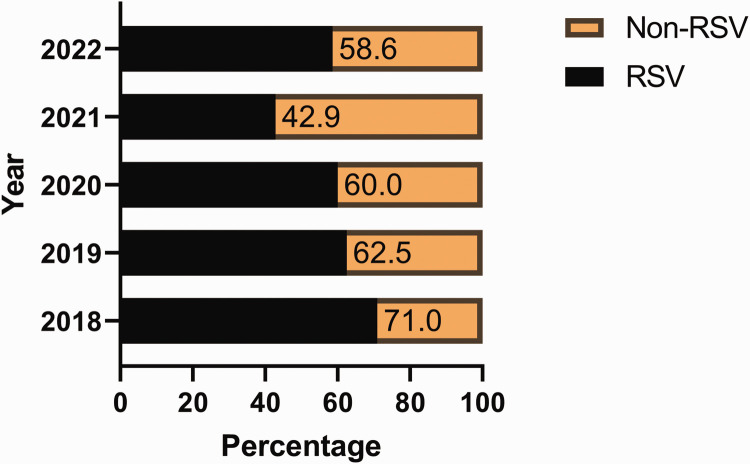
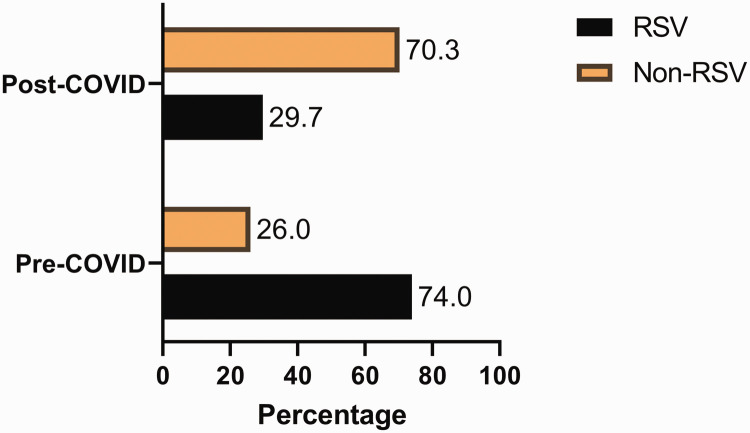
In total, 325 hospitalised paediatric patients with ALRTI were included. The incidence of RSV infections decreased from 71% in 2018 to 43% in 2021, but increased to 59% in 2022. (Figure 1). The rate of RSV infections decreased from 74% pre COVID to 30% post COVID (Figure 2). By contrast, non-RSV infections increased from 26% pre COVID to 70% post COVID.
Figure 1.
Distribution of in-patient respiratory infections from 2018 to 2022.
Figure 2.
Distribution of in-patient respiratory pre- and post-COVID pandemic.
Although age and sex ratio of the patients did not differ across the years, other clinical characteristics were significantly different (Table 1). For example, there were significant differences among, prematurity, low birth rate, breast feeding, infection exposure, smoking exposure, and incidence of chronic disease. Other clinical variables with significant differences included: SpO2; temperature at diagnosis; RR at diagnosis; LoS; respiratory distress; abnormal x-ray findings; PICU/NICU admissions; O2 supplementation; duration of O2 supplementation. The rate of medications (i.e., antibiotics, corticosteroids, antiviral drugs) was also significantly different across the years. Finally, the incidence of severe ALRTI significantly differed across the years.
Table 1.
Characteristics of participants by year of admission.
| Characteristics | Total(N = 325) | 2018(N = 45) | 2019(N = 58) | 2020(N = 80) | 2021(N = 47) | 2022(N = 95) | Statistical significance |
|---|---|---|---|---|---|---|---|
| Age, months | 5.0 (2.0–10.0) | 3.5 (1.5–8.0) | 5 (2.5–12.0) | 4 (1.6–9.0) | 6 (3.0–12.0) | 5 (1.5–11.0) | ns |
| Sex, M: F | 191/134 | 23/22 | 38/20 | 47/33 | 31/16 | 52/43 | ns |
| Prematurity | 62/301 (21) | 11 (24) | 16 (36) | 13 (17) | 5 (11) | 17 (19) | P = 0.047 |
| Low birth weight | 69/291 (24) | 10 (22) | 19 (41) | 12 (17) | 8 (17) | 20 (24) | P = 0.033 |
| NICU admission | 102/313 (33) | 12 (27) | 19 (39) | 25 (33) | 11 (23) | 35 (37) | ns |
| Breastfeeding | 39/144 (27) | 12 (100) | 2 (100) | 4 (10) | 11 (32) | 10 (19) | P < 0.000 |
| Daycare attendance | 19/176 (11) | 0 (0) | 1 (10) | 7 (11) | 3 (8) | 8 (16) | ns |
| Infection exposure | 99/186 (53) | 2 (17) | 3 (27) | 37 (54) | 24 (67) | 33 (57) | P = 0.015 |
| Smoking exposure | 40/122 (33) | 0 (0) | 1 (14) | 13 (33) | 10 (29) | 16 (50) | P = 0.048 |
| Chronic disease | 80/325 (25) | 14 (31) | 23 (40) | 17 (21) | 3 (6) | 23 (24) | P = 0.002 |
| Flu vaccine | 3/265 (1) | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 1 (1) | NA |
| Antibiotics (previous) | 37/266 (14) | 1 (5) | 6 (13) | 15 (20) | 2 (5) | 13 (16) | ns |
| SpO2 | 91.2 (88.0–95.0) | 86.5 (80.0–89.5) | 90 (86.0–94.0) | 91 (88.0–96.0) | 95 (93.0–97.0) | 93 (89.0–96.0) | P < 0.000 |
| Temperature at admission, °C | 37.0 (36.6–38.0) | 38.5 (38.0–38.5) | 38.7 (38.5–39.2) | 37 (36.7–37.1) | 37.7 (36.6–38.0) | 36.8 (36.6–37.6) | P < 0.000 |
| RR at admission, breaths/min | 50.0 (40.0–60.0) | 50 (47.0–65.0) | 53 (40.0–65.0) | 50 (40.0–60.0) | 50 (44.0–50.0) | 45 (33.5–55.0) | P = 0.011 |
| Length of stay, days | 6 (4–9) | 7 (5–9) | 6 (4–8) | 7 (5–9) | 4 (3–5) | 6 (4–10) | P < 0.000 |
| Respiratory distress | 204/323 (63) | 42 (93) | 34 (59) | 40 (50) | 22 (47) | 66 (71) | P < 0.000 |
| Abnormal X-ray | 211/287 (74) | 38 (95) | 42 (81) | 45 (60) | 27 (61) | 59 (78) | P = 0.000 |
| X-ray findings | NA | ||||||
| Missing | 38/325 (12) | 5 (11) | 6 (10) | 5 (6) | 3 (6) | 19 (20) | ns |
| Consolidation | 43/325 (13) | 7 (16) | 9 (16) | 14 (18) | 0 (0) | 13 (14) | ns |
| Hyperinflation | 62/325 (19) | 10 (22) | 11 (19) | 25 (31) | 11 (23) | 5 (5) | ns |
| Interstitial infiltrates | 106/325 (33) | 21 (47) | 22 (38) | 6 (8) | 16 (34) | 41 (43) | ns |
| Normal | 76/325 (23) | 2 (4) | 10 (17) | 30 (38) | 17 (36) | 17 (18) | ns |
| PICU/NICU admission | 156/322 (48) | 27 (61) | 18 (31) | 55 (71) | 6 (13) | 50 (53) | P < 0.000 |
| Mechanical ventilation | 23/323 (7) | 5 (11) | 3 (5) | 7 (9) | 0 (0) | 8 (8) | ns |
| O2 supplementation | 240/273 (88) | 44 (98) | 39 (77) | 77 (99) | 40 (87) | 40 (76) | P < 0.000 |
| Duration O2 supplementation, days | 4 (2–7) | 5 (3–7) | 5 (2–8) | 5 (3–7) | 3 (2–4) | 4 (2–7) | P < 0.000 |
| Antibiotics | 251/315 (80) | 41 (93) | 53 (91) | 63 (81) | 28 (68) | 66 (70) | P = 0.001 |
| Corticosteroids | 186/315 (59) | 16 (36) | 38 (66) | 54 (69) | 29 (71) | 49 (52) | P = 0.001 |
| Antiviral drugs | 67/314 (21) | 16 (36) | 13 (22) | 26 (33) | 6 (15) | 6 (7) | P < 0.000 |
| severe ALRTI | 244/322 (76) | 43 (96) | 44 (77) | 62 (78) | 23 (51) | 72 (76) | P < 0.000 |
Data are expressed as n/N (%) or median (interquartile range).
NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; SpO2, oxygen saturation; RR, respiratory rate; ALRTI, acute lower respiratory tract infection; not statistically significant
Comparison of clinical variables over the years separated into severe and non-severe ALRTI groups, showed significant differences between the groups (Table 2). For example, by comparison with patients with non-severe disease, patients with severe disease were more likely to be younger (P < 0.001), premature (P < 0.001), have low birth weight (P = 0.007), and a history of NICU admissions (P = 0.001). In terms of clinical characteristics, the non-severe disease group had significantly higher SpO2, lower RR, lower LoS, fewer cases of respiratory distress, and fewer abnormal X-ray findings (all P < 0.001) than the severe disease group. Importantly, patients in the severe disease group had significantly more PICU/NICU admissions (P < 0.001), requirement for mechanical ventilation (P = 0.02), O2 supplementation (P < 0.001), and greater duration of O2 supplementation (P < 0.001) than patients in the non-severe disease group. In terms of medications, by comparison with patients in the non-severe group, patients with severe disease had more antibiotics (P = 0.028) and antiviral drugs (P = 0.012).
Table 2.
Characteristics of participants stratified by clinical status (N = 322*).
| Characteristics | Non severe ALRTI(N = 78) | Severe ALRTI(N = 244) | Statistical significance |
|---|---|---|---|
| Age, months | 7 (4.0–15.0) | 4 (1.6–9.0) | P < 0.000 |
| Sex, M: F | 48/30 | 141/103 | ns |
| Prematurity | 3 (4) | 58 (25) | P < 0.000 |
| Low birth weight | 7 (11) | 62 (28) | P = 0.007 |
| NICU admission | 13 (18) | 89 (38) | P = 0.001 |
| Breastfeeding | 9 (24) | 30 (29) | ns |
| Daycare attendance | 6 (15) | 13 (10) | ns |
| Infection exposure | 30 (63) | 68 (50) | ns |
| Smoking exposure | 11 (28) | 28 (35) | ns |
| Chronic disease | 21 (27) | 59 (24) | ns |
| Flu vaccine | 0 (0) | 3 (2) | NA |
| Antibiotics (previous) | 10 (15) | 26 (13) | ns |
| SpO2 | 95 (94.0–97.0) | 90 (85.0–95.0) | P < 0.000 |
| Temperature at admission, °C | 37 (36.7–38.0) | 37 (36.6–38.0) | ns |
| RR at admission, breaths/min | 40 (32.0–50.0) | 50 (44.0–62.0) | P < 0.000 |
| Length of stay, days | 4 (3.0–5.0) | 7 (5.0–10.0) | P < 0.000 |
| Respiratory distress | 1 (1) | 203 (83) | P < 0.000 |
| Abnormal X-ray | 35 (52) | 176 (81) | P < 0.000 |
| X-ray findings | NA | ||
| Missing | 10 (13) | 27 (11) | |
| Consolidation | 4 (5) | 39 (16) | |
| Hyperinflation | 13 (17) | 49 (20) | |
| Interstitial infiltrates | 18 (23) | 88 (36) | |
| Normal | 33 (42) | 41 (17%) | |
| PICU/NICU admission | 9 (12) | 147 (61) | P < 0.000 |
| Mechanical ventilation | 1 (1) | 22 (9) | P = 0.020 |
| O2 supplementation | 45 (69) | 194 (95) | P < 0.000 |
| Duration O2 supplementation, days | 2 (1.0–4.0) | 5 (3.0–7.0) | P < 0.000 |
| Antibiotics | 52 (71) | 199 (83) | P = 0.028 |
| Corticosteroids | 45 (62) | 140 (58) | ns |
| Antiviral drugs | 8 (11) | 59 (25) | P = 0.012 |
Data are expressed as n/N (%) or median (interquartile range).
Data missing for 3 patients
NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; SpO2, oxygen saturation; RR, respiratory rate; ALRTI, acute lower respiratory tract infection; not statistically significant
Interestingly, the rate of co-infection with bacteria or other viruses did not significantly differ between groups (non-severe, 28%, severe 29%). Co-infection with rhinovirus was most frequently observed in both groups (non-severe, 14%, severe 11%). Next most common was adenovirus (non-severe, 4%, severe 2%) followed by coronavirus SARS-CoV-2 (non-severe, 1%, severe 4%).
Compared with patients diagnosed pre-COVID, fewer patients diagnosed post-COVID were premature or breastfed (P < 0.05). Moreover, patients diagnosed post-COVID had significantly higher SpO2, less chronic disease, lower temperature and RR at admission and LoS compared with those diagnosed pre-COVID (all P < 0.05; Table 3). Furthermore, patients diagnosed post-COVID were significantly less likely to have abnormal X-rays (P < 0.001), PICU/NICU admission (P = 0.016), require O2 supplementation long-term (P < 0.001), require antibiotics (P < 0.001), or antiviral drugs (P < 0.001) compared with those diagnosed pre-COVID. Interestingly, patients diagnosed post-COVID had reduced rates of severe ALRTI (P = 0.033).
Table 3.
Characteristics of participants stratified according to pandemic status.
| Characteristics | Pre-COVID(n = 148) | Post-COVID(n = 177) | Statistical significance |
|---|---|---|---|
| Age, months | 5 (2.0–10.0) | 5 (1.7–10.0) | ns |
| Sex, M: F | 92/56 | 99/78 | ns |
| Prematurity | 34 (26) | 28 (17) | P = 0.044 |
| Low birth weight | 37 (28) | 32 (20) | ns |
| NICU admission | 44 (32) | 58 (33) | ns |
| Breastfeeding | 16 (47) | 23 (21) | P = 0.003 |
| Daycare attendance | 8 (13) | 11 (10) | ns |
| Infection exposure | 27 (44) | 72 (58) | ns |
| Smoking exposure | 10 (30) | 30 (34) | ns |
| Chronic disease | 51 (35) | 29 (16) | P < 0.000 |
| Flu vaccine | 2 (2) | 1 (1) | ns |
| Antibiotics (previous) | 12 (11) | 25 (16) | ns |
| SpO2 | 88.5 (85.0–92.0) | 95 (90.0–96.0) | P < 0.000 |
| Temperature at admission, °C | 37.5 (37.0–38.7) | 36.9 (36.6–37.9) | P < 0.000 |
| RR at admission, breaths/min | 52 (45.0–63.0) | 47 (37.0–55.5) | P < 0.000 |
| Length of stay, days | 7 (4.0–9.0) | 5 (4.0–8.0) | P = 0.017 |
| Respiratory distress | 97 (66) | 107 (61) | ns |
| Abnormal X-ray | 112 (84) | 99 (65) | P < 0.000 |
| X-ray findings | NA | ||
| Missing | 14 (10) | 24 (14) | |
| Consolidation | 26 (18) | 17 (10) | |
| Hyperinflation | 42 (28) | 20 (11) | |
| Interstitial infiltrates | 44 (30) | 62 (35) | |
| Normal | 22 (15) | 54 (31) | |
| PICU/NICU admission | 81 (56) | 75 (42) | P = 0.016 |
| Mechanical ventilation | 13 (9) | 10 (6) | ns |
| O2 supplementation | 126 (90) | 114 (86) | ns |
| Duration O2 supplementation, days | 5 (3.0–7.0) | 4 (2.0 –5.0) | P = 0.002 |
| Antibiotics | 130 (88) | 121 (72) | P < 0.000 |
| Corticosteroids | 91 (62) | 95 (57) | ns |
| Antiviral drugs | 46 (31) | 21 (13) | P < 0.000 |
| severe ALRTI | 51 (35) | 42 (24) | P = 0.033 |
Data are expressed as n/N (%) or median (interquartile range).
NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; SpO2, oxygen saturation; RR, respiratory rate; ALRTI, acute lower respiratory tract infection; not statistically significant
Multivariate analysis showed that febrile status at admission (OR: 2.92; 95%CI: 1.01–8.41), abnormal X-ray findings (OR: 3.63; 95% CI: 1.49–8.91), PICU/NICU admissions (OR: 4.31; 95% CI: 1.46–12.80), O2 supplementation (OR: 5.10; 95% CI: 1.30–19.93), and increased length of hospitalization (OR: 1.21; 95% CI: 1.00–1.46) were significantly positive predictors of severe ALRTI (P < 0.05; Table 4).
Table 4.
Predictors of severe ALRTI.
| Variable | Odds Ratio | 95% CIs | Statistical significance |
|---|---|---|---|
| Prematurity | 3.21 | 0.48–21.7 | ns |
| Low birth weight | 0.94 | 0.25–3.49 | ns |
| Febrile at admission | 2.92 | 1.02–8.41 | P = 0.047 |
| Abnormal X-ray findings | 3.64 | 1.49–8.91 | P = 0.005 |
| PICU/NICU admission | 4.32 | 1.46–12.80 | P = 0.008 |
| O2 supplementation | 5.10 | 1.30–19.93 | P = 0.019 |
| Antibiotic use | 1.16 | 0.42–3.20 | ns |
| Corticosteroid use | 0.97 | 0.41–2.28 | ns |
| Antiviral drug use | 1.24 | 0.39–3.99 | ns |
| Length of hospital stay | 1.21 | 1.00–1.46 | P = 0.050 |
ALRTI, acute lower respiratory tract infection; NICU, neonatal intensive care unit; PICU, paediatric intensive care unit; CIs, confidence intervals; not statistically significant
Discussion
The results of this study indicate that the incidence of ALRTI fluctuated throughout the data collection period. It decreased during the COVID-19 pandemic years, with a gradual return to pre-pandemic levels beginning in 2022. Patients with severe ALRTI were younger, premature at birth, had lower birth weight, and required more interventions than patients with non-severe ALRTI. However, co-infection rates did not differ by disease severity. Multivariate analysis showed predictors of severe disease included febrile status and abnormal X-rays at admission, PICU/NICU stay, and oxygen supplementation. Interestingly, patients diagnosed pre-pandemic with severe ALRTI, experienced more severe disease, longer hospitalization, and higher antibiotic and antiviral use compared with those diagnosed post-pandemic.
The shift in the epidemiology and seasonality of RSV during the COVID-19 pandemic has been reported elsewhere.1,3,16–19 Indeed, analysis of the Virus Detection Surveillance System data showed that weekly RSV cases in the U.S. and Canada decreased by nearly 100-fold. 3 Furthermore, several South American and European nations reported no cases of RSV during the COVID-19 pandemic. 3 This phenomenon was linked to several factors, primarily the introduction of stringent public health measures, intra-viral competition and viral interference, as well as pandemic-related disruptions in healthcare logistics.1,4,12 Social restrictions, such as physical distancing, school closures, and limits on public interactions, were identified as primary factors driving changes in RSV seasonality. Complemented by increased hygiene standards during the pandemic, these social restrictions contributed to the reduction of RSV transmission through droplets or contact. 12 However, the decrease in RSV cases may have been due to the purely logistical issue of a globally over-burdened healthcare system. For example, during the pandemic, patients with mild-to-moderate disease were less likely to attend face-to-face services, would only visit emergency departments for life-threatening cases and, tended to rely on tele-consultations. 4 While reduced testing could have been a potential explanation for the decline in RSV cases, this was not the case, as the number of RSV tests actually increased during that period.20,21
Intra-viral competition and its impact on RSV cases has previously been demonstrated in a number of non-COVID instances. For example, during the 2009 influenza pandemic, the Hong Kong H1N1 outbreak, and the adenovirus epidemic in Hunan, delayed peaks in RSV activity were observed in comparison.22–24 Significantly, viral interactions, particularly those involving rhinovirus, were thought to have played a role in delaying the onset of COVID-19 in Europe. 25 Therefore, it is conceivable that once pandemic-related viral interactions stabilized, they may have influenced RSV epidemics.
Consistent with global trends, we have observed a notable decline in severe RSV cases in Jordan during the years following the pandemic. This particular phenomenon is poorly understood and has been attributed to a number of different factors. For instance, some studies have linked the reduction in severity to the older age of infected individuals, as older patients tend to have more resilient airways and stronger immune responses than younger patients. 26 This aligns with the typical age-related patterns of RSV, which tends to cause mild illness in older individuals but can result in more severe or life-threatening outcomes in young children, particularly those with weakened immune systems. 27 Additionally, it has been suggested that variations in RSV severity could be linked to reduced viral activity or geographic factors that may impact the spread and development of different RSV strains. 11 Finally, one study suggested that this shift could be due to the new hygiene practices adopted by parents during the pandemic. 12
Interestingly, we observed an increase in non-RSV cases in the post-pandemic years. Therefore, our findings are consistent with studies that indicated that the flu virus was more effectively suppressed during the COVID period than RSV. 4 This observation implies that the transmission dynamics and viral burden, in the context of implemented public health measures and viral interactions, may differ substantially from one virus to another. However, co-infection is a complex phenomenon, with reported rates varying widely, from nearly absent in some studies to significantly high in others. 3 Additionally, it has been observed that pre-pandemic RSV infections were often complicated by secondary bacterial infections, especially in young patients. 28 Notably, we did not observe a statistically significant effect of co-infections on disease severity of ALRTI.
The pattern of RSV resurgence in several countries during the summer of 2021 has been documented in other studies.1,3 In contrast to historical trends, these atypical RSV resurgences began earlier in the year and lasted longer than previously noted. This phenomenon has been attributed to a number of factors. For example, the relaxation and inconsistent enforcement of social restrictions played a key role. This factor also underscored the potential contribution of adults in the transmission of RSV. 29 It was observed that the increase in RSV cases coincided more with the easing of social restrictions and the reopening of borders than with the reopening of schools and childcare facilities. Additionally, the low level of RSV activity left previously unaffected children vulnerable to an ‘immunity debt’, which impaired their ability to handle severe disease thereafter.30,31 This also resulted in decreased adult immunity to RSV. Another factor could be the changes in climate and the age distribution of the participants, which were believed to have impacted both the transmission and severity of RSV.32,33 It is important to highlight that in our study, the median age at RSV diagnosis did not show a significant difference between the pre- and post-pandemic periods. Overall, the current literature does not link this resurgence to the emergence of a new RSV strain.3,34
In the context of Jordan, the kingdom implemented early and highly stringent lockdown measures. In early March 2020, the Jordanian government imposed a full national lockdown, which was later relaxed to a curfew allowing only designated family members to travel for essential purchases. 35 Additionally, the use of cars was restricted, with exceptions made only for healthcare workers and those in essential sectors. Jordan also imposed fines ranging from $30 to $70 for individuals who failed to adhere to social distancing measures, particularly the requirement to wear masks in public spaces. While these initial measures helped control the COVID-19 infection rate, the relaxation of restrictions in the subsequent months, coupled with the reopening of the economy, contributed to a surge in COVID-19 cases and increased associated case-fatality rates. 36 During the pandemic period, both private and public hospitals were primarily focused on treating COVID-19 cases or high-risk clinical cases with COVID-19-related complications. In addition, technical resources were redirected towards monitoring and analysing COVID-19 data within the kingdom. We are of the opinion that while the efforts to minimize the risk of COVID-19 were commendable, their shortcomings ultimately proved detrimental not only to the economy but also to the entire Jordanian healthcare system. The impact led to a shift in the responsibility for managing common illnesses, such as respiratory infections, from healthcare institutions to individuals. As a result, the burden of these diseases could either have been reduced due to decreased transmission pathways associated with strict preventive measures, or it may have been obscured due to insufficient follow-up and the overwhelming strain placed on the healthcare system by COVID-19.
Our study had several limitations, one of which was the absence of data from the last two seasons, covering the period from December 2022 to 2024. Other notable limitations of our study included a modest sample size, data from a single centre, its retrospective design, and reliance on data from electronic medical records. Moreover, the study lacked RSV typing, and we were unable to demonstrate viral or bacterial distribution among the participants. We were also unable to distinguish between superinfections and early co-infections. Further studies involving large numbers of patients and control groups are required to confirm our results.
In conclusion, consistent with global findings, the COVID-19 pandemic and the socio-behavioural changes it brought about had a significant impact on the epidemiology and clinical manifestations of RSV at a tertiary hospital in Jordan. Further research is needed to uncover the molecular mechanisms behind inter- and intra-virus interactions, as their consequences influence clinical, diagnostic, and therapeutic management decisions. Nevertheless, given the dynamic nature of viral infections, including variations in transmission, shedding duration, host immune responses, and socio-environmental factors, modelling the interactions between viruses is extremely challenging. It is anticipated that populations living in different climates will experience distinct epidemic dynamics, particularly in terms of disease progression and severity.
Footnotes
The authors declare there are no conflicts of interest.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
ORCID iD
Farah A. Khraisat https://orcid.org/0000-0003-0863-8941
References
- 1.Xie LY, Wang T, Yu T, et al. Seasonality of respiratory syncytial virus infection in children hospitalized with acute lower respiratory tract infections in Hunan, China, 2013–2022. Virol J 2024; 21: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi H, Chung CH. Respiratory Syncytial Virus Outbreak in Infants and Young Children during COVID-19 Pandemic in Taiwan. Children (Basel) 2023; 10: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang YC, Lin KP, Wang LA, et al. The Impact of the COVID-19 Pandemic on Respiratory Syncytial Virus Infection: A Narrative Review. Infect Drug Resist 2023; 16: 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu W, Zheng C, Huang S, et al. Epidemiological Trend of RSV Infection Before and During COVID-19 Pandemic: A Three-Year Consecutive Study in China. Infect Drug Resist 2022; 15: 6829–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35: 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutinho Baldoto Gava Chakr V. Which should we fear more in preschoolers and infants: SARS-CoV-2 or respiratory syncytial virus? Postgrad Med J 2022; 98: e7. [DOI] [PubMed] [Google Scholar]
- 7.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory Syncytial Virus Seasonality: A Global Overview. J Infect Dis 2018; 217: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 8.Barbati F, Moriondo M, Pisano L, et al. Epidemiology of Respiratory Syncytial Virus-Related Hospitalization Over a 5-Year Period in Italy: Evaluation of Seasonality and Age Distribution Before Vaccine Introduction. Vaccines (Basel) 2020; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of Coronavirus Disease 2019 Public Health Measures on Detections of Influenza and Respiratory Syncytial Virus in Children During the 2020 Australian Winter. Clin Infect Dis 2021; 72: 2199–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitzer VE, Viboud C, Alonso WJ, et al. Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States. PLoS Pathog 2015; 11: e1004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren L, Lin L, Zhang H, et al. Epidemiological and clinical characteristics of respiratory syncytial virus and influenza infections in hospitalized children before and during the COVID‐19 pandemic in Central China. Influenza Other Respir Viruses 2023; 17: e13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaiut W, Sapbamrer R, Dacha S, et al. Characteristics of Respiratory Syncytial Virus Infection in Hospitalized Children Before and During the COVID-19 Pandemic in Thailand. J Prev Med Public Health 2023; 56: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Eleventh revision of the International Classification of Diseases. World Health Organ [Internet]. 2019. Apr; Available from: https://apps.who.int/gb/ebwha/pdf_files/WHA72/A72_29-en.pdf.
- 15.Bradley JS, Byington CL, Shah SS, et al. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramzali M, Salimi V, Cheraghali F, et al. Epidemiology and clinical features of respiratory syncytial virus (RSV) infection in hospitalized children during the COVID‐19 pandemic in Gorgan, Iran. Health Sci Rep 2024; 7: e1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guadalupe-Fernández V, Martínez-Solanas E, Sabrià-Sunyé A; Epidemiological Surveillance Network of Catalonia et al. Investigating epidemiological distribution (temporality and intensity) of respiratory pathogens following COVID-19 de-escalation process in Catalonia, September 2016–June 2021: Analysis of regional surveillance data. PLOS ONE 2024; 19: e0285892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matera L, Manti S, Petrarca L, et al. An overview on viral interference during SARS-CoV-2 pandemic. Front Pediatr 2023; 11: 1308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong B, Koç U, Bandeira T, et al. Changes in the global hospitalisation burden of respiratory syncytial virus in young children during the COVID-19 pandemic: a systematic analysis. Lancet Infect Dis 2024; 24: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardsley M, Morbey RA, Hughes HE, et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis 2023; 23: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britton PN, Hu N, Saravanos G, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health 2020; 4: e42–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mak GC, Wong AH, Ho WYY, et al. The impact of pandemic influenza A (H1N1) 2009 22, on the circulation of respiratory viruses 2009–2011. Influenza Other Respir Viruses 2012; 6: e6–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Wang X, Msosa T, et al. The impact of the 2009 influenza pandemic on the seasonality of human respiratory syncytial virus: A systematic analysis. Influenza Other Respir Viruses 2021; 15: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nenna R, Matera L, Licari A; ICHRIS Group et al. An Italian Multicenter Study on the Epidemiology of Respiratory Syncytial Virus During SARS-CoV-2 Pandemic in Hospitalized Children. Front Pediatr 2022; 10: 930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickbakhsh S, Mair C, Matthews L, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A 2019; 116: 27142–27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agha R, Avner JR. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics 2021; 148: e2021052089. [DOI] [PubMed] [Google Scholar]
- 27.Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now 2021; 51: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorburn K, Harigopal S, Reddy V, et al. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 2006; 61: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binns E, Koenraads M, Hristeva L, et al. Influenza and respiratory syncytial virus during the COVID-19 pandemic: Time for a new paradigm? Pediatr Pulmonol 2022; 57: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Raya B, Viñeta Paramo M, Reicherz F, et al. Why has the epidemiology of RSV changed during the COVID-19 pandemic? eClinicalMedicine 2023; 61: 102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodi L, Catamerò F, Voarino M, et al. Epidemiology of respiratory syncytial virus in hospitalized children over a 9-year period and preventive strategy impact. Front Pharmacol 2024; 15: 1381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebastien CJ, Javouhey E, Ploin D, et al. Delayed Start of the Respiratory Syncytial Virus Epidemic at the End of the 20/21 Northern Hemisphere Winter Season. Lyon, France [Internet]. medRxiv; 2021. [cited 2024 Apr 2]. p. 2021.03.12.21253446. Available from: https://www.medrxiv.org/content/10.1101/2021.03.12.21253446v1.
- 33.Foley DA, Yeoh DK, Minney-Smith CA, et al. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin Infect Dis Off Dis 2021; 73: e2829–e2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pappa S, Haidopoulou K, Zarras C, et al. Early initiation of the respiratory syncytial virus season in 2021-2022, Greece. J Med Virol 2022; 94: 3453–3456. [DOI] [PubMed] [Google Scholar]
- 35.Khatatbeh M. The Battle Against COVID-19 in Jordan: From Extreme Victory to Extreme Burden. Front Public Health 2020; 8: 634022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Sabbagh MQ, Al-Ani A, Mafrachi B, et al. Predictors of adherence with home quarantine during COVID-19 crisis: the case of health belief model. Psychol Health Med 2022; 27: 215–227. [DOI] [PubMed] [Google Scholar]