Abstract
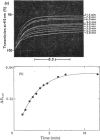
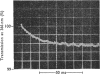
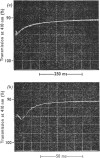
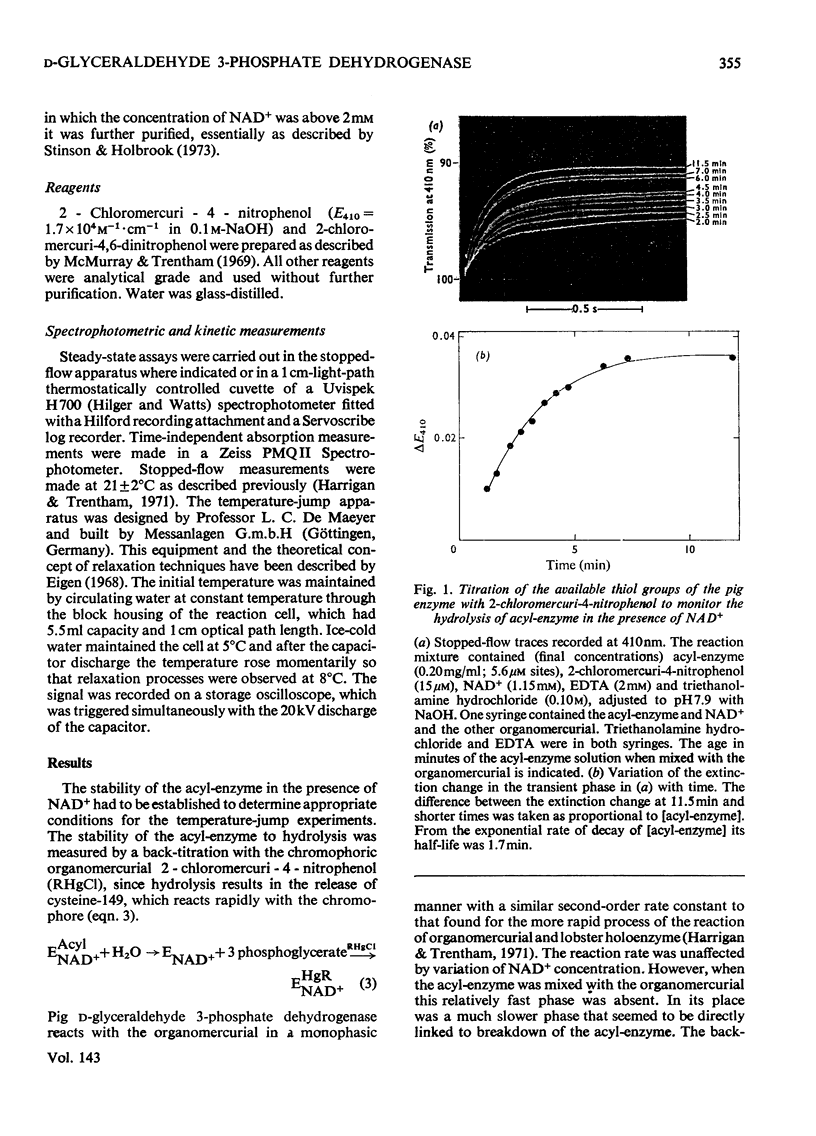
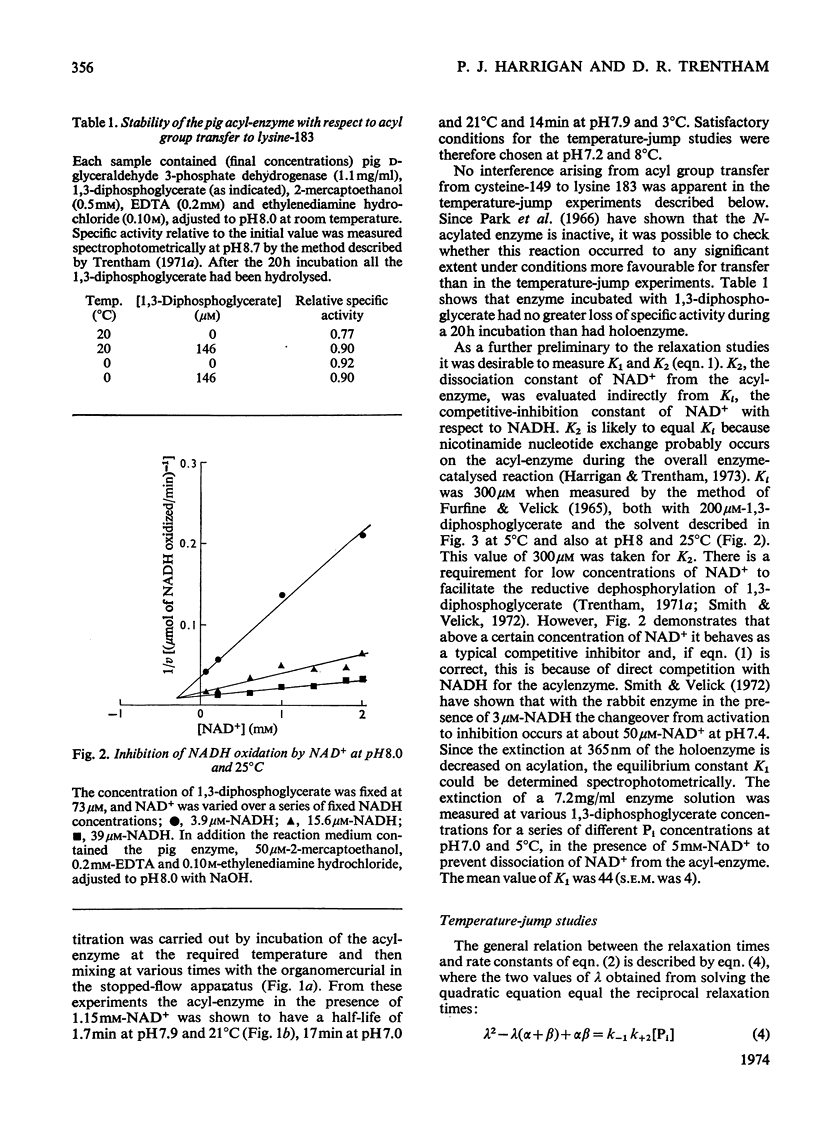
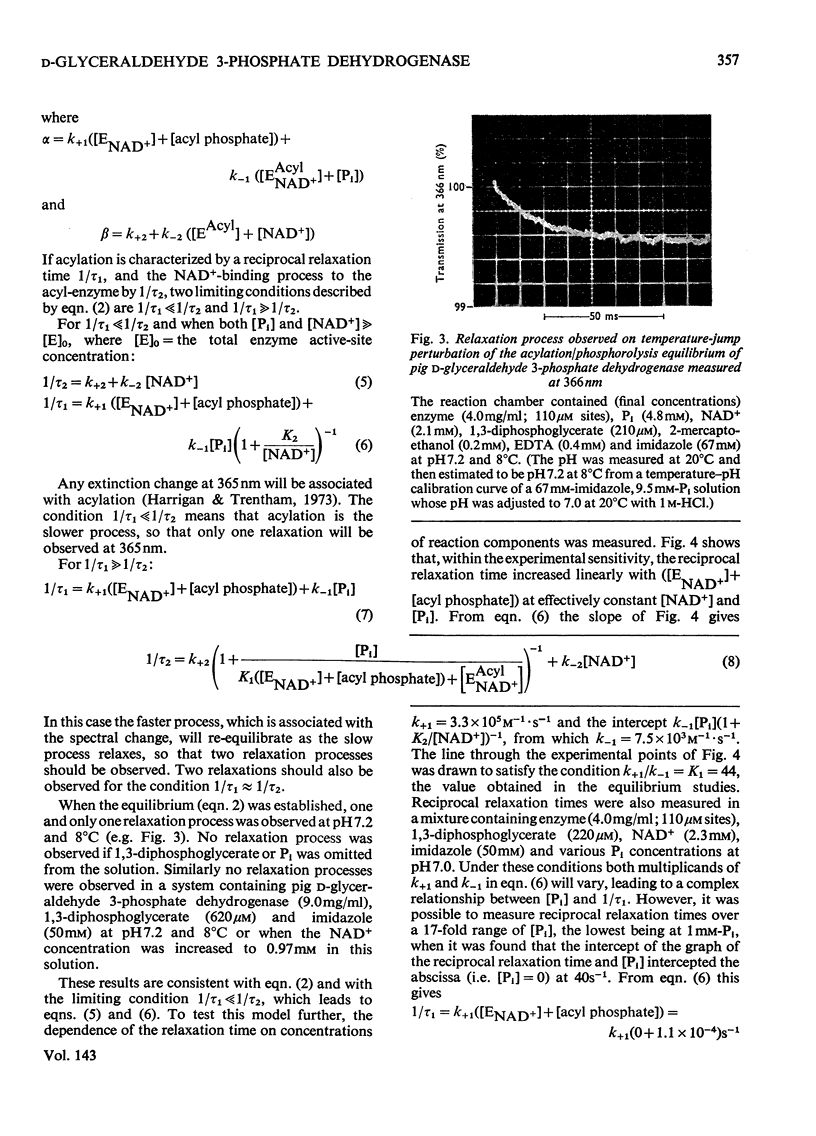
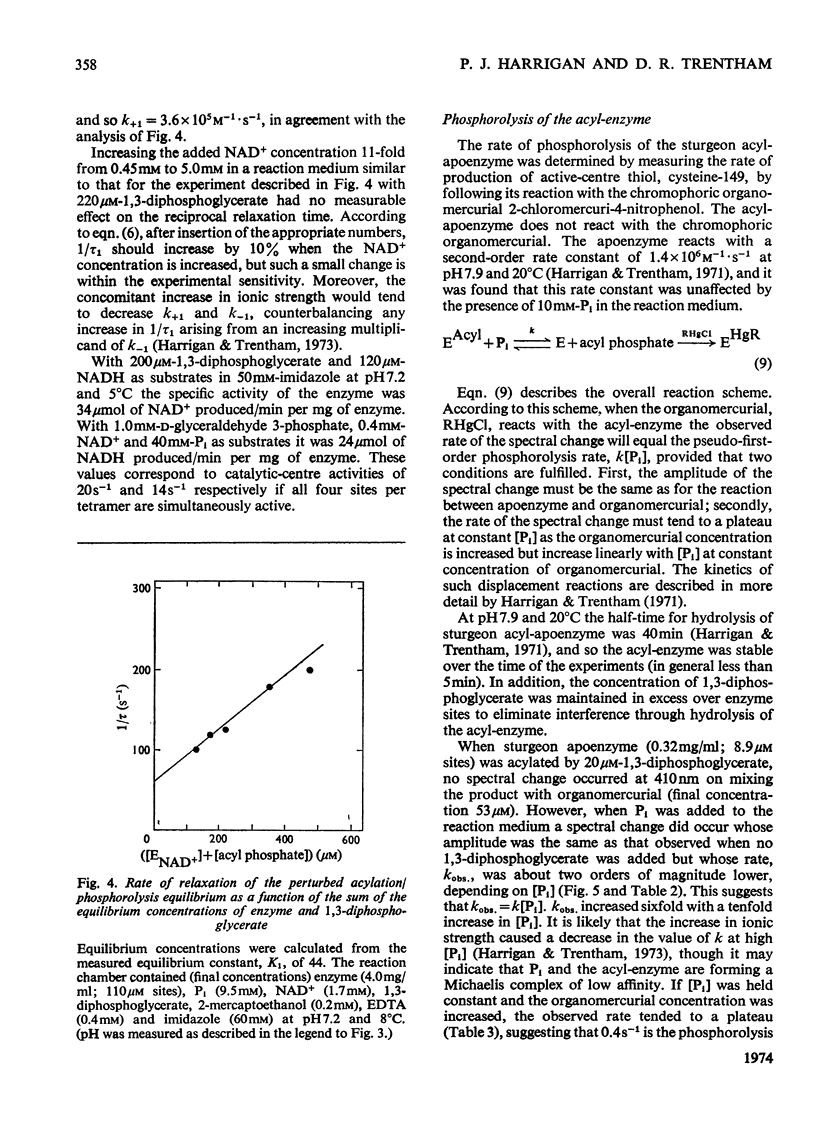
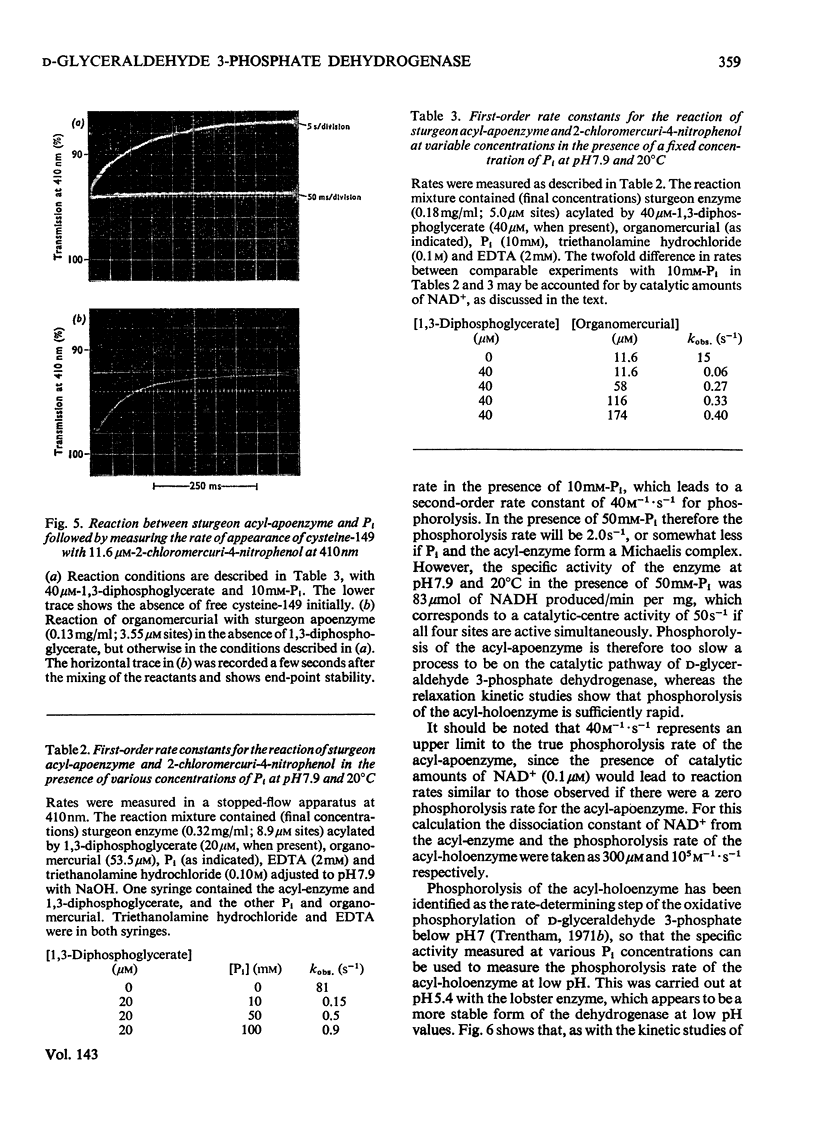
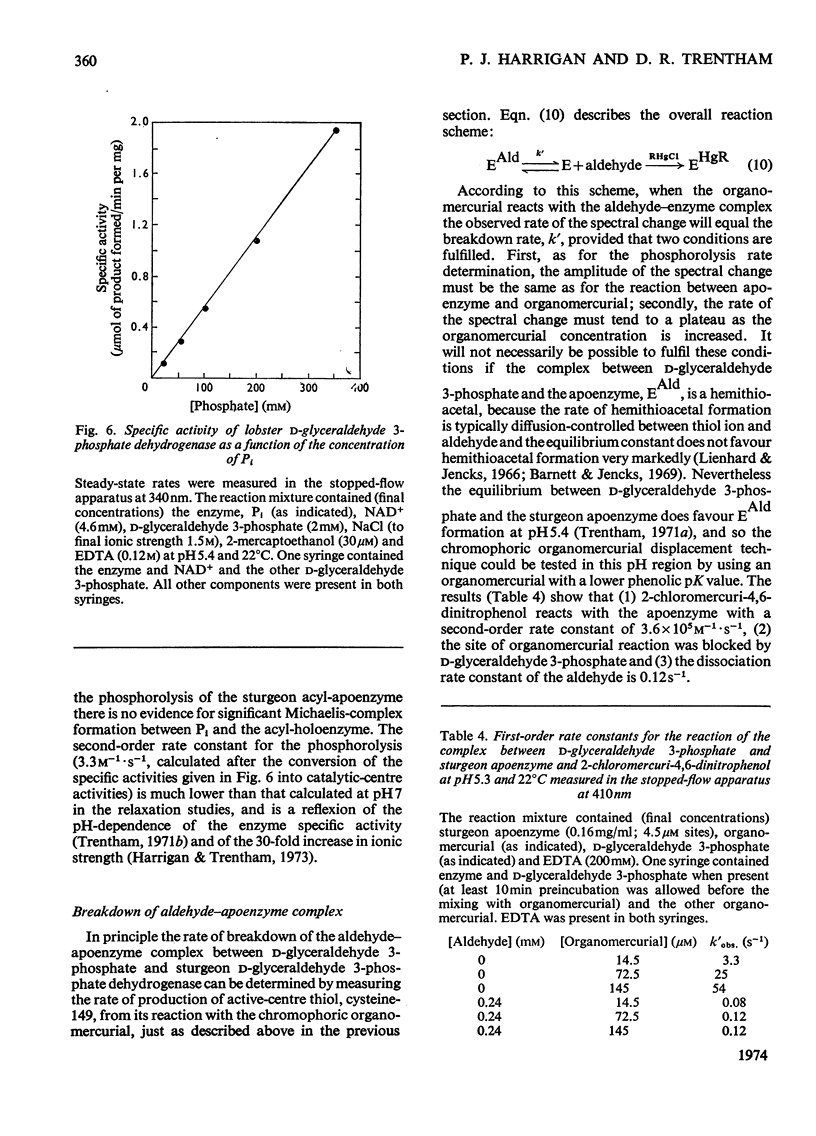
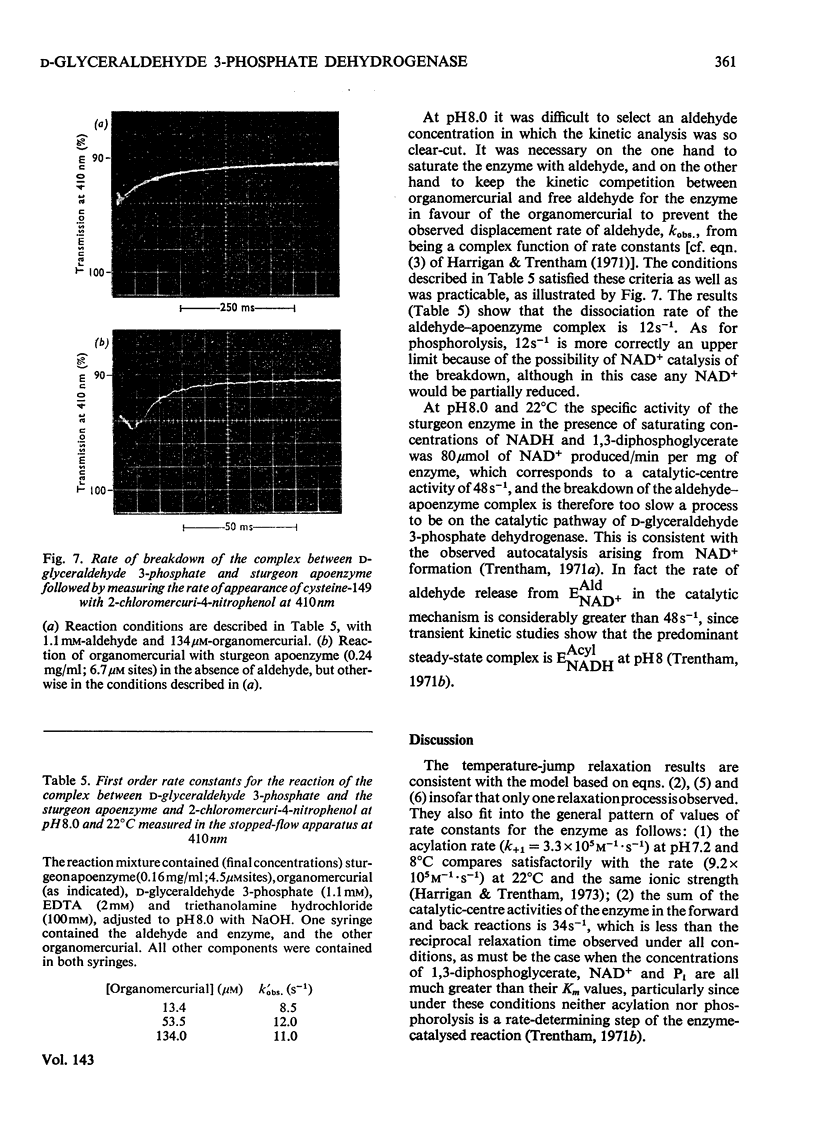
The kinetics of the acylation of d-glyceraldehyde 3-phosphate dehydrogenase from pig muscle by 1,3-diphosphoglycerate in the presence of NAD+ has been analysed by using the relaxation temperature-jump method. At pH7.2 and 8°C the rate of acylation of the NAD+-bound (or holo-) enzyme was 3.3×105m−1·s−1 and the rate of phosphorolysis, the reverse reaction, was 7.5×103m−1·s−1. After a temperature-jump perturbation the equilibrium of NAD+ binding to the acyl-enzyme was re-established more rapidly than that of the acylation. The rate of phosphorolysis of the apoacylenzyme from sturgeon muscle and of aldehyde release from the d-glyceraldehyde 3-phosphate–apoenzyme complex were ≤40m−1·s−1 and ≤12s−1 respectively at pH8.0 and 22°C, which means that both processes are too slow to contribute significantly to the reaction pathway of the reversible NAD+-linked oxidative phosphorylation of d-glyceraldehyde 3-phosphate. Phosphorolysis of both acyl-apoenzyme and acyl-holoenzyme was first-order in Pi up to 100mm-Pi and more. PO43− could be the reactive species of the phosphorolysis of the acyl-holoenzyme, in which case phosphorolysis is a diffusion-controlled reaction, although other kinetically indistinguishable rate equations for the reaction are possible.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON W. S., KAPLAN N. O. THE COMPARATIVE ENZYMOLOGY OF TRIOSEPHOSPHATE DEHYDROGENASE. J Biol Chem. 1964 Jul;239:2140–2152. [PubMed] [Google Scholar]
- Bloch W., MacQuarrie R. A., Bernhard S. A. The nucleotide and acyl group content of native rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. J Biol Chem. 1971 Feb 10;246(3):780–790. [PubMed] [Google Scholar]
- Cseke E., Boross L. Factors affecting the reactivity of the activated SH-group of D-glyceraldehyde 3-phosphate dehydrogenase. Acta Biochim Biophys Acad Sci Hung. 1970;5(4):385–397. [PubMed] [Google Scholar]
- Davidson B. E. The identification of a reactive lysine residue in lobster glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1970 Jul;14(3):545–548. doi: 10.1111/j.1432-1033.1970.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Nicotinamide adenine dinucleotide-specific glyceraldehyde 3-phosphate dehydrogenase from Pisum sativum. Assay and steady state kinetics. J Biol Chem. 1974 Jan 10;249(1):167–174. [PubMed] [Google Scholar]
- Eigen M. New looks and outlooks on physical enzymology. Q Rev Biophys. 1968 May;1(1):3–33. doi: 10.1017/s0033583500000445. [DOI] [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Gutfreund H. Transients and relaxation kinetics of enzyme reactions. Annu Rev Biochem. 1971;40:315–344. doi: 10.1146/annurev.bi.40.070171.001531. [DOI] [PubMed] [Google Scholar]
- Harrigan P. J., Trentham D. R. Kinetic studies of the acylation of pig muscle D-glyceraldehyde 3-phosphate dehydrogenase by 1,3-diphosphoglycerate and of proton uptake and release in the overall enzyme mechanism. Biochem J. 1973 Dec;135(4):695–703. doi: 10.1042/bj1350695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan P. J., Trentham D. R. Reactions of D-glyceraldehyde 3-phosphate dehydrogenase with chromophoric thiol reagents. Biochem J. 1971 Sep;124(3):573–580. doi: 10.1042/bj1240573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhard G. E., Jencks W. P. Thiol addition to the carbonyl group. Equilibria and kinetics. J Am Chem Soc. 1966 Sep 5;88(17):3982–3994. doi: 10.1021/ja00969a017. [DOI] [PubMed] [Google Scholar]
- McMurray C. H., Trentham D. R. A new class of chromophoric organomercurials and their reactions with D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1969 Dec;115(5):913–921. doi: 10.1042/bj1150913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H., Agnello C. F., Mathew E. S-N transfer and dual acetylation in the S-acetylation and N-acetylation of 3-phosphoglyceraldehyde dehydrogenase by substrates. J Biol Chem. 1966 Feb 10;241(3):769–771. [PubMed] [Google Scholar]
- Polgár L. The effect of coenzyme on the S--N acyl migration in glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1966 May 5;118(2):276–284. doi: 10.1016/s0926-6593(66)80036-x. [DOI] [PubMed] [Google Scholar]
- RACKER E., KRIMSKY I. The mechanism of oxidation of aldehydes by glyceralde-hyde-3-phosphate dehydrogenase. J Biol Chem. 1952 Oct;198(2):731–743. [PubMed] [Google Scholar]
- Smith C. M., Velick S. F. The glyceraldehyde 3-phosphate dehydrogenases of liver and muscle. Cooperative interactions and conditions for functional reversibility. J Biol Chem. 1972 Jan 10;247(1):273–284. [PubMed] [Google Scholar]
- Stinson R. A., Holbrook J. J. Equilibrium binding of nicotinamide nucleotides to lactate dehydrogenases. Biochem J. 1973 Apr;131(4):719–728. doi: 10.1042/bj1310719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Aspects of the chemistry of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1968 Oct;109(4):603–612. doi: 10.1042/bj1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Rate-determining processes and the number of simultaneously active sties of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1971 Mar;122(1):71–77. doi: 10.1042/bj1220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R. Reactions of D-glyceraldehyde 3-phosphate dehydrogenase facilitated by oxidized nicotinamide-adenine dinucleotide. Biochem J. 1971 Mar;122(1):59–69. doi: 10.1042/bj1220059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F. Coenzyme binding and the thiol groups of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1953 Aug;203(2):563–573. [PubMed] [Google Scholar]
- VELICK S. F., HAYES J. E., Jr Phosphate binding and the glyceraldehyde-3-phosphate dehydrogenase reaction. J Biol Chem. 1953 Aug;203(2):545–562. [PubMed] [Google Scholar]