The timing of restarting oral anticoagulation (OAC) for patients with atrial fibrillation (AF) after experiencing an acute ischaemic stroke (AIS) is a complex challenge for clinicians. This decision involves balancing the need to minimise the risk of early ischaemic stroke recurrence with the risk of symptomatic intracranial haemorrhage. There is significant uncertainty in decision-making, particularly for patients who have suffered large infarct cores.1
Traditionally, the 1-3-6-12 day rule, also known informally as ‘Diener’s law’, has been widely used by clinicians to guide the timing of restarting OAC after a transient ischaemic attack (TIA) or ischaemic stroke2.This expert consensus suggests adjusting the timing of anticoagulation based on stroke severity as measured by the National Institute of Health Stroke Scale (NIHSS). The rule is predicated on the observation that the risk of haemorrhagic transformation is linked to infarct size, which closely correlates with the NIHSS.3 According to Diener’s law, anticoagulation should be resumed on day 1 for TIA, day 3 for minor strokes, day 6 for moderate strokes and day 12 for severe strokes.
While the risk of haemorrhagic transformation is a major concern, patients with AIS who develop AF face a high risk of reinfarction or new embolic events in other cerebral vascular territories shortly after the initial stroke.4 Data from randomised trials suggest that early direct OAC initiation in patients who had ischaemic stroke with AF is safe and did not increase the risk of symptomatic intracranial haemorrhage.5 6 The TIMING trial (2022) was the first randomised clinical trial to test the paradigm of the 1-3-6-12 rule; patients were randomly allocated to either early initiation of OAC within 4 days of ischaemic stroke or delayed initiation 5–10 days6. The early initiation group had non-inferior outcomes with respect to the recurrence of ischaemic stroke and symptomatic intracerebral haemorrhage. Another landmark trial, ELAN, was published in 2023, which similarly suggested that earlier initiation of OAC than suggested by guidelines did not significantly increase the risk of haemorrhagic transformation and may even reduce the risk of composite endpoints, including the risk of reinfarction.5
There has also been observational data to suggest that early initiation of OAC may reduce the risk of ischaemic stroke without a significant increased bleeding risk1.Kimura et al demonstrated that the risk of stroke or systemic embolism was reduced when OAC therapy was initiated earlier than the median day according to the 1-3-6-12 day rule.1 Additionally, Chang et al found that in cases of mild and moderate strokes, early or routine initiation of OAC (using the 1-3-6-12 day rule) did not increase the risk of bleeding and was linked to a decrease in recurrence of ischaemic stroke and adverse cardiovascular outcomes.7 However, early initiation of OAC in severe stroke cases was associated with a 1.67-fold (95% CI 1.30 to 2.13) risk of safety outcomes.7
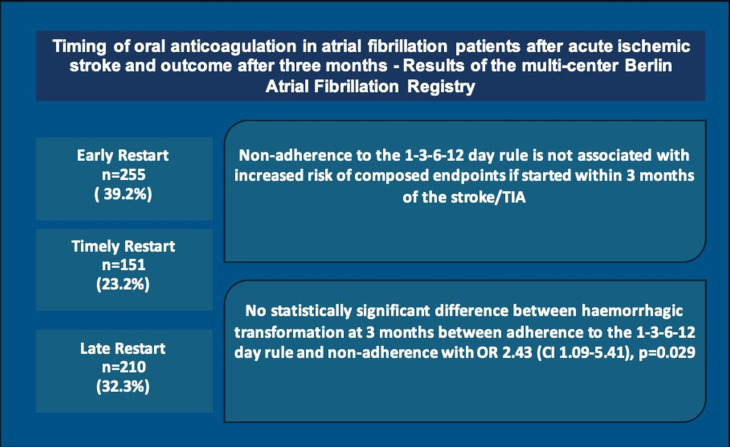
In this issue of Open Heart, Olma et al present a retrospective multicentre study involving 708 patients with AF from the Berlin AF Registry who experienced an AIS and were followed up for 3 months8. The study examined the impact of adhering to the 1-3-6-12 day rule for restarting anticoagulation, with nearly 40% of patients receiving timely initiation according to this guideline. In contrast, 151 patients (23.2%) initiated anticoagulation earlier than recommended by the guidelines, and 210 patients (32.3%) restarted OAC later than recommended by the guidelines. The primary findings showed that timely initiation of anticoagulation was not linked to a reduction in composite endpoints, including recurrent stroke, systemic embolism, myocardial infarction, major bleeding or all-cause mortality at 3 months. Furthermore, there was no increased risk of composite outcomes in the early restart group compared with the timely restart group, aligning with results from the ELAN and TIMING clinical trials.5 6 Nonetheless, it is important to highlight that in this study, the majority of patients evaluated in this study (86.6%) had mild strokes (NIHSS <8), with only around 3% having severe strokes.8 Additionally, due to the observational nature of this study, the non-adherence group had a higher proportion of patients with moderate-large infarct size on imaging compared with the adherence group (9.2% vs 3.3%) (figure 1).8
Figure 1. Visual abstract describing the results of the multicentre Berlin Atrial Fibrillation Registry.
The 1-3-6-12-day rule was designed to mitigate the risk of symptomatic intracranial haemorrhage by delaying anticoagulation based on stroke severity. However, several factors beyond stroke severity can influence the risk of haemorrhagic transformation, including age, the type of reperfusion therapy administered (thrombolysis or thrombectomy), recanalisation status, blood pressure variability and hyperglycaemia. Risk stratification approaches, such as those proposed by the ELAN trial based on neuroimaging criteria alone or the NIHSS-based stratification used in the 1-3-6-12 day rule, do not account for these additional factors. It is unlikely that there will be a one-size-fits-all approach and that the emerging evidence will reveal a need to tailor restarting anticoagulation to a range of individual patient factors. In the era of personalised medicine, leveraging big data could help in the multivariable risk versus benefit stratification for optimising the timing of OAC reinitiation in patients with AIS with AF (figure 1).
This real-world study by Olma et al highlights the significant variability in clinical practices regarding the timing of anticoagulation resumption after ischaemic stroke.8 It may be informative to explore further the factors influencing clinicians’ decisions to delay or initiate anticoagulation earlier. Besides stroke size and severity, other factors that could impact timing include whether the patient received reperfusion therapy and their individual systemic bleeding risk. Current clinical trials do not provide clear guidance on managing these specific subgroups. There are, however, observational data to suggest that early (within 2 weeks) restart of OAC in patients who have acute reperfusion therapy does not significantly increase the risk of symptomatic haemorrhagic transformation or reinfarction.9
Stroke populations display diverse clinical phenotypes, necessitating larger trials to determine the optimal timing of restarting anticoagulation across different demographics. Future studies, such as OPTIMAS and START, may provide further clarity on the optimal timing for resuming anticoagulation in patients who had ischaemic stroke.10 11Indeed, OPTIMAS showed that early Direct Oral Anticoagulation (DOAC) initiation within 4 days after ischaemic stroke associated with atrial fibrillation was non-inferior to delayed initiation for the composite outcome of ischaemic stroke, intracranial haemorrhage, unclassifiable stroke, or systemic embolism at 90 days.12 Similarly, START did not identify a clearly superior day to initiate DOAC for secondary stroke prevention in AF, although early DOAC initiation was better than at later times within the first 2 weeks after stroke onset.13
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Commissioned; internally peer reviewed.
Contributor Information
Ashwin Balu, Email: ashwin.balu@nhs.net.
Sia Ching Hui, Email: mdcschi@nus.edu.sg.
Benjamin Yong-Qiang Tan, Email: benjaminyqtan@gmail.com.
Gregory Y H Lip, Email: gregory.lip@liverpool.ac.uk.
References
- 1.Kimura S, Toyoda K, Yoshimura S, et al. Practical '1-2-3-4-Day' Rule for Starting Direct Oral Anticoagulants After Ischemic Stroke With Atrial Fibrillation: Combined Hospital-Based Cohort Study. Stroke. 2022;53:1540–9. doi: 10.1161/STROKEAHA.121.036695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diener H-C, Aisenberg J, Ansell J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J. 2017;38:860–8. doi: 10.1093/eurheartj/ehw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paciaroni M, Agnelli G, Ageno W, et al. Timing of anticoagulation therapy in patients with acute ischaemic stroke and atrial fibrillation. Thromb Haemost. 2016;116:410–6. doi: 10.1160/TH16-03-0217. [DOI] [PubMed] [Google Scholar]
- 4.Seiffge DJ, Werring DJ, Paciaroni M, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18:117–26. doi: 10.1016/S1474-4422(18)30356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer U, Koga M, Strbian D, et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. N Engl J Med. 2023;388:2411–21. doi: 10.1056/NEJMoa2303048. [DOI] [PubMed] [Google Scholar]
- 6.Oldgren J, Åsberg S, Hijazi Z, et al. Early Versus Delayed Non-Vitamin K Antagonist Oral Anticoagulant Therapy After Acute Ischemic Stroke in Atrial Fibrillation (TIMING): A Registry-Based Randomized Controlled Noninferiority Study. Circulation. 2022;146:1056–66. doi: 10.1161/CIRCULATIONAHA.122.060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang P-Y, Wang W-T, Wu W-L, et al. Oral Anticoagulation Timing in Patients with Acute Ischemic Stroke and Atrial Fibrillation. Thromb Haemost. 2022;122:939–50. doi: 10.1055/a-1669-4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olma MC, Tütüncü S, Hansen K, et al. Timing of oral anticoagulation in atrial fibrillation patients after acute ischaemic stroke and outcome after 3 months: results of the multicentre Berlin Atrial Fibrillation Registry. Open Heart. 2024;11:e002688. doi: 10.1136/openhrt-2024-002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giustozzi M, Acciarresi M, Agnelli G, et al. Safety of Anticoagulation in Patients Treated With Urgent Reperfusion for Ischemic Stroke Related to Atrial Fibrillation. Stroke. 2020;51:2347–54. doi: 10.1161/STROKEAHA.120.030143. [DOI] [PubMed] [Google Scholar]
- 10.King BT, Lawrence PD, Milling TJ, et al. Optimal delay time to initiate anticoagulation after ischemic stroke in atrial fibrillation (START): Methodology of a pragmatic, response-adaptive, prospective randomized clinical trial. Int J Stroke. 2019;14:977–82. doi: 10.1177/1747493019870651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arram L, et al. Optimas: A randomised controlled trial to establish the optimal timing of anticoagulation after acute ischaemic stroke. Eur Stroke J. 2021;6 [Google Scholar]
- 12.Werring DJ, Dehbi HM, Ahmed N, et al. Optimal timing of anticoagulation after acute ischaemic stroke with atrial fibrillation (OPTIMAS): a multicentre, blinded-endpoint, phase 4, randomised controlled trial. Lancet. 2024;23:02197–4. doi: 10.1016/S0140-6736(24)02197-4. [DOI] [PubMed] [Google Scholar]
- 13.Warach SJ, Davis LA, Lawrence P, et al. Abstract 66: Optimal Delay Time to Initiate Anticoagulation After Ischemic Stroke in Atrial Fibrillation: Primary Results of the START Trial. Stroke. 2024;55:A66. doi: 10.1161/str.55.suppl_1.66. [DOI] [Google Scholar]