Abstract
Background
This study aims to address the timing of repair for severe congenital diaphragmatic hernia (CDH) without the use of extracorporeal membrane oxygenation (ECMO) and to determine the feasibility of an earlier intervention to avoid deaths associated with non-repair in patients who are more challenging to stabilize without ECMO.
Methods
This single-center retrospective study was conducted on neonates with CDH from 2013 to 2023. Based on the timing of surgery, the patients were classified into three groups: <24 hours (group A), 24–48 hours (group B) and ≥48 hours (group C). The 90-day survival rates were analyzed using Kaplan-Meier curves and compared among groups via log-rank tests. The independent factors related to survival assessed using the multivariate Cox regression model.
Results
Of 132 CDH infants, the overall 90-day survival rate was 81.8% (108/132), with a median operative time of 26.00 (24.00, 38.50) hours. A significant difference was observed in the 90-day survival rate among the three groups: 60.5% (23/38) in group A vs. 91.3% (74/81) in group B vs. 84.6% (11/13) in group C (log-rank p<0.001). In mild and severe cases and those with an oxygen index ≥7.5, group A resulted in significantly reduced survival rates. Multivariate Cox regression analysis indicated that surgical timing <24 hours remained an independent mortality-related risk factor in infants with CDH.
Conclusions
Repair surgery should be performed at least 24 hours after birth. The optimal timing for CDH neonates in non-ECMO centers appears to be 24–48 hours after birth, which can prevent the loss of treatment opportunities for severe cases.
Keywords: Neonatology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
Most CDH children can reach a good state after 24 hours of birth with proper fluid management, the rational use of vasoactive drugs and persistent pulmonary hypertension-specific therapeutic drugs. If a child has reached initial stability within 24 hours, surgical intervention could also be performed 24–48 hours after birth.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
For those centers where ECMO is not available or not used, repair surgery should be performed at least 24 hours after birth. The 24–48-hour window after birth is an appropriate time for CDH surgery once the patient reaches physiologic stability, which can avoid the loss of treatment opportunities for some severe children.
Introduction
Congenital diaphragmatic hernia (CDH) is a severe and rare birth defect, with an incidence rate of 2.3 per 10 000 live births.1 It is mainly caused by the incomplete development of the fetal diaphragm during pregnancy and herniation of abdominal organs into the thoracic cavity, resulting in abnormal lung development and persistent pulmonary hypertension (PAH). Despite significant advancements in neonatal surgical techniques and intensive care over the past two decades, neonatal mortality rates for those diagnosed with CDH remain extremely high, generally around 30%, and as high as 70% in some middle-income countries.2 3 The mortality rate of CDH also varies depending on disease severity, with a mortality rate ranging from 10% to 25% for mild cases and from 50% to 70% for severe cases.4 5 Although previous studies have identified several prognostic factors, the optimal timing for surgical repair in CDH patients remains controversial.6
Traditionally, CDH was regarded as a surgical emergency requiring intervention within 24 hours of birth. However, many researchers have emphasized the importance of achieving physiological stability first, as they have found that early repair frequently leads to a deterioration in lung compliance and oxygenation.7 The strategy of delayed surgery has been increasingly accepted, with traditional practice suggesting that a delay of 3–4 days is necessary.8 However, recent studies have also revealed that approximately 20% of infants with CDH never undergo surgical repair due to strict surgical indications or traditional delay times. Aggressive surgical intervention, leading to a reduction in the unrepaired rate, is associated with improved mortality, while excessive delay in surgery can increase the risk of PAH. This is caused by limited pulmonary vascular bed and prolonged diastolic left ventricular failure, particularly in infants with severe CDH.9,11 In centers where extracorporeal membrane oxygenation (ECMO) is not available or not used, the traditional 3–4-day delay may lead to a higher non-repaired rate, as infants with severe CDH may have more difficulty achieving physiological stability without ECMO support. As reported in the recent literature, some non-ECMO centers have performed surgery within 24–72 hours after birth.12 It is hypothesized that a surgical intervention close to 24–48 hours after birth is feasible for non-ECMO centers.
This study aims to address the optimal timing of repair for severe CDH without the support of ECMO, assessing the feasibility of earlier intervention to prevent deaths associated with non-repair in patients who are more challenging to stabilize in the absence of ECMO.
Methods
Patients selection
A retrospective study was conducted in the department of pediatric surgery at the Children’s Hospital of the Capital Institute of Pediatrics in Beijing, China. Neonates with CDH who were treated in our center between September 2013 and December 2023 were involved in this study. The inclusion criteria were: (1) definite diagnosis of CDH through imaging examination; (2) neonates with respiratory distress who required intubation and ventilation immediately after birth; (3) admitted to our hospital within the first 24 hours of life; (4) underwent repair surgery. The exclusion criteria were: (1) incomplete medical records and follow-up data; (2) concomitant other structural malformations; (3) death without repair surgery before clinical stability; (4) postnatal diagnosis of CDH. The patients were stratified by the timing of surgery into three main groups: <24 hours (group A), 24–48 hours (group B) and ≥48 hours (group C).
Neonatal protocol
Our center has developed a standard management protocol based on the CDH EURO Consortium guidelines,13 which include gentle ventilation with permissive hypercapnia before surgery, strict fluid management, sedation and analgesia, standard use of PAH-specific therapeutic drugs combined with vasoactive agents, and antibiotic therapy. The initial ventilation mode is conventional mechanical mode, with a maximum peak inspiratory pressure of 25 cmH₂O, a respiratory rate of 40–60 breaths per minute and a positive end-expiratory pressure of 3–6 cmH₂O. High frequency oscillation ventilation is performed if oxygenation cannot be maintained.8 14 The PAH-specific therapeutic drugs used in our center mainly include inhaled nitric oxide (iNO), sildenafil and treprostinil.15 Vasoactive drugs including dopamine, dobutamine and epinephrine were administered to neonates with circulatory dysfunction.
Before surgery, the following physiological criteria must be met: urine output >1 mL/kg/hour; preductal oxygen saturation between 85% and 95% on FiO2 <0.5; normal mean arterial pressure for gestational age; lactate <3 mmol/L; estimated pulmonary artery pressures less than systemic pressure. Surgical repair was performed after achieving physiological stability, and thoracoscopic surgery was preferred. Children with large diaphragmatic defects undergo repair using either a patch or muscle flap technique. In our center, patients did not receive ECMO or fetoscopic endoluminal tracheal occlusion.
Data collection
The primary outcome measure was survival at 90 days after birth. Clinical data were collected via the medical record system. The preoperational information included: gender; birth weight, delivery mode, gestational age (GA) at birth, GA at CDH diagnosis (identified on the first ultrasound screening or MRI examination), observed-to-expected lung-to-head ratio (o/e LHR) and preoperative oxygen index (OI). The preoperative OI was calculated using an optimal blood gas results on the first day of life, with the equation: inhaled oxygen concentration (%)×mean airway pressure (cmH2O, 1 cm H2O=0.098 kPa)/oxygen partial pressure (mm Hg).16 According to previous studies, the OI was stratified based on a threshold of 7.5, with OI ≥7.5 indicating a severe condition.17 18 The surgical information included: timing of surgery, affected side, liver position, presence of hernial sac confirmed during surgery, type of surgery performed (thoracoscopic or traditional), classification of the diaphragmatic defect (A–D based on size) using criteria by Lally et al.,19 and whether a patch was used during the operation. Specifically, children exhibiting liver herniation or requiring patch repair were categorized into the severe group, whereas those without these characteristics were classified as belonging to the mild group.
Statistical analysis
The basic characteristics of patients were presented using descriptive statistics. Continuous data are expressed as mean ± standard deviation (SD) or median (interquartile range, IQR). Categorical data were expressed as number (percentage). Between-group comparisons were implemented using t-tests, rank-sum, or χ2 tests where appropriate. Overall survival rate within the first 90 days of life was calculated using Kaplan-Meier curves and compared among the three groups using a log-rank test. A Cox regression model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) to assess the timing of surgery for outcome (group A as reference). To examine the independent effect of the timing of surgery, multivariate Cox regression analysis was also employed, adjusting for potential confounders, which were related to mortality in the univariate analyses. The statistical analyses were performed using the STATA software (V.14.0, Stata Corp, Texas, USA) unless otherwise indicated. A two-sided p value less than 0.05 was considered statistically significant.
Results
Baseline characteristics and overall survival
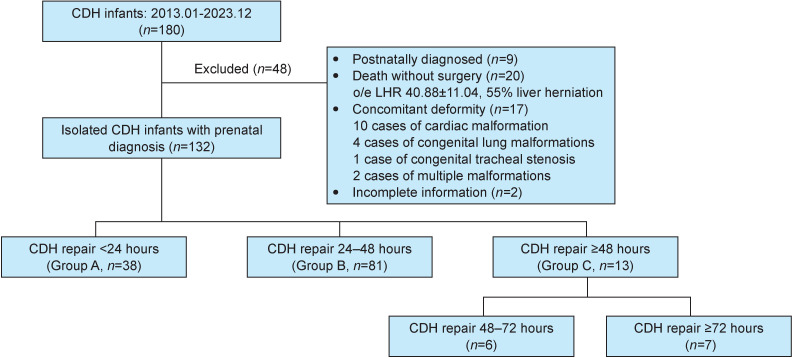
A total of 180 children with a prenatal diagnosis of CDH were treated for the first time in our hospital during the study period. Of these, 48 cases were excluded according to study criteria: 9 cases of postpartum diagnosis with life >24 hours at admission, 20 cases of death without repair surgery before clinical stability, 17 cases of concomitant severe structural malformations (9 cases of ventricular septal defect (VSD) or atrial septal defect (ASD), 1 case of pulmonary stenosis, 3 cases of congenital pulmonary airway malformation, 1 case of pulmonary sequestration and 2 cases with both pulmonary sequestration and ASD and 2 cases of incomplete data (figure 1). Of 132 included patients, 81.8% (108/132) of patients were discharged from hospital and survived within 90 days after birth, with an overall time to surgery was 26.00 (24.00, 38.50) hours. The association between baseline characteristics and mortality is shown in table 1. Open surgery (p<0.001), defect size (p=0.001), repair with a patch (p<0.001), liver herniation (p=0.008), o/e LHR (p<0.001), GA at diagnosis (p=0.002), OI score and group (both p<0.001) and surgical timing (p=0.016) were significantly associated with mortality.
Figure 1. The flowchart of patient selection in this study. CDH, congenital diaphragmatic hernia; o/e LHR, observed-to-expected lung-to-head ratio.
Table 1. The association between the baseline characteristics and survival.
| Variables | Overall (N=132) | Survival (n=108) | Death (n=24) | P value |
| Gender, n (%) | ||||
| Female | 61 (46.2) | 47 (43.5) | 14 (58.3) | 0.276 |
| Male | 71 (53.8) | 61 (56.5) | 10 (41.7) | |
| Delivery mode, n (%) | ||||
| Cesarean | 106 (80.3) | 86 (79.6) | 20 (83.3) | 0.897 |
| Eutocia | 26 (19.7) | 22 (20.4) | 4 (16.7) | |
| CDH affected side, n (%) | ||||
| Left | 109 (82.6) | 90 (83.3) | 19 (79.2) | 0.850 |
| Right | 23 (17.4) | 18 (16.7) | 5 (20.8) | |
| Open surgery, n (%) | 39 (29.5) | 24 (22.2) | 15 (62.5) | <0.001 |
| Defect size, n (%) | ||||
| A/B | 82 (62.1) | 75 (69.4) | 7 (29.2) | 0.001 |
| C/D | 50 (37.9) | 33 (30.6) | 17 (70.8) | |
| Repair with patch, n (%) | 24 (18.2) | 12 (11.1) | 12 (50.0) | <0.001 |
| Liver herniation, n (%) | 88 (66.7) | 78 (72.2) | 10 (41.7) | 0.008 |
| o/e LHR, mean±SD | 52.7±15.1 | 55.6±14.2 | 39.5±12.0 | <0.001 |
| GA at birth (week), median (IQR) | 37.5 (37.0, 38.0) | 37.5 (37.0, 38.0) | 37.3 (37.0, 38.6) | 0.646 |
| Birth weight (g), mean±SD | 2.9±0.5 | 3.0±0.5 | 2.9±0.6 | 0.470 |
| GA at diagnosis (week), median (IQR) | 25.0 (23.0, 31.0) | 27.0 (23.0, 31.3) | 23.0 (22.0, 24.0) | 0.002 |
| OI, median (IQR) | 3.9 (2.6, 6.2) | 3.5 (2.5, 5.3) | 9.7 (5.7, 22.8) | <0.001 |
| OI group, n (%) | ||||
| OI <7.5 | 107 (81.1) | 99 (91.7) | 8 (33.3) | <0.001 |
| OI ≥7.5 | 25 (18.9) | 9 (8.3) | 16 (66.7) | |
| Surgical timing (hours), median (IQR) | 26.0 (24.0, 38.5) | 26.5 (25.0, 41.0) | 22.5 (6.0, 29.8) | 0.016 |
CDHcongenital diaphragmatic herniaGAgestational ageo/e LHRobserved-to-expected lung-to-head ratioOIoxygen index
Survival curves among three groups and stratified analysis
There are 38 cases in group A, 81 cases in group B and 13 cases in group C, respectively. The background demographics of the three groups are detailed in table 2. The OI was significantly different among the three groups, with group A having worse OI (p=0.017). Other characteristics of patients among the three groups are equally distributed (all p>0.05).
Table 2. Demographic characteristics of CDH patients stratified by timing of surgery.
| Variables | Total (N=132) | Group A (n=38) | Group B (n=81) | Group C (n=13) | P value |
| Male, n (%) | 71 (53.8) | 22 (57.9) | 42 (51.8) | 7 (53.8) | 0.795 |
| Eutocia, n (%) | 26 (19.7) | 8 (21.1) | 14 (17.5) | 3 (23.1) | 0.835 |
| Left CDH, n (%) | 109 (82.6) | 32 (84.2) | 67 (82.7) | 10 (76.9) | 0.835 |
| Open surgery, n (%) | 39 (29.5) | 16 (42.1) | 19 (23.4) | 4 (30.8) | 0.150 |
| C/D defect phase, n (%) | 50 (37.9) | 14 (36.8) | 31 (38.3) | 5 (38.5) | 0.988 |
| Repair with patch, n (%) | 24 (18.2) | 6 (15.8) | 15 (18.5) | 3 (23.1) | 0.835 |
| Liver herniation, n (%) | 44 (33.3) | 13 (34.2) | 25 (30.9) | 6 (46.2) | 0.550 |
| o/e LHR, mean±SD | 52.7±15.1 | 50.9±15.8 | 53.0±15.0 | 56.3±14.6 | 0.532 |
| GA at birth (week), median (IQR) | 37.5 (37.0, 38.0) | 37.5 (36.3, 38.00) | 37.5 (37.0, 38.0) | 37.6 (37.0, 40.0) | 0.562 |
| Birth weight (g), mean±SD | 2.9±0.5 | 2.9±0.6 | 3.0±0.5 | 3.1±0.3 | 0.369 |
| GA at diagnosis (week), median (IQR) | 25.0 (23.0, 31.0) | 24.0 (22.3, 30.0) | 25.0 (23.0, 31.0) | 30.0 (24.0, 37.0) | 0.808 |
| OI, median (IQR) | 3.9 (2.6, 6.2) | 5.3 (3.2, 10.2) | 3.6 (2.5, 5.4) | 3.5 (2.0, 6.3) | 0.088 |
| OI ≥7.5, n (%) | 25 (18.9) | 13 (34.2) | 10 (12.3) | 2 (15.4) | 0.017 |
CDHcongenital diaphragmatic herniaGAgestational ageo/e LHRobserved-to-expected lung-to-head ratioOIoxygen index
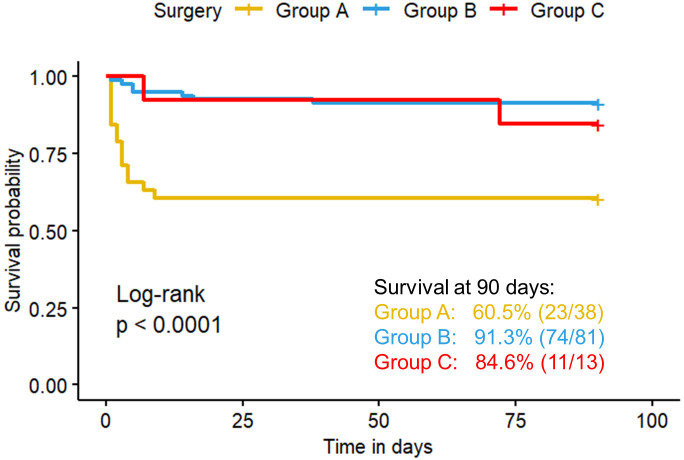
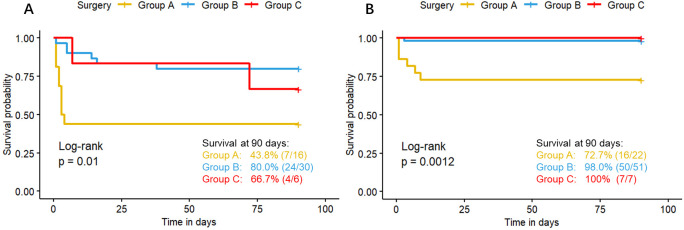
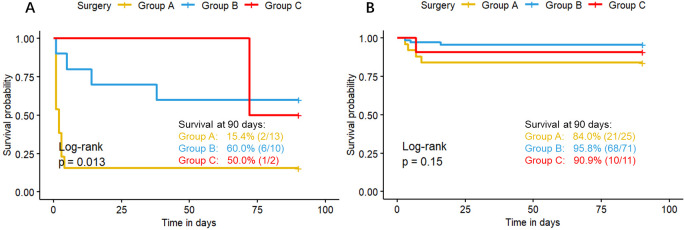
The Kaplan-Meier survival curves for surgical timing are presented in figure 2. The survival rates at 90 days for the groups A, B and C were 60.5% (23/38), 91.3% (74/81) and 84.6% (11/13), respectively, with a significant difference among these three groups (log-rank p<0.001). Stratified analysis revealed that the survival rate of group A is lower than group B in subgroup of severe cases (figure 3A) (43.8% vs. 80%, p=0.004), mild cases (figure 3B) (72.7% vs. 98.0%, p<0.001) and those with OI ≥7.5 (figure 4A) (15.4% vs. 60%, p=0.006). In patients with OI <7.5, there are no difference of survival rate among the three groups(figure 4B).
Figure 2. Overall survival curve within 90 days according to the timing of surgery after birth. The yellow, blue and red curves represent the survival curves of the Group A (<24 hours), Group B (24–48 hours) and Group C (≥48 hours), respectively. The plus sign at the end of each curve represents a censored value.
Figure 3. Survival curves stratified by severity (liver herniation and patch repair). (A) The survival curves of severe congenital diaphragmatic hernia (CDH) neonates who presented with liver herniation or required patch repair. (B) The survival curves of mild CDH neonates who underwent primary repair and did not have liver herniation. The yellow, blue and red curves represent the survival curves of the Group A (<24 hours), Group B (24–48 hours) and Group C (≥48 hours), respectively. The plus sign at the end of each curve represents a censored value.
Figure 4. Survival curves stratified by oxygen index (OI). (A) The survival curves of congenital diaphragmatic hernia (CDH) neonates with OI≥7.5 presented with liver herniation or required patch repair. (B) The survival curves of CDH neonates with OI<7.5. The yellow, blue and red curves represent the survival curves of the Group A (<24 hours), Group B (24–48 hours) and Group C (≥48 hours), respectively. The plus sign at the end of each curve represents a censored value.
Multivariate Cox regression analysis
Table 3 presented the results of multivariate Cox regression analyses. Compared with group A, group B had a significantly lower risk of mortality (HR=0.114, 95% CI: 0.043 to 0.313, p<0.001); while group C did not show a statistically significant difference in mortality risk compared with group A.
Table 3. Cox regression analysis of mortality within 90 days for CDH patients.
| Variables | HR | 95% CI | P value |
| Surgical timing 24–48 hours (<24 hours as ref) | 0.114 | 0.043 to 0.313 | <0.001 |
| Surgical timing ≥48 hours (<24 hours as ref) | 0.200 | 0.031 to 1.267 | 0.087 |
| GA at prenatal diagnosis | 0.862 | 0.78 to 0.953 | 0.004 |
| o/e LHR | 0.916 | 0.882 to 0.951 | <0.001 |
| Liver herniation | 3.369 | 1.397 to 8.129 | 0.007 |
| OI | 1.185 | 1.114 to 1.221 | <0.001 |
| Open surgery | 4.752 | 2.07 to 10.908 | 0.001 |
| Repair with patch | 5.709 | 2.365 to 13.779 | <0.001 |
| Defect phase | 4.44 | 1.823 to 10.826 | 0.001 |
CDHcongenital diaphragmatic herniaGAgestational ageo/e LHRobserved-to-expected lung-to-head ratioOIoxygen index
Discussion
This study retrospectively analyzed the surgical timing and prognosis of CDH patients in a non-ECMO center to determine the feasibility of an earlier surgical timing. First, most of the CDH patients in our center received repair surgery within 24–48 hours, while only 5% (7/132) neonates received surgery after 72 hours. During clinical observations, we found that after 24 hours of respiratory and circulatory support, as well as treatment to reduce PAH, most CDH neonates reached a relatively stable condition. Second, these data indicate that, whether in the overall or stratified analysis, the survival rate of the surgery group within 24 hours was the lowest, and this difference was statistically significant when compared with the 24–48-hour surgery group. The survival rates of CDH infants in the 24–48-hour surgery group and the ≥48-hour surgery group were relatively similar, with a slightly lower mortality rate observed in the ≥48-hour group for severe CDH cases, although this difference did not reach statistical significance. This may be attributed to the small sample size of the ≥48-hour surgery group. Therefore, this study demonstrates that surgery performed 24–48 hours after birth has significant advantages over surgery within 24 hours and similar outcomes to surgery after 48 hours. Consequently, earlier surgery is feasible, and 24–48 hours after birth may be an appropriate surgical timing for severe CDH neonates in non-ECMO centers.
The timing of delayed surgery for CDH has evolved over time, and surgical indications continue to be updated. Initially, there was no consensus on surgical indications for CDH, and surgery was often delayed until at least 5 days after birth to improve lung tolerance.20 In 2015, the CDH EURO Consortium Consensus proposed the following factors for surgical indications: normal mean arterial blood pressure for GA, preductal oxygen saturation of 85%–95% on FiO2 below 0.5, lactate <3 mmol/L and urine volume >1 mL/kg/hour.13 With reference to the above indications, a clinical practice guideline proposed by the Canadian Congenital Diaphragmatic Hernia Collaborative in 2018 added the criterion of estimated pulmonary artery pressures less than systemic pressure.21 According to the latest data from the Congenital Diaphragmatic Hernia Study Group (CDHSG), the median time to surgery for patients not requiring extracorporeal life support was 4 days.22 However, previous studies had also shown that an excessive delay in surgery can increase the risk of worsening PAH, due to the limited pulmonary vascular bed and prolonged diastolic left ventricular failure, especially in severe CDH infants.9 10 Many severe neonates may die before surgery, missing the opportunities for surgery as a result of the strict pre-established indication, especially in cases where ECMO is not an option.11 Notably, a study showed that one-third of survivors who did not meet preoperative stability criteria achieved favorable postoperative outcomes. Therefore, withholding surgery from patients who failed to meet these criteria may deprive potentially viable candidates of treatment. Consequently, some experts suggest that surgical repair should be considered for all infants, even in the absence of preoperative stability criteria.23
In recent years, some centers have explored the improvement of surgical indications, leading to a progressive advancement in the timing of surgery. In 2021, Shinno et al. improved the indicator to estimate pulmonary artery pressure, adopting the percentage of velocity–time integral (VTI) of the left-to-right flow of patent ductus arteriosus as a criterion. They established that a VTI ratio greater than 50% indicated readiness for surgery. The surgical timing of patients selected by this protocol was 1.6±1.3 days while the control group was 4.3±7.3 days. Importantly, an earlier operation time under the new strategy did not decrease the survival rate, with rates of 95.0% and 88.9% in the protocol and control groups, respectively.9 Cox et al. conducted a continuous observation of preoperative OI for the first time, and suggested that an OI≤9.4 may be an indicator of physiological stability prior to repair. They found that 81% of patients achieved preoperative stability within 24 hours after birth, with an additional 12% achieving it between 25 and 48 hours of life. Delaying CDH repair beyond initial stability was associated with increased ventilator days and age of discharge, without any survival benefit.12
Most patients in our hospital received surgery within 24–48 hours after achieving physiological stability, which is similar to recent international reports. Our data also showed that the ≥48-hour group had a higher proportion of CDH patients with liver hernia and patch repair. This suggests that severe cases may need more time to achieve physiological stability. Through long-term clinical observation, we found that with proper fluid management and rational application of vasoactive drugs, particularly the PAH-specific therapeutic drug treprostinil, most CDH patients achieved optimal physiological parameters at 24–48 hours after birth, including optimal blood pressure, urine volume, PO2, PCO2, blood oxygen saturation, blood lactate and OI. However, if the operation is excessively delayed after achieving physiological stability, the blood vessels of the herniated organs will continue to be stretched and compressed by inflatable intestines, causing inadequate blood supply to the organs and gradually aggravating edema. Maintaining physiological stability for these children becomes significantly more challenging when ECMO support is not available. The compression of organs leads to a continuous increase in systemic vascular resistance. Simultaneously, the edematous organs compress the central veins, leading to reduced venous return and dropped systemic pressure, which further aggravate organ ischemia. Over time, patients may develop irreversible hypotension and hypoxia, accumulation of carbon dioxide and lactate, and eventually die from severe hypoxemia and hypercapnia.
In addition, our study showed that performing surgery within less than 24 hours, associated with a higher mortality rate, may not represent the optimal timing for CDH infants. The impact of surgical timing on survival outcomes has been controversial in previous studies. Two Japanese studies found that performing repair between 24 and 72 hours yields significant benefits in terms of improved survival rates and reduced complications.24 25 Conversely, two studies based on CDHSG data suggested that different timing of surgery did not affect the prognosis of infants with CDH who did not require ECMO before surgery.6 22 Our results are largely consistent with the studies from Japan, likely due to both centers being non-EMCO. Most severe CDH patients in CDHSG studies received ECMO support, which resulted in less severe CDH in the enrolled children, thus reducing the impact of surgical timing. Notably, our data shows that the main difference between the <24-hour surgery group and the other two groups lies in OI. The proportion of open surgeries in the <24-hour group was also slightly higher, though there was no statistical difference. This indicates that although the children in <24-hour group met the minimum requirements of surgical indications before surgery, the physiological conditions of some children may not have reached complete stability, making them less tolerant to thoracoscopic surgery. As demonstrated in the study by Cox et al.,12 the OI not only predicts the severity of CDH, but also reflects the preoperative physiological status of neonates with CDH. The poorer OI observed in the <24-hour group represented the children with more severe hypoxemia and uncorrected PAH before surgery, conditions that may further deteriorate with early surgical intervention. The decision to perform emergency surgery on some neonates was made due to the occurrence of incarcerated hernia. Four patients had a sudden deterioration after reaching initial stability. Based on hemodynamics and ultrasound findings, we suspected the occurrence of incarceration of the herniated organ, and proceeded with emergency surgery. Therefore, although the mortality rate in <24-hour group was significantly higher, it is plausible that early surgery may have saved those children who would have otherwise succumbed before surgery.23 These findings suggest that more thorough evaluations of preoperative OI should be undertaken in future work. More importantly, if the condition of the patient permits, surgery should be performed at least 24 hours after birth.
Our study proposes the optimal surgical timing for non-ECMO centers by analyzing the prognosis of CDH newborns grouped according to different surgical timings, thereby addressing the gaps in the existing literature. However, we acknowledge that several limitations in our study. First, this study was a retrospective study from a single institution and lacked external verification. The generalizability and practical clinical value of our findings need to be further verified by subsequent prospective cohort studies. Second, our findings may have limited applicability to ECMO centers, but still provide a reference for organizations that use ECMO less frequently. Thirdly, the absence of lung volume measurement in fetal MRI for CDH patients in this study posed limitations to the analysis and grouping. We will continue to strengthen our collaboration with obstetrics in future work and make concerted efforts to refine the measurement and collection of this crucial indicator.
Taken together, our findings suggest that performing surgery in the 24–48-hour window after birth may be appropriate for severe CDH infants in the absence of ECMO support. This timing allows for earlier relief of organ compression while avoiding the loss of surgical opportunities by waiting excessively.
Acknowledgements
We acknowledge the support and trust of all patients and their parents.
Footnotes
Funding: This project was supported in part by the National Key Research and Development Program of China 341 (2018YFC1002503) and the Beijing Municipal Natural Science Foundation (7224321).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics approval: Ethics approval was obtained from the ethics committee of the Children’s Hospital of Capital Institute of Pediatrics (approval number: SHERLLM2022035). All methods were carried out in accordance with relevant guidelines and regulation. As a retrospective and observational study, the need for informed consent was waived by the ethics committee of the Children’s Hospital of Capital Institute of Pediatrics.
Contributor Information
Zhong Feng, Email: leosu24@163.com.
Yan-Dong Wei, Email: wyd0720@126.com.
Ying Wang, Email: qtails@hotmail.com.
Jing-Na Li, Email: lijingna86@163.com.
Chao Liu, Email: paddy_fifish@163.com.
Hui Zhang, Email: zhanghui7012@aliyun.com.
Fei Wang, Email: wangfei@shouer.com.cn.
Tao Wu, Email: wutao19811010@163.com.
Yu-Lin Jiang, Email: yulinj@gmail.com.
Lishuang Ma, Email: malishuang2006@126.com.
Data availability statement
Data are available on reasonable request.
References
- 1.Chatterjee D, Ing RJ, Gien J. Update on Congenital Diaphragmatic Hernia. Anesth Analg. 2020;131:808–21. doi: 10.1213/ANE.0000000000004324. [DOI] [PubMed] [Google Scholar]
- 2.Lally PA, Miller CC, Hirschl RB, et al. Mortality in Congenital Diaphragmatic Hernia: A Multicenter Registry S tudy of Over 5000 Patients Over 25 Years. ANN SURG. 2021 doi: 10.1097/SLA.0000000000005113. [DOI] [PubMed] [Google Scholar]
- 3.Scavacini Marinonio AS, Harumi Miyoshi M, Testoni Costa-Nobre D, et al. Congenital diaphragmatic hernia in a middle-income country: Persistent high lethality during a 12-year period. PLoS ONE . 2023;18:e0281723. doi: 10.1371/journal.pone.0281723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz‐Martínez R, Etchegaray A, Molina‐Giraldo S, et al. A multicentre study to predict neonatal survival according to lung‐to‐head ratio and liver herniation in fetuses with left congenital diaphragmatic hernia (CDH): Hidden mortality from the Latin American CDH Study Group Registry. Prenat Diagn. 2019;39:519–26. doi: 10.1002/pd.5458. [DOI] [PubMed] [Google Scholar]
- 5.Jancelewicz T, Paton EA, Jones J, et al. Risk-stratification enables accurate single-center outcomes assessment in congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2019;54:932–6. doi: 10.1016/j.jpedsurg.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Hollinger LE, Lally PA, Tsao K, et al. A risk-stratified analysis of delayed congenital diaphragmatic hernia repair: Does timing of operation matter? Surgery . 2014;156:475–82. doi: 10.1016/j.surg.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Horn-Oudshoorn EJJ, Knol R, Te Pas AB, et al. Perinatal stabilisation of infants born with congenital diaphragmatic hernia: a review of current concepts. Arch Dis Child Fetal Neonatal Ed . 2020;105:449–54. doi: 10.1136/archdischild-2019-318606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang MJ, Russell KW, Yoder BA, et al. Congenital diaphragmatic hernia: a narrative review of controversies in neonatal management. Transl Pediatr . 2021;10:1432–47. doi: 10.21037/tp-20-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinno Y, Terui K, Endo M, et al. Optimization of surgical timing of congenital diaphragmatic hernia using the quantified flow patterns of patent ductus arteriosus. Pediatr Surg Int . 2021;37:197–203. doi: 10.1007/s00383-020-04788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inamura N, Kubota A, Nakajima T, et al. A proposal of new therapeutic strategy for antenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 2005;40:1315–9. doi: 10.1016/j.jpedsurg.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Harting MT, Hollinger L, Tsao K, et al. Aggressive Surgical Management of Congenital Diaphragmatic Hernia: Worth the Effort? Ann Surg. 2018;267:977–82. doi: 10.1097/SLA.0000000000002144. [DOI] [PubMed] [Google Scholar]
- 12.Cox KJ, Yang MJ, Fenton SJ, et al. Operative repair in congenital diaphragmatic hernia: How long do we really need to wait? J Pediatr Surg. 2022;57:17–23. doi: 10.1016/j.jpedsurg.2022.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Snoek KG, Reiss IKM, Greenough A, et al. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus - 2015 Update. Neonatology . 2016;110:66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]
- 14.Bhombal S, Patel N. Diagnosis & management of pulmonary hypertension in congenital diaphragmatic hernia. Semin Fetal Neonat Med. 2022;27:101383. doi: 10.1016/j.siny.2022.101383. [DOI] [PubMed] [Google Scholar]
- 15.De Bie FR, Avitabile CM, Flohr S, et al. Treprostinil in Neonates with Congenital Diaphragmatic Hernia-Related Pulmonary Hypertension. J Pediatr. 2023;259:113420. doi: 10.1016/j.jpeds.2023.113420. [DOI] [PubMed] [Google Scholar]
- 16.Tan Y-W, Ali K, Andradi G, et al. Prognostic value of the oxygenation index to predict survival and timing of surgery in infants with congenital diaphragmatic hernia. J Pediatr Surg. 2019;54:1567–72. doi: 10.1016/j.jpedsurg.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Ruttenstock E, Wright N, Barrena S, et al. Best Oxygenation Index on Day 1: A Reliable Marker for Outcome and Survival in Infants with Congenital Diaphragmatic Hernia. Eur J Pediatr Surg. 2015;25:3–8. doi: 10.1055/s-0034-1393960. [DOI] [PubMed] [Google Scholar]
- 18.Sinha CK, Islam S, Patel S, et al. Congenital diaphragmatic hernia: prognostic indices in the fetal endoluminal tracheal occlusion era. J Pediatr Surg. 2009;44:312–6. doi: 10.1016/j.jpedsurg.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 19.Lally KP, Lasky RE, Lally PA, et al. Standardized reporting for congenital diaphragmatic hernia – An international consensus. J Pediatr Surg. 2013;48:2408–15. doi: 10.1016/j.jpedsurg.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Lally KP. Congenital diaphragmatic hernia – the past 25 (or so) years. J Pediatr Surg. 2016;51:695–8. doi: 10.1016/j.jpedsurg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Puligandla PS, Skarsgard ED, Offringa M, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ . 2018;190:E103–12. doi: 10.1503/cmaj.170206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta VS, Shepherd ST, Ebanks AH, et al. Association of timing of congenital diaphragmatic hernia repair with survival and morbidity for patients not requiring extra-corporeal life support. NPM . 2022;15:759–65. doi: 10.3233/NPM-221072. [DOI] [PubMed] [Google Scholar]
- 23.Beres AL, Puligandla PS, Brindle ME. Stability prior to surgery in Congenital Diaphragmatic Hernia: Is it necessary? J Pediatr Surg. 2013;48:919–23. doi: 10.1016/j.jpedsurg.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Yamoto M, Ohfuji S, Urushihara N, et al. Optimal timing of surgery in infants with prenatally diagnosed isolated left-sided congenital diaphragmatic hernia: a multicenter, cohort study in Japan. Surg Today . 2021;51:880–90. doi: 10.1007/s00595-020-02156-7. [DOI] [PubMed] [Google Scholar]
- 25.Oluyomi-Obi T, Kuret V, Puligandla P, et al. Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH) J Pediatr Surg. 2017;52:881–8. doi: 10.1016/j.jpedsurg.2016.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.