Abstract
Objective
Retinopathy of prematurity (ROP) is a leading cause of preventable childhood blindness in preterm infants with low birth weight. The efficacy and safety of prophylactic agents, including vitamin A, propranolol and lipids, in reducing ROP incidence remain unclear. This systematic review and meta-analysis evaluated the effectiveness and safety of these agents in preventing ROP.
Methods and Analysis
A systematic search was conducted in Embase, MEDLINE and CENTRAL databases. Eight randomised controlled trials involving 1101 preterm infants were included. We assessed the incidence of ROP at any stage, severe ROP, adverse events and mortality. Subgroup analyses were performed for each prophylactic agent. Data were pooled using the inverse variance weighting method and reported as risk ratios (RRs) with 95% CI.
Results
No significant reduction in ROP incidence at any stage was found in the intervention groups compared with placebo (RR=0.83; 95% CI= (0.69 to 1.00); p=0.05; I²=0%). Lipids significantly reduced severe ROP incidence (RR=0.48; 95% CI= (0.28 to 0.80); p=0.005), while vitamin A (RR=1.14; 95% CI= (0.51 to 2.54); p=0.75) and propranolol (RR=0.69; 95% CI= (0.29 to 1.65); p=0.41) did not. There were no significant differences in adverse events (RR=0.83; 95% CI= (0.59 to 1.17); p=0.28) or mortality (RR=0.93; 95% CI= (0.67 to 1.30); p=0.68) across all groups.
Conclusion
Lipids show promise in reducing severe ROP in preterm infants, while vitamin A and propranolol were not effective. Further research is needed to confirm these findings and explore the potential role of lipids in clinical practice.
Keywords: Visual perception, Vision, Treatment Medical, Retina, Child health (paediatrics)
WHAT IS ALREADY KNOWN ON THIS TOPIC
Retinopathy of prematurity (ROP) is a leading cause of preventable childhood blindness, particularly in preterm infants with low birth weight. Previous studies have investigated various prophylactic agents, such as vitamin A, propranolol and lipids, to reduce the incidence and severity of ROP, but their efficacy and safety remain inconclusive.
WHAT THIS STUDY ADDS
This systematic review and meta-analysis demonstrated that while vitamin A, propranolol and lipids did not significantly reduce the incidence of ROP at any stage, lipids were effective in significantly reducing the rates of severe ROP.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings support the use of lipids as a potential prophylactic agent against severe ROP in preterm infants, suggesting a need for further research.
Introduction
Retinopathy of prematurity (ROP), a vasoproliferative condition, is considered one of the leading aetiologies of preventable childhood blindness globally,1 affecting the retina of preterm newborns with low birth weight.2 Retinal vessel immaturity makes the retinas of such infants more prone to developing pathological extraretinal neovascularisation, particularly when undergoing oxygen therapy at high and fluctuating rates.1 ROP severity can be classified into five stages (stages 1–5), with the need for treatment varying according to the severity and location of the disease.3 The estimated pooled prevalence of ROP ranges from 21.8% to 36.5% in preterm babies with a gestational age <32 weeks.4 Patients with ROP are at high risk of developing lifelong visual impairment or blindness.4 Therefore, preventive strategies and interventions have been investigated to reduce the incidence and complication rates of ROP.4
Although the pathogenesis of ROP is not completely understood, multiple factors along with retinal vessel immaturity and oxygen therapy are considered to contribute to the risk of developing ROP, including intermittent hypoxia, oxidative distress, inflammation and dysregulated vascular endothelial growth factor (VEGF). For instance, retinal astrocytes, under intermittent hypoxic conditions, release VEGF, which in turn contributes to neovascularisation by promoting the migration of endothelial cells in the retina and aids in their differentiation and proliferation.5 These factors are targeted by multiple pharmacological agents, including vitamin A, propranolol and lipids, to interfere with ROP pathogenesis and prevent its occurrence or progression.5 Recent studies have highlighted the potential ability of vitamin A and propranolol to reduce the rates and severe stages of ROP among supplemented patients by downregulating VEGF expression.5,7 Furthermore, lipids such as arachidonic acid and docosahexaenoic acid have been found to have a prophylactic effect against ROP by inhibiting the pathological neovascularisation process.8
Although vitamin A, propranolol and lipids have shown promising results as ROP prophylactic agents, no consensus has been reached regarding their true efficacy and safety in clinical practice.5 6 9 Additionally, no previous systematic reviews have evaluated the safety and efficacy of these drugs. This systematic review and meta-analysis investigated the efficacy and safety of lipids, vitamin A and propranolol in reducing the incidence of ROP, adverse events and mortality rates in preterm infants with low birth weight.
Methods
This systematic review and meta-analysis were designed and carried out in accordance with a predefined protocol published in PROSPERO (CRD42022344800). This paper has been conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist10 (see online supplemental file 2).
Eligibility criteria
This paper assessed randomised controlled trials (RCTs) that specifically examined preterm infants born with a gestational age below 32 weeks and a birth weight less than 1500 grams who received lipids, vitamin A, or propranolol and compared them with placebo or no supplementation arms with regard to the rates of the following prespecified outcomes: ROP at any stage, ROP stage 1, ROP stage 2, severe ROP, adverse events and mortality. The rationale to specifically include preterm infants born with a gestational age below 32 weeks and a birth weight less than 1500 g is grounded in consistent evidence from previous literature indicating that this population is at the highest risk of developing ROP.4
RCTs that were not placebo-controlled or did not include a no-supplementation arm were excluded. Studies that included patients who were previously diagnosed with ROP or who had received previous non-surgical or surgical management for ROP were also excluded. Previously, ROP management included retinal laser therapy or intravitreal anti-VEGF therapy. In this study, severe ROP was characterised by cases falling within ROP stages 3–5, prethreshold ROP type 1 or ROP requiring treatment. These cases are linked to a heightened risk of enduring permanent visual impairment or blindness, often requiring urgent care.3 11 12 Prethreshold ROP type 1 was defined as stage 3 ROP without plus disease in zone I, any stage ROP with plus disease in zone I, or stage 2+ or 3+ in zone II.3
Search strategy
A systematic search was performed in Embase, MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) from the inception of each database to 10 July 2022, without any limitations on language or date. The search was carried out using the following keywords: “infant,” “premature,” “low birth weight,” “retinopathy of prematurity,” “retrolental fibroplasia,” “vitamin A,” “retinol,” “fat,” “fatty acids,” “omega 3,” “omega 6,” “lipids,” “propranolol,” “inderal,” “anthralin,” “obsidian,” “obsidian,” and “beta blockers”. Online supplemental data demonstrates the full search strategy. Reference lists of the eligible studies were also manually screened for any potential eligible RCTs not noticed in the original search. The search was updated in October 2024 to identify possible new studies.
Study selection and data extraction
Two authors independently conducted screening of titles, abstracts and full-text assessments to determine the eligibility of studies. They independently extracted data from the eligible RCTs. Any disagreements were resolved through discussion until a consensus was reached or, if necessary, by consulting a third author’s opinion.
Meta-analysis
The random-effects model in RevMan (Review Manager) V.5.3 (Cochrane Collaboration) was used for the meta-analysis. As a threshold for statistical significance, we used a 95% confidence level and a p<0.05. A p value of the χ2 test for heterogeneity and I2 were used to assess the heterogeneity. The inverse variance weighting method was used to pool dichotomous outcomes, such as of ROP at any stage, ROP stage 1, ROP stage 2, severe ROP, adverse events and mortality rates. These outcomes were represented as risk ratios (RRs). Subgroup analysis was done according to the following interventions: lipids, vitamin A and propranolol. Subgroup analyses were used to compare the interventions and provide evidence of each intervention’s superiority over the remaining interventions in terms of efficacy and safety. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria were used to assess the evidence quality for each outcome in the paper.
Risk of bias assessment
Two authors independently evaluated the risk of bias in the included trials using the Revised Cochrane Risk of Bias Assessment Tool.13 This tool uses prespecified and standardised criteria to assess the risk of bias in each RCT according to five domains: the randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported results.
Following the paired assessment, each of the included studies was categorised as either high risk, low risk or of concern. Any discrepancies between the authors were resolved through discussion, leading to a consensus or by seeking the input of a third author when necessary. The possibility of publication bias was not investigated using funnel plots because this meta-analysis included fewer than 10 studies.
Results
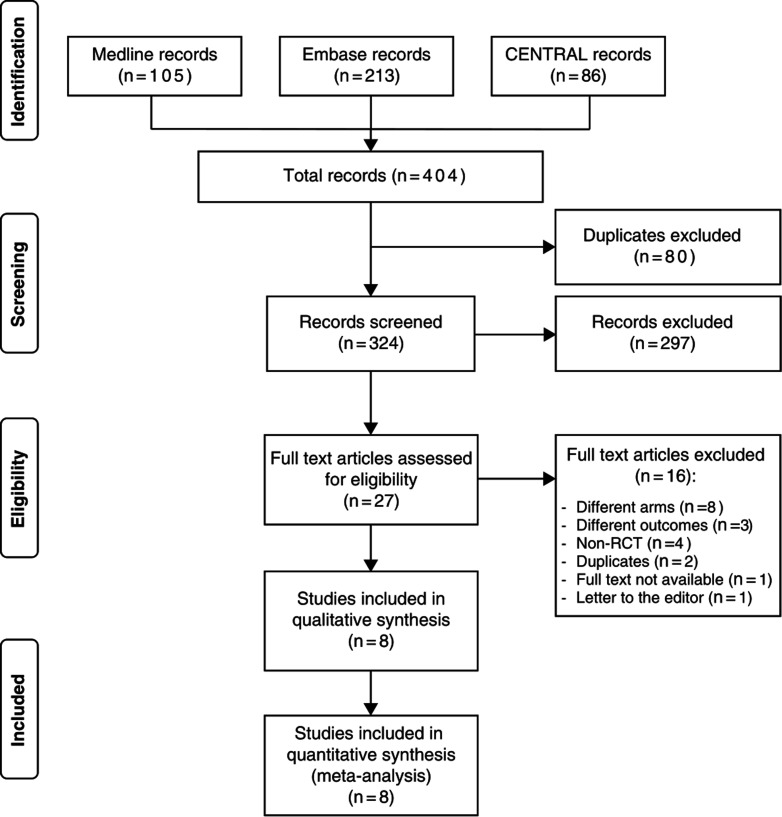
Figure 1 illustrates the flow chart depicting the inclusion process of the study and provides a rationale for excluding specific studies. The search strategy employed in this review initially yielded 380 articles, out of which only 8 RCTs were deemed eligible and included in the meta-analysis, with 4 focusing on vitamin A, 3 on propranolol and 1 on lipids.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart demonstrating the process of screening and selecting studies. RCT, randomised controlled trial.
Trial characteristics
A total of 1101 participants were included in this meta-analysis. Of these, 479 (43.5%) were assigned vitamin A, 416 (37.8%) were assigned propranolol and 206 (18.7%) lipids. The mean gestational age ranged between 26 and 30.9 weeks in the vitamin A arm, 29 and 29.54 weeks in the propranolol arm, and approximately 25.5 weeks in the lipid arm. The mean birth weight varied within the range of 782–1185 g in the vitamin A-arm, 1054–1235 g in the propranolol arm and approximately 797 g in the lipid arm. Detailed characteristics of the included RCTs are presented in online supplemental table.
Risk of bias assessment
According to the Revised Cochrane Risk of Bias Assessment Tool, four out of the eight included RCTs exhibited a low risk of bias, while three RCTs raised concerns. One RCT was deemed to have a high risk of bias, primarily attributed to challenges in outcome measurement. Detailed risk of bias assessments for these RCTs are presented in online supplemental figures 1 and 2.
ROP at any stage
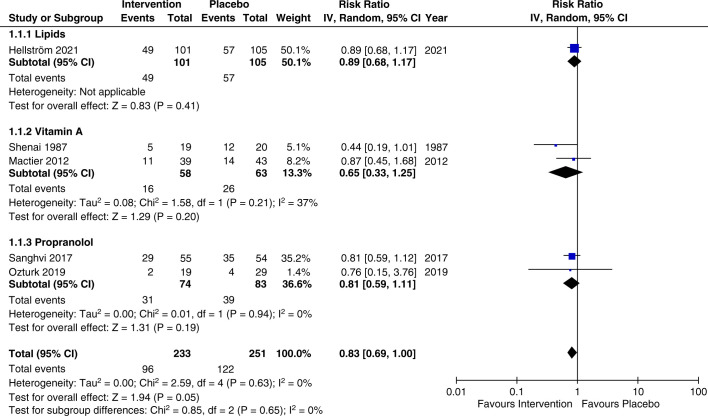
Five RCTs (n=580) reported ROP rates at any stage.814,17 No statistically significant differences were observed between vitamin A, propranolol and lipids in reducing or preventing the rate of ROP at any stage in comparison with the control group (RR=0.83, 95% CI 0.69 to 1.00, p=0.05, I2=0%). The subgroup analysis based on the received intervention revealed no significant difference in the vitamin A (RR=0.65, 95% CI 0.33 to 1.25, p=0.2, I2=37%), propranolol (RR=0.81, 95% CI 0.59 to 1.11, p=0.19, I2=0%) or lipid (RR=0.89, 95% CI 0.68 to 1.17, p=0.41, I2=not applicable) subgroups (figure 2). The overall certainty of evidence according to the GRADE assessment was deemed moderate for ROP at any stage (supplementary GRADE).
Figure 2. Meta-analysis and subgroup analysis of ROP of any stage by intervention comparing lipids, vitamin A, and propranolol. ROP, retinopathy of prematurity; IV, inverse variance.
ROP stage 1
Four RCTs (n=540) reported the rates of ROP stage 1.815,17 All intervention arms, including vitamin A, propranolol and lipids, showed no significant differences in comparison to the control group in reducing the rates of ROP stage 1 (RR=1.13, 95% CI 0.72 to 1.79, p=0.59, I2=27%). Subgroup analyses revealed comparable results with no significant differences in the vitamin A (RR=0.5, 95% CI 0.17 to 1.47, p=0.21, I2=not applicable), propranolol (RR=1.04, 95% CI 0.57 to 1.90, p=0.89, I2=0%) or lipids (RR=1.56, 95% CI 0.98 to 2.48, p=0.06, I2=not applicable) subgroups (online supplemental figure 3). The GRADE overall certainty of the evidence was rated low for ROP stage 1 (supplementary GRADE).
ROP Stage 2
Four RCTs (n=540) reported data on the rates of ROP stage 2.815,17 Similar to ROP stage 1, the incidence rates of ROP stage 2 were comparable between the intervention and control groups, with no significant differences noted (RR=1.04, 95% CI 0.54 to 2.02, p=0.9, I2=26%). The subgroup analysis also revealed no significant differences within the vitamin A (RR=0.33, 95% CI 0.01 to 7.85, p=0.5, I2=not applicable), propranolol (RR=0.62, 95% CI 0.25 to 1.54, p=0.3, I2=0%) or lipids (RR=1.56, 95% CI 0.98 to 2.48, p=0.06, I2=not applicable) subgroups (online supplemental figure 4). The overall certainty of evidence according to the GRADE assessment was deemed low for ROP stage 2 (supplementary GRADE).
Severe ROP
Seven RCTs (n=1061) reported rates of severe ROP.78 15,19 Participants in the intervention arm exhibited a significant reduction in the rates of severe ROP compared with the control arm (RR=0.63, 95% CI 0.46 to 0.86, p=0.004, I2=6%). The lipids subgroup demonstrated a significant reduction in severe ROP among its participants (RR=0.48, 95% CI 0.28 to 0.80, p=0.005, I2=not applicable), while the remaining groups, including vitamin A (RR=1.14, 95% CI 0.51 to 2.54, p=0.75, I2=0%) and propranolol (RR=0.69, 95% CI 0.29 to 1.65, p=0.41, I2=12%) subgroups did not show a significant rate reduction (online supplemental figure 5). The overall certainty of evidence according to the GRADE assessment was deemed low for severe ROP (supplementary GRADE).
Adverse events
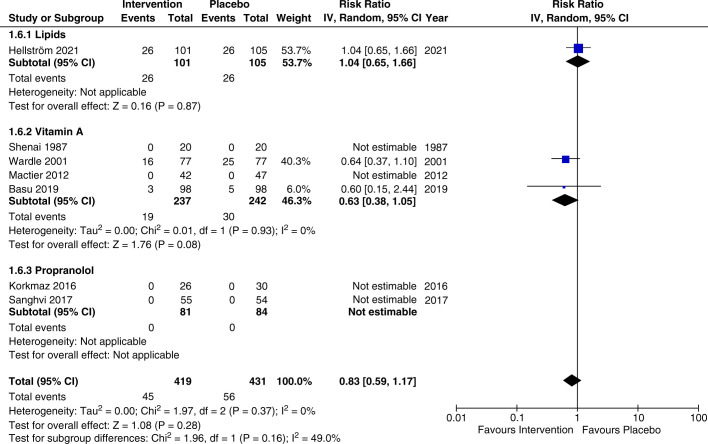
Seven RCTs (n=965) reported rates of adverse events.78 14 15 17,19 The rate of adverse events was comparable between the intervention and control arms, with no significant difference found (RR=0.83, 95% CI 0.59 to 1.17, p=0.28, I2=0%). The subgroup analysis for lipids (RR=1.04, 95% CI 0.65 to 1.66, p=0.87, I2=not applicable) and vitamin A (RR=0.63, 95% CI 0.38 to 1.05, p=0.08, I2=0%) did not reveal significant differences (figure 3). The overall certainty of evidence according to the GRADE assessment was deemed moderate for adverse events (supplementary GRADE).
Figure 3. Meta-analysis and subgroup analysis of adverse events by intervention comparing lipids, vitamin A, and propranolol. IV, inverse variance.
Mortality rate
Seven RCTs (n=930) reported the mortality rate.814,19 Comparable rates were observed between the intervention and control groups, with no significant differences (RR=0.93, 95% CI 0.67 to 1.30, p=0.68, I2=6%). The subgroup analysis based on the intervention also revealed no significant differences in the vitamin A (RR=0.83, 95% CI 0.51 to 1.37, p=0.47, I2=21%), propranolol (RR=1.31, 95% CI 0.31 to 5.58, p=0.72, I2=not applicable) or lipids (RR=1.28, 95% CI 0.65 to 2.52, p=0.48, I2=not applicable) subgroups (online supplemental figure 6). The overall certainty of evidence according to the GRADE assessment was deemed moderate for the mortality rate (supplementary GRADE).
Discussion
This systematic review and meta-analysis comprised 8 RCTs with a total of 1101 premature infants with low birth weight and assessed the efficacy and safety of lipids, vitamin A and propranolol in preventing the incidence of ROP and severe ROP. The pooled effect estimate revealed a statistically significant decrease in the rates of severe ROP among patients in the intervention arm compared with those in the control arm. The subgroup analysis revealed a significant reduction in severe ROP only in the lipid subgroup. Nevertheless, there were no notable differences observed between the intervention and control arms concerning ROP at any stage, ROP stages 1, ROP stage 2, adverse events or mortality rates.
Nutritional and pharmaceutical agents have been the focus of recent RCTs to identify effective and safe agents for ROP prevention.78 14,19 Vitamin A, propranolol and lipids are the main agents that have a consistent ability to reduce ROP or severe ROP, as demonstrated in recent RCTs.78 14,19 Therefore, our study focused solely on these three agents and compared them to reach a consensus on the prophylactic agent of choice for treating ROP. As our study was the first to concurrently include these agents, no comparative data are available to assess them against the pooled effect we estimated.
Our subgroup analysis revealed the ability of lipids to reduce the incidence of severe ROP. This finding contradicts the conclusions reached by Fang et al20 and Raghuveer and Zackula21 in their meta-analyses, which suggested that lipids have no impact on ROP at any stage or severe ROP. This disparity can be attributed to differences in the inclusion criteria between our study and those of Fang et al20 and Raghuveer and Zackula.21 Specifically, our study included only placebo-controlled RCTs, whereas Fang et al20 and Raghuveer and Zackula21 included RCTs comparing different lipid emulsions in both study arms. In addition, Hellström et al,8 the only lipid-related RCT in our study, used triglyceride oil containing DHA from algae and AA from fungi, whereas Fang et al20 and Raghuveer and Zackula21 investigated oils sourced from soybeans, fish and olives. Further investigations into the impact of different types of oil sources, including fungi and algae, on ROP prevention may be beneficial.
Our results also indicated that lipids did not affect the risk of ROP development at any stage. This is in line with a recent systematic review that investigated the effects of long-chain polyunsaturated fatty acids AA and DHA on ROP, which also showed no influence on ROP incidence.22 A possible explanation for the ability of lipids to reduce severe ROP, but not ROP at any stage, is that severe ROP has a wider definition and includes multiple categories. For example, our study included ROP stages 3–5, threshold ROP type 1, or severe ROP requiring treatment, resulting in a larger sample of patients in the severe ROP category; the remaining categories, such as ROP at any stage, stage 1 and stage 2, were limited by one specific definition per category. Regarding the safety profile of lipids, similar rates of adverse events and mortality have been observed between the lipid and placebo arms.8 This indicates that lipid supplementation did not contribute to a higher risk of death or adverse events. Both study arms included 26 patients who developed serious adverse events including bronchopulmonary dysplasia, necrotising enterocolitis, patent ductus arteriosus and intraventricular haemorrhage. However, these adverse events are well-known complications of prematurity.23 Therefore, given the similar rates of adverse events in both arms (intervention and control), it is more plausible to associate these events with participant prematurity.
Our vitamin A subgroup analysis demonstrated vitamin A failed to reduce the rate of ROP at any stage.15 18 Wardle et al18 proposed that insufficient serum retinol concentrations may explain the failure of vitamin A. This is possible because the optimal serum retinol levels remain unknown in premature babies. Therefore, not knowing the optimal measurements can result in inadequate vitamin A supplementation. This is supported by the fact that Mactier et al15 and Wardle et al18 who both reported the failure of vitamin A, had a retinol serum concentration ≤0.7 µmol/L in most of their intervention arms for participants on the 28th day of life. Serum retinol levels ranging from 0.35 to 0.7 µmol/L are considered a reflection of vitamin A deficiency.24 In contrast to our findings, the RCT conducted by Shenai et al14 reported a reduction in severe ROP rates in preterm infants supplemented with vitamin A. This RCT reported that in all subsequent serum retinol measurements, the intervention arm had a higher serum retinol concentration than the control arm. In Shenai et al’s14 RCT, participants consistently maintained serum retinol levels >20 µg/dL (3.58 µmol/L) in all measurements, while participants in the studies by and Mactier et al15 and Wardle et al18 exhibited lower values. This finding further supports the argument that adequate serum retinol levels have a crucial effect on the efficacy of vitamin A in preventing ROP. Consistent with our results, a previous meta-analysis reported that the rates of mortality and adverse events of vitamin A were similar between infants supplemented with vitamin A and those in the control group, indicating the safety of vitamin A supplementation in premature infants.25 It is important to know that the adverse events reported by the included RCTs in our study were seizures and vomiting.18 19
Our results showed that propranolol did not reduce the rates of ROP at any stage or severe ROP.7 16 17 A Cochrane review published in 2018 concluded that there is limited evidence suggesting the ability of propranolol to reduce progression towards stage 3 ROP and ROP requiring treatment, with insufficient evidence to determine the efficacy and safety of propranolol due to a high risk of bias and lack of long-term outcomes in the included studies.26 Due to the clear need for further investigation of propranolol, our study included an RCT by Ozturk and Korkmaz,16 which was published in 2019 and reported the failure of propranolol to prevent ROP’s occurrence. In addition, to investigate the true efficacy of propranolol as a prophylactic agent for ROP, our inclusion criteria included only outcomes that reported ROP rates in infants who were at risk of developing ROP but were not diagnosed, whereas Kaempfen et al26 included infants with confirmed stage 1 or stage 2 ROP in their analysis. Additional RCTs and more focused inclusion criteria may provide additional evidence that a Cochrane review should be performed to better evaluate the prophylactic abilities of propranolol. In line with the results of Kaempfen et al26 and Stritzke et al,27 our study showed no differences in the rates of adverse events or mortality between the intervention and control groups, indicating the safety of propranolol in these patients.
Our results agreed with the previous study by Shafique et al who found propranolol to be associated with a lower overall risk of ROP.28 However, we built on them by exploring other prophylactic agents than propranolol. Mohammadi et al found that the use of propranolol in infants with stage 1 and 2 ROP was beneficial in preventing severe stages of ROP which further supports our results.29 Recent studies have provided evidence on the safety of arachidonic and docosahexaenoic acid in preterm infants. Wackernagel et al found that arachidonic and docosahexaenoic acid supplementation was safe with no effect on pulmonary morbidity, a concern usually raised in the use of this combination.30 These polyunsaturated fatty acids were also correlated with several proteins that are involved in the inflammation process which may explain some of their protective effects.31
This systematic review and meta-analysis was the first and most comprehensive study to concurrently investigate vitamin A, propranolol and lipids as prophylactic agents for ROP through RCTs. This study investigated the effect of interventions on all possible stages of ROP to provide more thorough evidence for the efficacy of these agents. The subgroup analysis conducted based on the intervention, not only furnishes evidence regarding the superiority of one intervention over another but also augments the clinical relevance of our findings by offering additional guidance for physicians in selecting the optimal prophylactic agent for ROP. This study also provided the GRADE criteria for each outcome. The GRADE criteria ensured a transparent and comprehensive evaluation of each outcome’s certainty of evidence, thereby allowing us to provide reliable and practical recommendations for clinical practice.
We acknowledge the limitations of our study. First, variability in the doses, routes and time of initiation of each intervention may have influenced the pooled results that reflected the efficacy of each agent. Second, some of the included RCTs did not adequately define severe ROP in their trial. Missing definitions or variable definitions may have affected the results of these RCTs, hindering their generalisability. Third, one study had an overall high risk of bias due to issues in the measurement of outcomes and selection of the reported results. Finally, the small number of included studies and the lack of data on long-term outcomes are also limitations of our review, potentially impacting the generalisability of the results.
Conclusion
Although our results indicated that none of the prophylactic agents affected the risk of ROP in general, lipids showed promise in reducing severe ROP. Further research is needed to confirm these findings and explore the potential role of lipids in clinical practice.
supplementary material
Acknowledgements
We express our gratitude to Editage (www.editage.com) for their assistance in editing the English language.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Contributor Information
Waleed T Batais, Email: Wal.batais@hotmail.com.
Nada O Taher, Email: Omartahernada@hotmail.com.
Abeer K Alhindi, Email: alhindiabeer@gmail.com.
Abdullah A Ghaddaf, Email: abdullahg.official@gmail.com.
Anas Alamoudi, Email: anasalamoudi99@gmail.com.
Sarah A Al-Ghamdi, Email: Sarah.aboetrah@gmail.com.
Jumanah J Homsi, Email: jumanah.homsi@gmail.com.
Hashem S Almarzouki, Email: Ha.almarzouki@gmail.com.
Mansour A Qurashi, Email: qurashim@ksau-hs.edu.sa.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Azad R, Chandra P. Retinopathy of prematurity. J Indian Med Assoc. 2005;103:370–2. [PubMed] [Google Scholar]
- 3.Agarwal K, Jalali S. Classification of retinopathy of prematurity: from then till now. Community Eye Health. 2018;31:S4–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H, Lawn JE, Vazquez T, et al. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74 Suppl 1:35–49. doi: 10.1038/pr.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aranda JV, Qu J, Valencia GB, et al. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin Perinatol. 2019;43:360–6. doi: 10.1053/j.semperi.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Garofoli F, Barillà D, Angelini M, et al. Oral vitamin A supplementation for ROP prevention in VLBW preterm infants. Ital J Pediatr. 2020;46:77. doi: 10.1186/s13052-020-00837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korkmaz L, Baştuğ O, Ozdemir A, et al. The Efficacy of Propranolol in Retinopathy of Prematurity and its Correlation with the Platelet Mass Index. Curr Eye Res. 2017;42:88–97. doi: 10.3109/02713683.2016.1158272. [DOI] [PubMed] [Google Scholar]
- 8.Hellström A, Nilsson AK, Wackernagel D, et al. Effect of Enteral Lipid Supplement on Severe Retinopathy of Prematurity: A Randomized Clinical Trial. JAMA Pediatr. 2021;175:359–67. doi: 10.1001/jamapediatrics.2020.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vayalthrikkovil S, Bashir RA, Rabi Y, et al. Parenteral Fish-Oil Lipid Emulsions in the Prevention of Severe Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Am J Perinatol. 2017;34:705–15. doi: 10.1055/s-0036-1597131. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong EH, Shin YU, Cho H. Retinopathy of prematurity: a review of epidemiology and current treatment strategies. Clin Exp Pediatr . 2022;65:115–26. doi: 10.3345/cep.2021.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Shenai JP, Kennedy KA, Chytil F, et al. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–77. doi: 10.1016/s0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 15.Mactier H, McCulloch DL, Hamilton R, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr. 2012;160:954–9. doi: 10.1016/j.jpeds.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Ozturk MA, Korkmaz L. The efficacy of propranolol in very preterm infants at the risk of retinopathy of prematurity: Which newborn and when? Int Ophthalmol. 2019;39:1921–30. doi: 10.1007/s10792-018-1018-8. [DOI] [PubMed] [Google Scholar]
- 17.Sanghvi KP, Kabra NS, Padhi P, et al. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial) Arch Dis Child Fetal Neonatal Ed. 2017;102:F389–94. doi: 10.1136/archdischild-2016-311548. [DOI] [PubMed] [Google Scholar]
- 18.Wardle SP, Hughes A, Chen S, et al. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child - Fetal Neonatal Ed. 2001;84:9F–13. doi: 10.1136/fn.84.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu S, Khanna P, Srivastava R, et al. Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. Eur J Pediatr. 2019;178:1255–65. doi: 10.1007/s00431-019-03412-w. [DOI] [PubMed] [Google Scholar]
- 20.Fang JL, Sorita A, Carey WA, et al. Interventions To Prevent Retinopathy of Prematurity: A Meta-analysis. Pediatrics. 2016;137:e20153387. doi: 10.1542/peds.2015-3387. [DOI] [PubMed] [Google Scholar]
- 21.Raghuveer TS, Zackula R. Strategies to Prevent Severe Retinopathy of Prematurity: A 2020 Update and Meta-analysis. Neoreviews. 2020;21:e249–63. doi: 10.1542/neo.21-4-e249. [DOI] [PubMed] [Google Scholar]
- 22.Diggikar S, Aradhya AS, Swamy RS, et al. Effect of Enteral Long-Chain Polyunsaturated Fatty Acids on Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Neonatology. 2022;119:547–57. doi: 10.1159/000525266. [DOI] [PubMed] [Google Scholar]
- 23.Atalay D, Salihoğlu O, Can E, et al. Short-term outcomes of very low birth weight infants born at a tertiary care hospital, istanbul, Turkey. Iran J Pediatr. 2013;23:205–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Sun H, Cheng R, Wang Z. EARLY VITAMIN A SUPPLEMENTATION IMPROVES THE OUTCOME OF RETINOPATHY OF PREMATURITY IN EXTREMELY PRETERM INFANTS. Retina . 2020;40:1176–84. doi: 10.1097/IAE.0000000000002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Y, Yang X, Zhao J, et al. Early Vitamin A Supplementation for Prevention of Short-Term Morbidity and Mortality in Very-Low-Birth-Weight Infants: A Systematic Review and Meta-Analysis. Front Pediatr. 2022;10:788409. doi: 10.3389/fped.2022.788409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaempfen S, Neumann RP, Jost K, et al. Beta-blockers for prevention and treatment of retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev. 2018;3:CD011893. doi: 10.1002/14651858.CD011893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stritzke A, Kabra N, Kaur S, et al. Oral propranolol in prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol. 2019;39:1584–94. doi: 10.1038/s41372-019-0503-x. [DOI] [PubMed] [Google Scholar]
- 28.Shafique MA, Haseeb A, Uddin MMN, et al. Effectiveness of Propranolol in Preventing Severe Retinopathy of Prematurity: A Comprehensive Systematic Review and Meta-Analysis. Am J Ophthalmol. 2024;259:141–50. doi: 10.1016/j.ajo.2023.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi P, Babaei H, Mohsenpour H, et al. Efficacy of Oral Propranolol in Prevention of Severe Retinopathy of Prematurity: A Randomized Clinical Trial Study. Iran J Neonatol. 2023;14:1–7. doi: 10.22038/ijn.2023.70115.2361. [DOI] [Google Scholar]
- 30.Wackernagel D, Nilsson AK, Sjöbom U, et al. Enteral supplementation with arachidonic and docosahexaenoic acid and pulmonary outcome in extremely preterm infants. Prostaglandins Leukot Essent Fatty Acids. 2024;201:102613. doi: 10.1016/j.plefa.2024.102613. [DOI] [PubMed] [Google Scholar]
- 31.Klevebro S, Kebede Merid S, Sjöbom U, et al. Arachidonic acid and docosahexaenoic acid levels correlate with the inflammation proteome in extremely preterm infants. Clin Nutr. 2024;43:1162–70. doi: 10.1016/j.clnu.2024.03.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.