Abstract
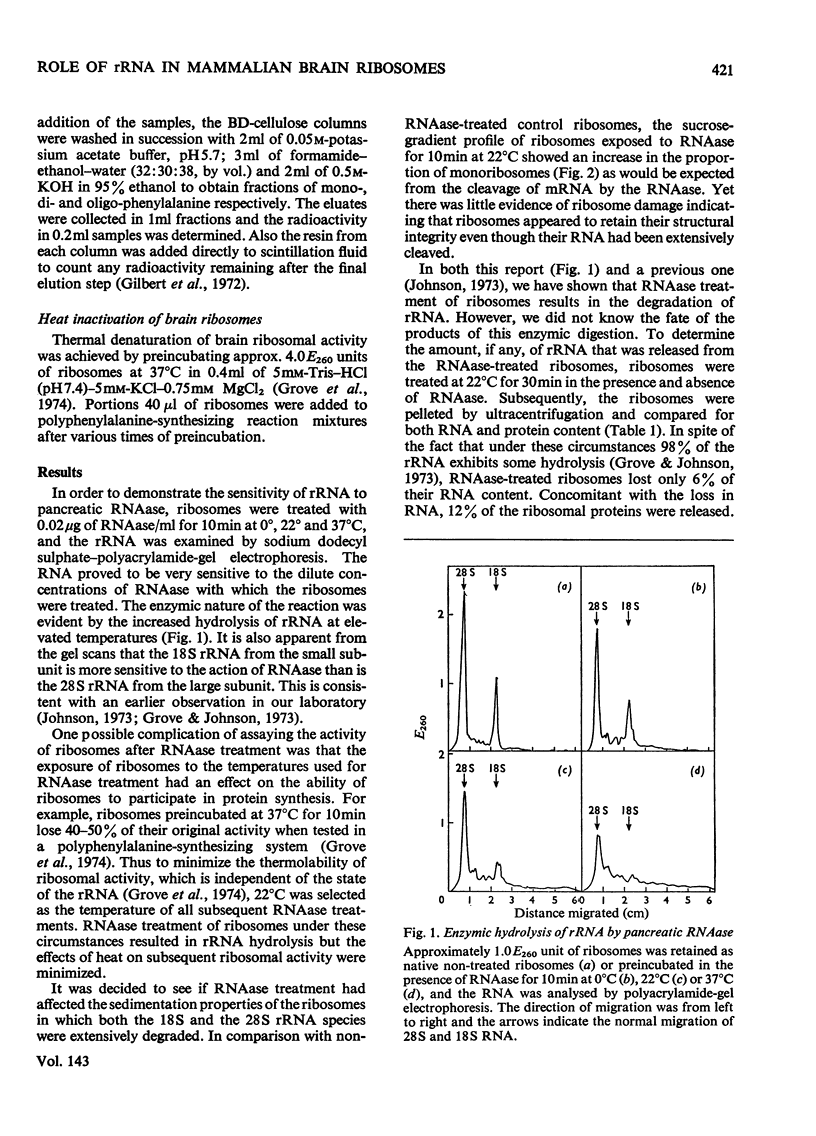
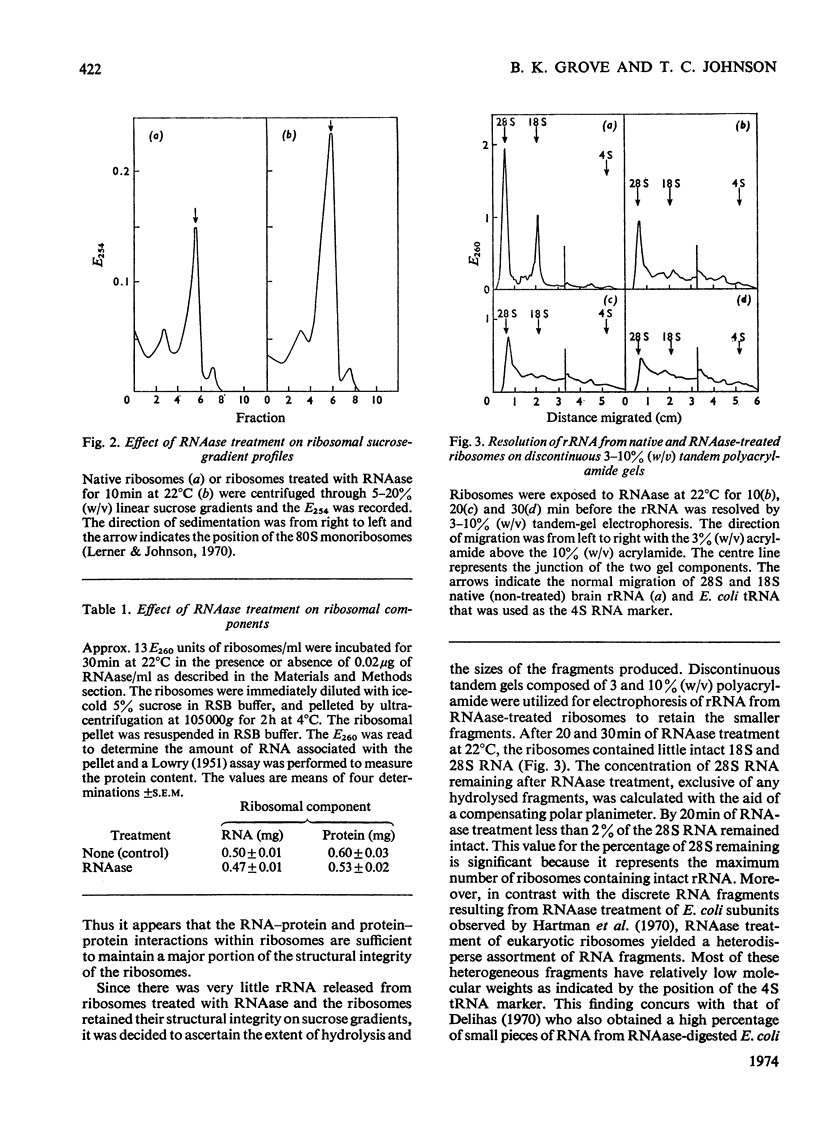
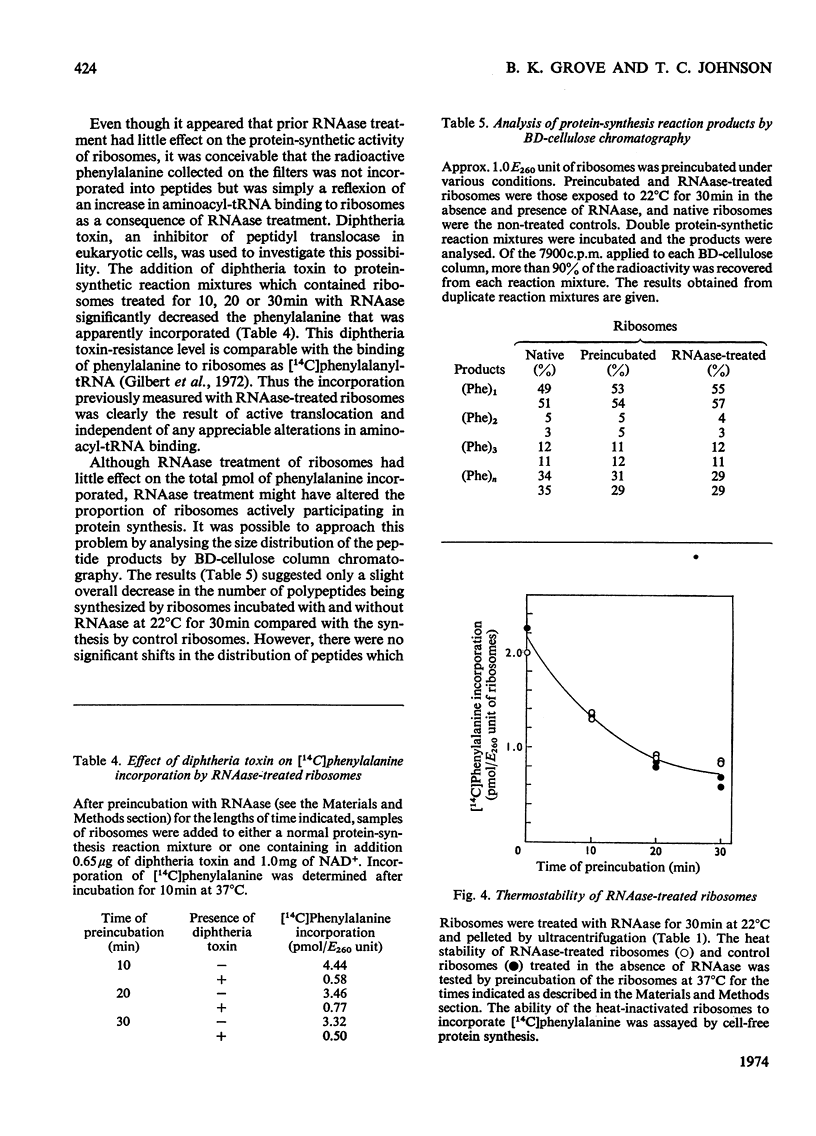
In order to resolve the functional role of intact rRNA in polypeptide chain elongation mouse brain ribosomes were treated with dilute pancreatic or T1 RNAase (ribonuclease). After RNAase treatment, several physical–chemical properties as well as the functional activity of the ribosomes were measured. RNAase treatment resulted in the extensive hydrolysis of both 18S and 28S rRNA; however, the sedimentation properties of mono-ribosomes were unaltered and more than 90% of the relatively low-molecular-weight RNA fragments remained associated with ribosome particles. Analysis of the ability of RNAase-treated ribosomes to participate in cell-free protein synthesis showed that ribosomes with less than 2% intact rRNA retained more than 85% of their activity in polyphenylalanine incorporation. Proof that the incorporation of phenylalanine by ribosomes with hydrolysed rRNA actually represented active translocation was obtained by the effective inhibition of incorporation by diphtheria toxin. In addition, the oligopeptide products of protein synthesis could be identified by BD (benzoylated diethylaminoethyl)-cellulose column chromatography. Analysis of the size distribution of oligopeptides synthesized by normal and RNAase-treated ribosomes showed no significant differences which indicated that there was no change in the proportion of ribosomes engaged in protein synthesis. Thus strong RNA–protein and protein–protein interactions must serve to maintain the functional integrity of ribosomes even when the rRNA is extensively degraded. The ability of the enzyme-treated ribosomes to efficiently incorporate amino acids clearly demonstrated that `intact' rRNA is not required for protein-synthetic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avadhani N. G., Buetow D. E. A proposed role for 5S ribosomal RNA. Biochem Biophys Res Commun. 1973 Jan 23;50(2):443–451. doi: 10.1016/0006-291x(73)90860-7. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. The effect of pancreatic ribonuclease on rabbit reticulocyte ribosomes and its interpretation in terms of ribosome structure. Biochem J. 1969 Oct;114(4):753–767. doi: 10.1042/bj1140753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Delihas N. Effect of ribonuclease on Escherichia coli ribosomes. Biochem Biophys Res Commun. 1970 Jun 5;39(5):905–910. doi: 10.1016/0006-291x(70)90409-2. [DOI] [PubMed] [Google Scholar]
- Delihas N. Liver ribosomal ribonucleic acid structural studies. Characterization of fragments from partial nuclease digestion. Biochemistry. 1967 Nov;6(11):3356–3362. doi: 10.1021/bi00863a004. [DOI] [PubMed] [Google Scholar]
- Delihas N., Zorn G. A., Strobel E. The reaction of Escherichia coli ribosomes with kethoxal. Biochimie. 1973;55(10):1227–1234. doi: 10.1016/s0300-9084(74)80327-5. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Gilbert B. E., Grove B. K., Johnson T. C. Characteristics and products of a cell-free polypeptide synthesizing system from neonatal and adult mouse brain. J Neurochem. 1972 Dec;19(12):2835–2842. doi: 10.1111/j.1471-4159.1972.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Grove B. K., Johnson T. C., Gilbert B. E. Thermostability of mammalian brain ribosomes and the effects of nucleoside triphosphates on their heat-sensitivity. Biochem J. 1974 Feb;137(2):291–298. doi: 10.1042/bj1370291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove B. K., Johnson T. C. The effect of ribonuclease on ribosomal RNA and subsequent polypeptide synthesis. Biochem Biophys Res Commun. 1973 Nov 1;55(1):45–51. doi: 10.1016/s0006-291x(73)80057-9. [DOI] [PubMed] [Google Scholar]
- Hartman K. A., Amaya J., Schachter E. M. Structure of RNA in ribosomes. Science. 1970 Oct 9;170(3954):171–173. doi: 10.1126/science.170.3954.171. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Hüvös P., Vereczkey L., Gaál O. Incorporating activity of ribosomes and integrity of ribosomal RNA. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1020–1026. doi: 10.1016/0006-291x(70)90187-7. [DOI] [PubMed] [Google Scholar]
- Kuechler E., Bauer K., Rich A. Protein synthesis with ribonuclease digested ribosomes. Biochim Biophys Acta. 1972 Sep 14;277(3):615–627. doi: 10.1016/0005-2787(72)90106-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B., Fahnestock S. R., Nomura M. Alteration of 23 S ribosomal RNA and erythromycin-induced resistance to lincomycin and spiramycin in Staphylococcus aureus. J Mol Biol. 1973 Feb 15;74(1):67–72. doi: 10.1016/0022-2836(73)90355-0. [DOI] [PubMed] [Google Scholar]
- Lerner M. P., Johnson T. C. Regulation of protein synthesis in developing mouse brain tissue. Alteration in ribosomal activity. J Biol Chem. 1970 Mar 25;245(6):1388–1393. [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Assembly of bacterial ribosomes. Science. 1973 Mar 2;179(4076):864–873. doi: 10.1126/science.179.4076.864. [DOI] [PubMed] [Google Scholar]
- Santer M., Székely M. Nuclease action on Escherichia coli ribosomes and its application to sequence studies on ribosomal ribonucleic acid. Biochemistry. 1971 May 11;10(10):1841–1846. doi: 10.1021/bi00786a018. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W. Enzymatic degradation of ribosomal RNA in isolated purified ribosomes. Biochem Biophys Res Commun. 1969 Jun 6;35(5):653–658. doi: 10.1016/0006-291x(69)90454-9. [DOI] [PubMed] [Google Scholar]



