Abstract
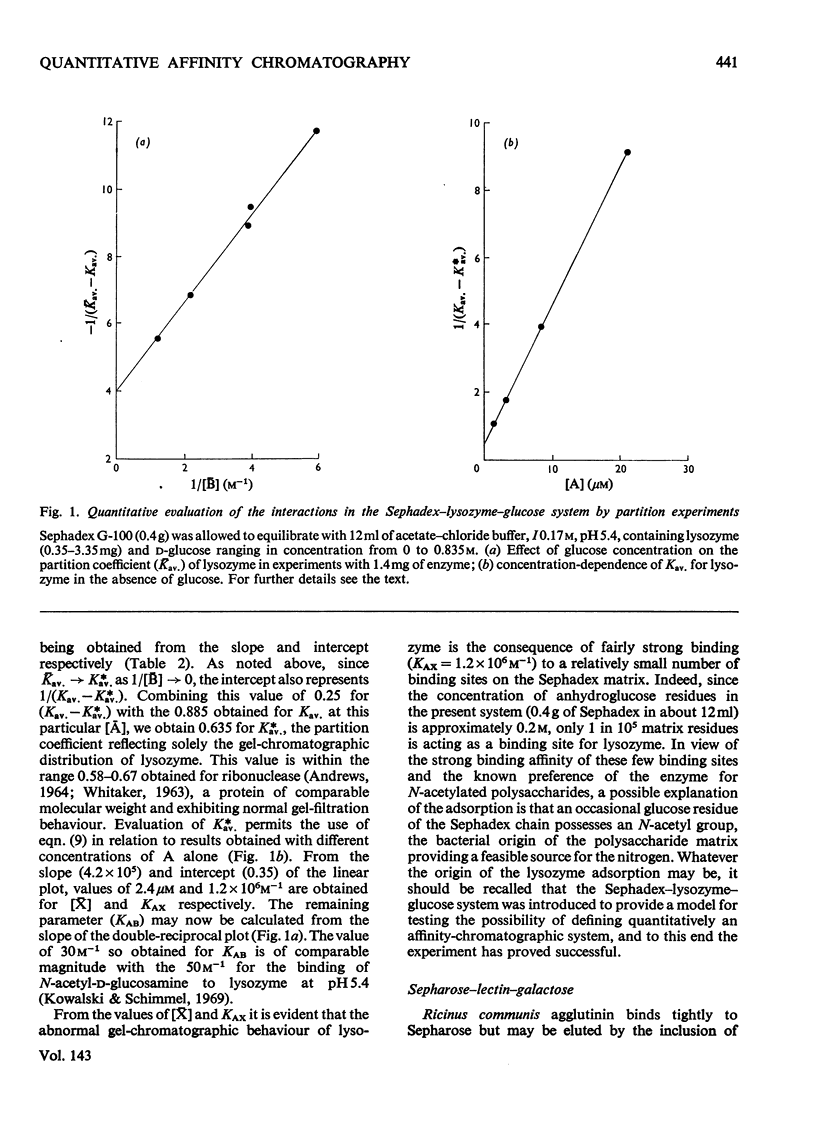
Theoretical expressions are derived for affinity chromatography of systems comprising an acceptor A with one binding site for attachment to a functional group X on the column matrix and one site for interaction with a small ligand B that specifically affects its elution. From a general relationship covering all possible interactions between A, B and X simpler expressions are derived for affinity systems in which only two equilibria operate. Methods are suggested whereby these simpler systems may be characterized in terms of the two pertinent equilibrium constants and the concentration of matrix-bound constituent. The means by which the theory may be adapted to affinity chromatography of acceptors with multiple binding sites for ligand is also illustrated. Results of partition experiments on the Sephadex G-100–lysozyme–d-glucose system in acetate–chloride buffer (I=0.17m), pH5.4, are used to demonstrate the feasibility of evaluating quantitatively affinity-chromatography interactions. Values of 30m−1 and 1.2×106m−1 are obtained for the equilibrium constants for the reactions of lysozyme with glucose and Sephadex respectively, there being only an occasional binding site in the polysaccharide matrix (approximately 1 in 105 glucose residues). In a second experimental study the phytohaemagglutinin from Ricinus communis is subjected to frontal chromatography on Sepharose 4B in the presence of different concentrations of d-galactose, the results illustrating some of the difficulties and limitations that are likely to be encountered in quantitative studies of affinity-chromatographic systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P., Kitchen B. J., Winzor D. J. Use of affinity chromatography for the quantitative study of acceptor-ligand interactions: The lactose synthetase system. Biochem J. 1973 Dec;135(4):897–900. doi: 10.1042/bj1350897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. M., Chaiken I. M. Quantitative affinity chromatography. Determination of binding constants by elution with competitive inhibitors. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2382–2385. doi: 10.1073/pnas.71.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond E., Farquhar S., Dunstone J. R., Ogston A. G. The osmotic behaviour of Sephadex and its effects on chromatography. Biochem J. 1968 Aug;108(5):755–763. doi: 10.1042/bj1080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert G. A., Kellett G. L. Interacting systems of the type A + B = C. J Biol Chem. 1971 Oct 10;246(19):6079–6086. [PubMed] [Google Scholar]
- Goldberg I., Bloch K. Fatty acid synthetases in Euglena gracilis. J Biol Chem. 1972 Nov 25;247(22):7349–7357. [PubMed] [Google Scholar]
- Kowalski C. J., Schimmel P. R. Interaction of lysozyme with alpha-N-acetyl-D-glucosamine. J Biol Chem. 1969 Jul 10;244(13):3643–3646. [PubMed] [Google Scholar]
- Masters C. J., Sheedy R. J., Winzor D. J., Nichol L. W. Reversible adsorption of enzymes as a possible allosteric control mechanism. Biochem J. 1969 May;112(5):806–808. doi: 10.1042/bj1120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Ogston A. G., Silpananta P. The thermodynamics of interaction between Sephadex and penetrating solutes. Biochem J. 1970 Jan;116(2):171–175. doi: 10.1042/bj1160171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., RHODES C. K., HOLCOMB D. N., VAN HOLDE K. E. Physical studies of lysozyme. I. Characterization. J Biol Chem. 1962 Apr;237:1107–1112. [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VANHOLDE K. E. PHYSICAL STUDIES OF MURAMIDASE (LYSOZYME). II. PH-DEPENDENT DIMERIZATION. J Biol Chem. 1964 Aug;239:2516–2524. [PubMed] [Google Scholar]
- WINZOR D. J., SCHERAGA H. A. STUDIES OF CHEMICALLY REACTING SYSTEMS ON SEPHADEX. I. CHROMATOGRAPHIC DEMONSTRATION OF THE GILBERT THEORY. Biochemistry. 1963 Nov-Dec;2:1263–1267. doi: 10.1021/bi00906a016. [DOI] [PubMed] [Google Scholar]


