Abstract
Background
Recently, there have been an increasing number of reports on the association between inflammatory markers and the prognosis of malignant tumors. However, the current inflammatory indicators have limited accuracy. We aimed to develop a new scoring system for predicting endometrial cancer recurrence using inflammatory markers, tumor markers, and histological diagnoses.
Methods
Patients with primary, previously untreated, and suspected endometrial cancer who underwent surgery at the Nara Medical University Hospital between January 2007 and December 2020 were included and followed up until March 2024. Items were divided into positive and negative using scores based on cutoff values and placed into the new scoring system, the endometrial tumor-related (ETR) score.
Results
We found that positive postoperative histological examination of lymph node metastasis and myometrial invasion, high levels of carcinoembryonic antigen and D-dimer in preoperative blood tests, and a large difference in preoperative and postoperative white blood cell counts were significantly associated with recurrence. The sensitivity and specificity of recurrence prediction using the ETR score were not inferior to those using the International Federation of Gynecology and Obstetrics staging system, which is considered the best prognostic factor for survival.
Conclusions
The ETR score is a significant prognostic marker of recurrence in patients who have undergone staging surgery, with complete surgical tumor removal.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12905-024-03528-8.
Keywords: Endometrial cancer, Recurrence, Disease-free survival rate, ETR score, D-dimer, White blood cell count
Background
Endometrial cancer (EC) is the second most prevalent gynecological cancer after cervical cancer in women [1, 2]. The morbidity of EC has been increasing globally; in 2018, almost 90,000 deaths due to EC were reported worldwide [3]. EC is known to recur in 18% of all patients [4], and the International Federation of Gynecology and Obstetrics (FIGO) staging system is considered one of the best prognostic indicators for survival [5]. FIGO (2008) stage I includes tumors confined to the corpus uteri, stage II includes those that invade the cervical stroma but do not extend beyond the uterus, stage III includes local and/or regional spread of the tumor, and stage IV includes tumors that invade the ladder/bowel mucosa and/or distant metastases [5]. The 5-year disease-free survival rates were reportedly 85%, 75%, 45%, and 25% for stages I, II, III, and IV, respectively [5]. In addition, the histological types of EC are known to correlate with the prognosis [6]. These include endometrioid carcinoma, serous carcinoma, clear cell carcinoma, mixed carcinoma, undifferentiated carcinoma, carcinosarcoma, other unusual types, and gastrointestinal mucinous carcinomas [6]. These histological types are divided into two groups, non-aggressive and aggressive, based on their prognostic value [6]. Non-aggressive types include endometrioid carcinoma grades 1 and 2, whereas the other types are aggressive [6, 7]. EC is typically treated with surgery, including hysterectomy and bilateral salpingo-oophorectomy [8, 9]. Lymphadenectomy is also known to be associated with prognosis in patients with intermediate- and high-risk EC [8, 10, 11].
In recent years, there have been an increasing number of reports on the association between inflammatory markers and the prognosis of malignant tumors [12, 13]. The inflammatory markers used include the systemic immune inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR), all of which are calculated using blood cell counts [12–14]. The Glasgow Prognostic Score (GPS) is an inflammation-based prognostic marker calculated using C-reactive protein and albumin levels [15]. We previously reported the association of the SII, NLR, and PLR and the prognosis of EC for all stages in 2021 and concluded that elevated SII was a better indicator of overall survival and progression-free survival in patients with EC than PLR or NLR [12].
Current indicators have limited accuracy because they rely only on one timepoint before surgery. To enhance accuracy, we aimed to develop a prediction system using preoperative and postoperative data rather than just one pretreatment timepoint. To ensure consistency in clinical background, we focused on cases that included lymphadenectomy. We aimed to develop a new scoring system to predict EC recurrence after complete tumor removal, including lymphadenectomy.
Methods
Patients
A list of patients with primary, previously untreated, and suspected EC who underwent surgery at the Nara Medical University Hospital (Kashihara, Japan) between January 2007 and December 2020 was generated from our institutional registry. The patients were followed up until March 2024. Informed consent for the use of the patients’ clinical data for research was obtained from all participants at their first hospitalization. After approval by the Ethics Review Committee of the Nara Medical Hospital (Kashihara, Japan), an opt-out form was provided through our institutional homepage. This study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Nara Medical University Hospital (protocol code: 3603).
Patients with suspected EC were included in this study. The inclusion criteria were as follows: (1) histologically confirmed EC after surgery, (2) lymphadenectomy, and (3) did not undergo chemotherapy or radiotherapy before the first surgery. The exclusion criteria were as follows: (1) stage IV, (2) combined with other malignant tumors or hematologic diseases, (3) lost to follow-up, and (4) insufficient preoperative and postoperative serum data, which included no blood tests within 14–60 days or performed while infection occurred.
Collection of candidates predicting recurrence
The following data were collected through a chart review of the patients’ medical records: age, body mass index, parity, and postoperative diagnosis, including the FIGO stage, histological type, myometrial invasion, lymphovascular invasion, ascites cytology, lymph node metastasis, and distant metastasis. In addition, data concerning the use of adjuvant chemotherapy and the results of preoperative and postoperative blood tests were collected.
Examination of prognosis using past prognostic indicators
We examined the efficacy of past inflammation-based prognostic indices such as SII, NLR, MLR, PLR, and GPS. These indicators were calculated using the following formulas: SII = platelet count×neutrophil count/lymphocyte count, NLR = neutrophil count/lymphocyte count, MLR = monocyte count/lymphocyte count, and PLR = platelet count/lymphocyte count. If an elevated C-reactive protein level (> 1.0 mg/dL) and hypoalbuminemia (< 3.5 g/dL) were present, the GPS was 2. Patients with only one of these were assigned a score of 1, and those with none were assigned a score of 0.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statics for Windows, version 29.0 (IBM Corp., Armonk, NY, USA). Differences in each factor were compared using Student’s t-test or the Mann–Whitney U test after assessing whether the distribution was normal. The receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff value for predicting poor prognosis. The cutoff value was based on the highest Youden index (i.e., sensitivity + specificity – 1). Next, logistic regression analyses were used to assess the risk factors for recurrence and death. Kaplan–Meier life table analysis and log-rank tests were used to assess the disease-free and overall survival rates. We also performed a Kaplan-Meier life table analysis for the groups that underwent only pelvic lymphadenectomy and up-to para-aortic lymphadenectomy. A two-sided p < 0.05 was considered statistically significant. Multivariate analyses of prognostic factors for disease-free and overall survivals were performed using the Cox proportional hazard regression model.
Results
Patients
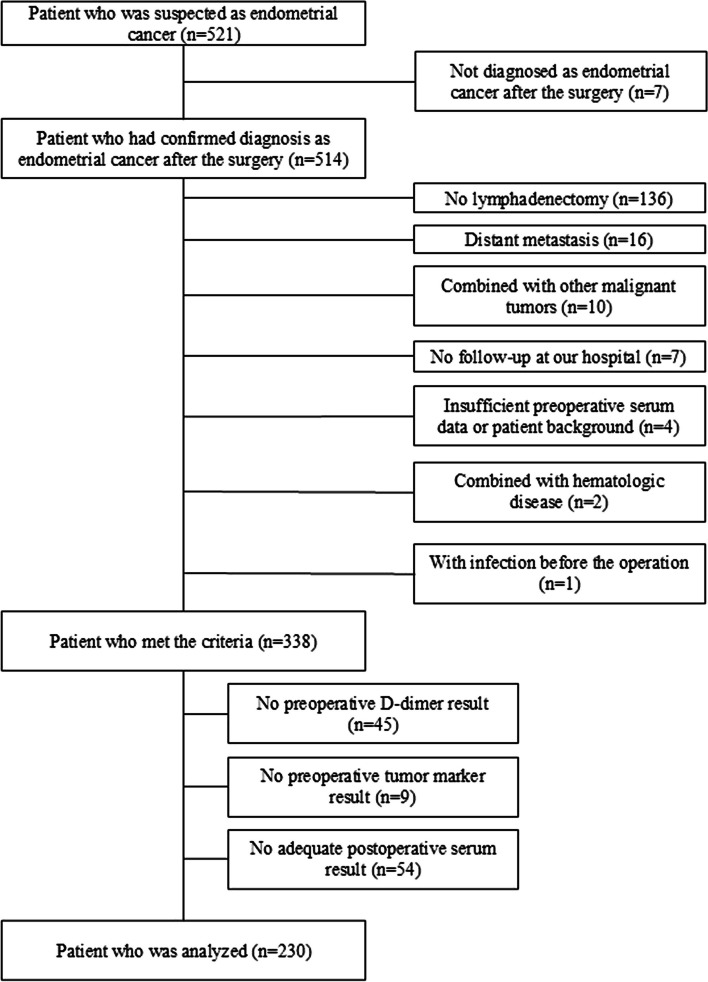
A total of 521 patients were suspected of having EC during the study period, of whom 230 met the inclusion criteria (Fig. 1). The median patient age was 59 years (range, 23–79 years), and the median follow-up period was 84.5 months (range, 12–195 months). The patients’ peripheral blood data were collected before the operation, and the median number of days until the operation was 37 (range, 1–92 days). In addition, the postoperative blood data before adjuvant therapy were collected, and the median number of days between the operation date and the blood test was 26 days (range, 14–59 days). The overall recurrence rate was 15.7% (n = 36). Table 1 and Additional file 1 present the demographic and clinical characteristics of the study cohort. The surgeries performed in the study cohort are outlined in Additional file 2.
Fig. 1.
Participant selection. Of the 338 women who met the inclusion criteria, 108 were excluded as they met the exclusion criteria, while 230 patients participated in the study
Table 1.
Clinical characteristics of the study cohort
| Total n = 230 | No recurrence n = 194 | Recurrence n = 36 | p - value | |
|---|---|---|---|---|
| Age (years)a | 59 (23–79) | 59 (23–79) | 61.5 (43–77) | 0.105 |
| BMI (kg/m2)a | 23.65 (15.1–43.5) | 24.1 (15.1–43.5) | 22.8 (17.6–32.8) | 0.108 |
| Parity | ||||
| 0 | 50 (21.7%) | 42 (21.6%) | 8 (22.2%) | 0.675 |
| ≥1 | 180 (78.3%) | 152 (78.4%) | 28 (77.8%) | |
| FIGO Stage | ||||
| I | 169 (73.5%) | 152 (78.4%) | 17 (47.2%) | < 0.001*** |
| II | 23 (10.0%) | 19 (9.8%) | 4 (11.1%) | |
| III | 38 (16.5%) | 23 (11.9%) | 15 (41.7%) | |
| Tumor subtype | ||||
| EM G1/G2 | 155 (67.4%) | 135 (69.6%) | 20 (55.6%) | 0.152 |
| EM G3 | 25 (10.9%) | 20 (10.3%) | 5 (13.9%) | |
| CCC | 8 (3.5%) | 5 (2.6%) | 3 (8.3%) | |
| Serous carcinoma | 7 (3.0%) | 4 (2.1%) | 3 (8.3%) | |
| Carcinosarcoma | 10 (4.3%) | 7 (3.6%) | 3 (8.3%) | |
| Undifferentiated | 6 (2.6%) | 6 (3.1%) | 0 (0.0%) | |
| Mixed type | 17 (7.4%) | 15 (7.7%) | 2 (5.6%) | |
| Others | 2 (0.9%) | 2 (1.0%) | 0 (0.0%) | |
| Myometrial invasion | ||||
| < 1/2 | 140 (60.9%) | 124 (63.9%) | 16 (44.4%) | 0.028* |
| ≥ 1/2 | 90 (39.1%) | 70 (36.1%) | 20 (55.6%) | |
| Lymph vascular invasion | ||||
| Positive | 83 (36.1%) | 66 (34.0%) | 17 (47.2%) | 0.131 |
| Negative | 147 (63.9%) | 128 (66.0%) | 19 (52.8%) | |
| Ascites cytology | ||||
| Positive | 42 (18.3%) | 33 (17.0%) | 9 (25.0%) | 0.288 |
| Negative | 183 (79.6%) | 156 (80.4%) | 27 (75.0%) | |
| Lymph node metastasis | ||||
| Positive | 29 (12.6%) | 16 (8.2%) | 13 (36.1%) | < 0.001*** |
| Negative | 201 (87.4%) | 178 (91.8%) | 23 (63.9%) | |
| Adjuvant chemotherapy | ||||
| Yes | 146 (63.5%) | 119 (61.3%) | 27 (75.0%) | 0.119 |
| No | 84 (36.5%) | 75 (38.7%) | 9 (25.0%) | |
| Preoperative CEA | ||||
| < 2.4 (ng/mL) | 151 (65.7%) | 137 (70.6%) | 14 (38.9%) | < 0.001*** |
| ≥ 2.4 (ng/mL) | 79 (34.3%) | 57 (29.4%) | 22 (61.1%) | |
| Preoperative D-dimer | ||||
| < 1.1 (µg/mL) | 177 (77.0%) | 159 (82.0%) | 18 (50.0%) | < 0.001*** |
| ≥ 1.1 (µg/mL) | 53 (23.0%) | 35 (18.0%) | 18 (50.0%) | |
| WBC (pre – post operation) | ||||
| < 1,050 (/µL) | 120 (52.2%) | 111 (57.2%) | 9 (25.0%) | < 0.001*** |
| ≥ 1,050 (/µL) | 110 (47.8%) | 83 (42.8%) | 27 (75.0%) | |
BMI body mass index, FIGO The International Federation of Gynecology and Obstetrics, EM endometrioid carcinoma, G1 Grade 1, G2 Grade 2, G3 Grade 3, CCC clear cell carcinoma, WBC white blood cell count
amedian (range)
*p < 0.05, **p < 0.01, ***p < 0.001 calculated using a Mann-Whitney U test
Candidates predicting the recurrence of EC
The results of the ROC curve based on recurrence were used to determine the value of the new scoring system in predicting EC recurrence. Candidates varied according to patient characteristics; histological findings after the surgery; and preoperative and postoperative serum data, including tumor markers, blood cell counts, differential counts of leukocytes, albumin, and D-dimer levels. The ROC analysis showed that lymph node metastasis, myometrial invasion, preoperative carcinoembryonic antigen (CEA) (ng/mL), preoperative D-dimer (µg/mL), and subtracted value of postoperative from preoperative white blood cell (WBC) counts (/µL) significantly predicted EC recurrence (Table 2). In addition, the cutoff value for evaluating whether a patient relapsed using these parameters alone was obtained from the cutoff value calculated with the highest Youden index. In the new scoring system, the endometrial tumor-related (ETR) score was assigned on a 5-point scale (range, 0–5), with each parameter scored as 1 for values greater than or equal to this cutoff value and 0 for those below this cutoff value.
Table 2.
Cutoff values for predicting recurrence
| Cut-off value | p-value | AUC | 95% CI | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Lymph node metastasis | - | 0.013* | 0.639 | 0.530–0.749 | 0.361 | 0.918 |
| Myometrial invasion | - | 0.014* | 0.620 | 0.525–0.715 | 0.556 | 0.639 |
| preoperative CEA (ng/mL) | 2.4 | 0.010* | 0.640 | 0.534–0.747 | 0.611 | 0.706 |
| preoperative D-dimer (µg/mL) | 1.1 | 0.003** | 0.657 | 0.533–0.761 | 0.500 | 0.820 |
| WBC (pre – post operation) (/µL) | 1050 | 0.010* | 0.637 | 0.533–0.741 | 0.750 | 0.572 |
AUC area under the curve, CI confidential interval, CEA carcinoembryonic antigen, WBC white blood cell. * p < 0.05, **:p < 0.01
Evaluation to predict the recurrence of EC using past prognostic indicators
The results of the ROC curve analysis based on recurrence were used to evaluate past prognostic indicators for predicting EC recurrence. The cutoff value was calculated using the highest Youden index. None of the past prognostic indicators showed significant differences between the recurrent and nonrecurrent groups (Table 3). However, the new scoring system, the ETR score, showed significant differences between the recurrent and nonrecurrent groups (Table 3). Some of the study cohort groups had missing data for calculating the past prognostic indicators. The percentages of analyzed and missing data are shown (Additional file 3).
Table 3.
Cutoff values of past predictive indicators to predict recurrence
| Cut-off value | p-value | AUC | 95% CI | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| SII | 574 | 0.102 | 0.593 | 0.482–0.704 | 0.706 | 0.508 |
| NLR | 2.72 | 0.070 | 0.599 | 0.492–0.706 | 0.618 | 0.608 |
| MLR | 0.41 | 0.500 | 0.537 | 0.429–0.645 | 0.147 | 0.931 |
| PLR | 121.9 | 0.084 | 0.587 | 0.488–0.686 | 0.941 | 0.286 |
| ETR score | 2.5 | < 0.001*** | 0.766 | 0.672–0.861 | 0.639 | 0.840 |
AUC area under the curve, CI confidential interval, PPV positive predictive value, NPV negative predictive value, SII systemic immune-inflammation index, NLR neutrophil to lymphocyte ratio, MLR monocyte to lymphocyte ratio, PLR platelet to lymphocyte ratio, GPS Glasgow prognostic score, ETR score endometrial cancer-tumor related score. ***p < 0.001
Evaluation of the new scoring system
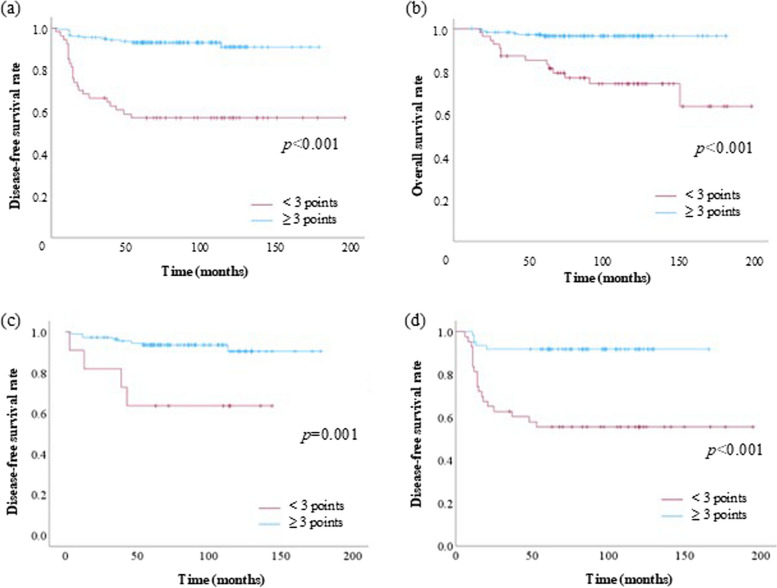
The ROC curve showed that ETR score was not an inferior prognostic tool compared to the FIGO staging system for predicting EC recurrence (Fig. 2). Specifically, the area under the curve for the ETR score and FIGO staging system was 0.766 and 0.669, respectively. The cutoff value of the ETR score and FIGO staging system was calculated as 2.5 and 1.5, respectively, using the Youden index. According to this cutoff value, we considered > 3 for the ETR score as the poor group, with a sensitivity and specificity of 63.9% and84.0%, respectively, for diagnosing recurrence. In contrast, > 2 was used for the FIGO staging system to define the poor group, with a sensitivity and specificity of 52.8% and 78.4%, respectively, for diagnosing recurrence. In addition, we used this cutoff point to evaluate the sensitivity and specificity for predicting death. The sensitivity and specificity were 70.0% and 81.0%, respectively. Kaplan–Meier life table analysis revealed significant differences in disease-free and overall survival rates using a cutoff value of 3 (Fig. 3a and b). Also, when we categorized the cases into only pelvic lymphadenectomy (Fig. 3c) and up-to paraaortic lymphadenectomy (Fig. 3d) groups, the KaplanMeier life table analysis showed significant differences in disease-free survival between the two groups. Especially, the up-to paraaortic lymphadenectomy group showed a more significant difference between in high and low ETR scores. Cox regression analyses demonstrated that the ETR score was a significant independent prognostic factor affecting the disease-free survival of patients with EC (Table 4).
Fig. 2.
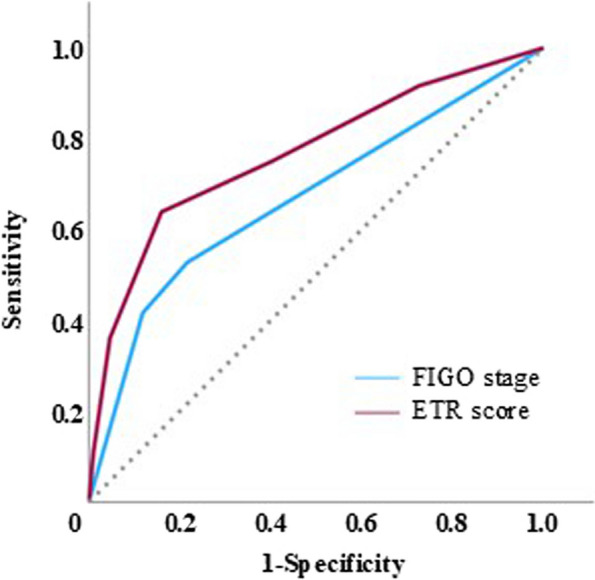
ROC curve of the FIGO staging system and new ETR score. The cutoff value was calculated as 2.5 using the Youden index. Using this cutoff value, the sensitivity and specificity were 63.9% and 84.0%, respectively. ROC, receiver operating characteristic curve; FIGO, International Federation of Gynecology and Obstetrics; ETR, endometrial tumor-related
Fig. 3.
Kaplan–Meier life table analysis of disease-free survival in patients with EC according to the ETR score using a cutoff value of 3 points (p < 0.01). a Disease-free survival and b overall survival of all cases. c Cases of only pelvic lymphadenectomy and d up-to paraaortic lymphadenectomy groups. FIGO, International Federation of Gynecology and Obstetrics; ETR, endometrial tumor-related; EC, endometrial cancer
Table 4.
Univariate and multivariate analyses predicting recurrence
| patients | Univariate analysis | Cox multivariate analysis | ||||
|---|---|---|---|---|---|---|
| n | HR | p-value | HR | 95% CI | p-value | |
| FIGO stage | ||||||
| ≥ III | 38 | 5.311 | < 0.001*** | |||
| < I – II | 192 | |||||
| Lymphovascular invasion | ||||||
| Yes | 83 | 1.735 | 0.133 | |||
| No | 147 | |||||
| Ascites cytology | ||||||
| Positive | 42 | 1.576 | 0.290 | |||
| Negative | 183 | |||||
| Myometrial invasion | ||||||
| ≥ 1/2 | 90 | 2.214 | 0.030* | |||
| < 1/2 | 140 | |||||
| Lymph node metastasis | ||||||
| Yes | 29 | 6.288 | < 0.001*** | |||
| No | 201 | |||||
| Preoperative CEA | ||||||
| ≥ 2.4 | 79 | 3.777 | < 0.001*** | |||
| < 2.4 | 151 | |||||
| Preoperative d-dimer | ||||||
| ≥ 1.1 | 53 | 4.543 | < 0.001*** | |||
| < 1.1 | 177 | |||||
| Preoperative-postoperative WBC (/µL) | ||||||
| ≥ 1,050 | 110 | 4.012 | < 0.001*** | |||
| < 1,050 | 120 | |||||
| ETR score | ||||||
| ≥ 3 | 176 | 9.303 | < 0.001*** | 9.017 | 4.127–19.701 | < 0.001*** |
| < 3 | 54 | |||||
HR hazard ratio, CI confidential interval, FIGO The international federation of gynecology and obstetrics, CEA carcinoembryonic antigen, WBC white blood cell, ETR endometrial cancer-tumor related
*p < 0.05, **p < 0.01, ***p < 0.001. Cox regression analysis was done
Discussion
In this study, we successfully developed a new scoring system, the ETR score, to predict EC recurrence after radical surgery. The score comprised lymph node metastasis, myometrial invasion, preoperative CEA levels, preoperative D-dimer levels, and WBC difference (preoperative–postoperative value). The strength of the ETR score is that it is easy to calculate because blood tests and histological findings have already been used worldwide. We previously reported that SII was essential marker for EC [12]. However, in above study, we examined a cohort of patients without distant metastasis and who had undergone a pelvic lymphadenectomy. Pecorino et al. reported that early-stage low-grade endometrioid cancer and synchronous endometrial-ovarian endometrioid cancer without apparent lymph node involvement at preoperative imaging have a relatively low rate of lymph node metastasis and similar relapse rate with or without lymphadenectomy [16]. Moreover, across all histological types, patients with distant metastasis and those who could not undergo lymphadenectomy for health reasons evidently have poor prognoses. Then, we believe that by separating the patient population, we developed a more meaningful scoring system. According to the result of the Kaplan-Meier life table analysis, more severe cases that need paraaortic lymphadenectomy show a more significant difference between ETR high and low.
Serum CEA, an item of the ETR score, is a diagnostic and prognostic marker of broad-spectrum malignant tumors, especially colon and rectal cancers [17, 18]. Although Cancer antigen 125 (CA125) is a well-established tumor marker for gynecologic cancer, particularly in ovarian cancer, preoperative CA125 has limited sensitivity in predicting the prognosis of EC [19]. However, Kozakiewicz et al. reported that postoperative CEA levels reflect recurrence or distant metastasis of EC [20], suggesting a relationship between EC prognosis and CEA level. This study included preoperative CEA levels in the predictive scoring system for recurrence, suggesting that these values can predict recurrence. It is reasonable to use CEA instead of CA125 as a predictive value.
There have been reports linking high WBC counts to aggressive tumors or poor prognosis. According to a large cohort study conducted by the UK Biobank, elevated WBC counts may indicate an overly active inflammatory response, which could contribute to the eventual onset of certain types of cancer [21]. In endometrial neoplasia, WBC counts were significantly higher in patients with cancer than in those with hyperplasia, according to a study that compared the hyperplasia, EC, and control groups [22]. Based on a few reports suggesting the usefulness of pretreatment peripheral WBC counts, this difference may help predict the prognosis. Our previous study showed that this difference contributes to the prognosis of ovarian cancer by comparing presurgical and postsurgical analyses [23, 24]. This study reaffirmed the usefulness of the differences in WBC counts in predicting recurrence.
Serum D-dimer level, an item of the ETR score, is a well-known biomarker of thrombosis, such as pulmonary embolism and venous thrombosis [25]. However, it is also known as a prognostic marker for several malignancies, such as ovarian [26], breast [27], lung [28], and other cancers [29–33]. According to a previous study, it is reasonable to include serum D-dimer levels in the new scoring system.
This study had a few limitations. First, a bias might exist owing to the nature of a retrospective and single-center study. Second, although serous and clear cell carcinomas have poor prognoses, the study cohort did not show a significant difference in histological types. Such patients ordinarily harbor an advanced stage and then receive chemotherapy rather than surgical treatment. ETR score could not reflect these small pathological groups. Third, although the latest classification of endometrial cancer includes molecular categorization, such as POLE, microsatellite instability, copy-number high, and copy-number low, we could not assess the relationships between these types and ETR score [16, 34]. However, since we demonstrated the usefulness of the ETR score in this retrospective study, we intend to add next-generation sequencing analysis and other methods to further investigate the relationship between the ETR score and molecular categorization.
Conclusions
The ETR score, comprising the presence of lymph node metastasis and myometrial invasion, preoperative CEA and D-dimer levels, and the difference in WBC levels (preoperative – postoperative value) WBC levels, is a good prognostic marker for patients with EC who have undergone complete surgery. Moreover, prospective multicenter studies are needed to validate our findings.
Electronic supplementary material
Acknowledgements
None.
Abbreviations
- ETR
Endometrial tumor-related
- EC
Endometrial caner
- SII
Systemic immune inflammation index
- NLR
Neutrophil-to-lymphocyte ratio
- PLR
Platelet-to-lymphocyte ratio
- GPS
The Glasgow Prognostic Score
- FIGO
The International Federation of Gynecology and Obstetrics
- ROC
Receiver operating characteristics
- CEA
Carcinoembryonic antigen
- WBC
White blood cell
- CA125
Cancer antigen 125
Authors’ contributions
Conceptualization, T.M.; methodology, T.M. and N.K.; validation, T.M. and N.K.; formal analysis, T.M. and N.K.; investigation, T.M. and N.K.; resources, T.M., N.K., R.K., and F.K.; data curation, T.M., and N.K.; writing—original draft preparation, T.M. and N.K.; writing—review and editing, T.M., N.K., R.K., and F.K.; visualization, T.M., J.K., M.M., and K.W.; supervision, F.K.; project administration, F.K. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in line with the principles of the Declaration of Helsinki. Informed consent for the use of the patients’ clinical data for research was obtained from all subjects at their first hospitalization. Approval was granted by the Institutional Ethics Committee of Nara Medical University Hospital (Kashihara, Japan) before the study began (protocol code: 3603). The opt-out form was provided through our institutional homepage.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (Lond). 2020;40:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Capozzi VA, Monfardini L, Maglietta G, Barresi G, De Finis A, Rosati A, et al. Pattern of recurrence in endometrial cancer. The murderer always returns to the scene of the crime. Eur J Surg Oncol. 2024;50:107985. [DOI] [PubMed] [Google Scholar]
- 5.Sabater S, Andres I, Lopez-Honrubia V, Marti-Laosa MM, Castro-Larefors S, Berenguer R, et al. Does postoperative irradiation improve survival in early-stage endometrial cancer? Brachytherapy. 2018;17:912–21. [DOI] [PubMed] [Google Scholar]
- 6.Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. J Gynecol Oncol. 2023;34:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. [DOI] [PubMed] [Google Scholar]
- 8.Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–79. [DOI] [PubMed] [Google Scholar]
- 9.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399:1412–28. [DOI] [PubMed] [Google Scholar]
- 10.Chan JK, Cheung MK, Huh WK, Osann K, Husain A, Teng NN, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107:1823–30. [DOI] [PubMed] [Google Scholar]
- 11.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–72. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara S, Mabuchi S, Takeda Y, Kawahara N, Kobayashi H. Prognostic value of pre-treatment systemic immune-inflammation index in patients with endometrial cancer. PLoS ONE. 2021;16:e0248871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng J, Wu F, Zhang L. Prognostic significance of pretreatment neutrophil-to- lymphocyte ratio, platelet-to-lymphocyte ratio, or monocyte-to-lymphocyte ratio in Endometrial neoplasms: a systematic review and Meta-analysis. Front Oncol. 2022;12:734948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Wang H, Liu CC, Lu Y, Tang H. The Glasgow Prognostic score (GPS) is a novel prognostic indicator in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer Res Clin Oncol. 2016;142:2339–45. [DOI] [PubMed] [Google Scholar]
- 16.Pecorino B, Laganà AS, Chiantera V, Ferrara M, Di Stefano AB, Di Donna MC, et al. Progression free survival, overall survival, and Relapse Rate in Endometrioid Ovarian Cancer and Synchronous Endometrial-Ovarian Endometrioid Cancer (SEO-EC): results from a large retrospective analysis. Med (Kaunas). 2022;58:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawahara N, Miyake R, Yamanaka S, Kobayashi H. A Novel Predictive Tool for discriminating Endometriosis Associated Ovarian Cancer from Ovarian Endometrioma: the R2 Predictive Index. Cancers (Basel). 2021;13:3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno H, Miyake H, Nagai H, Yoshioka Y, Shibata K, Asai S, et al. Optimal cutoff value of preoperative CEA and CA19-9 for prognostic significance in patients with stage II/III colon cancer. Langenbecks Arch Surg. 2021;406:1987–97. [DOI] [PubMed] [Google Scholar]
- 19.Sun S, Wei L, Zou L, Wang T, Liu Z, He J, et al. Preoperative serum CA125 level and age at diagnosis: an effective prognosis prediction tool for patients with early-stage endometrial cancer. Asia Pac J Clin Oncol. 2023;19:e258–66. [DOI] [PubMed] [Google Scholar]
- 20.Kozakiewicz B, Chądzyńska M, Dmoch-Gajzlerska E, Stefaniak M. Monitoring the treatment outcome in endometrial cancer patients by CEA and TATI. Tumour Biol. 2016;37:9367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song M, Graubard BI, Loftfield E, Rabkin CS, Engels EA. White Blood Cell Count, Neutrophil-to-lymphocyte ratio, and Incident Cancer in the UK Biobank. Cancer Epidemiol Biomarkers Prev. 2024;33:821–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acmaz G, Aksoy H, Unal D, Ozyurt S, Cingillioglu B, Aksoy U, et al. Are neutrophil/lymphocyte and platelet/lymphocyte ratios associated with endometrial precancerous and cancerous lesions in patients with abnormal uterine bleeding? Asian Pac J Cancer Prev. 2014;15:1689–92. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara N, Kawaguchi R, Waki K, Maehana T, Yamanaka S, Yamada Y, et al. The prognosis predictive score around primary debulking surgery (PPSP) improves diagnostic efficacy in predicting the prognosis of ovarian cancer. Sci Rep. 2002;12:22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawahara N, Yamanaka S, Sugimoto S, Kamibayashi J, Nishikawa K, Kawaguchi R, et al. The Prognosis Predictive score around neo adjuvant chemotherapy (PPSN) improves Diagnostic Efficacy in Predicting the prognosis of epithelial ovarian Cancer patients. Cancers (Basel). 2023;15:5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Es N, Takada T, Kraaijpoel N, Klok FA, Stals MAM, Büller HR, et al. Diagnostic management of acute pulmonary embolism: a prediction model based on a patient data meta-analysis. Eur Heart J. 2023;44:3073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Kawaguchi R, Iwai K, Niiro E, Morioka S, Tanase Y, et al. Preoperative plasma D-dimer level is a useful prognostic marker in ovarian cancer. J Obstet Gynaecol. 2020;40:102–6. [DOI] [PubMed] [Google Scholar]
- 27.Batschauer APB, Figueiredo CP, Bueno EC, Ribeiro MA, Dusse LMS, Fernandes AP, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21:1267–72. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Yin H, Zhang Y, Chen H, Xu J, Ren L. Plasma D-dimer and interleukin-6 are associated with treatment response and progression-free survival in advanced NSCLC patients on anti-PD-1 therapy. Cancer Med. 2023;12:15831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stender MT, Larsen TB, Sørensen HT, Thorlacius-Ussing O. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost. 2012;10:2027–31. [DOI] [PubMed] [Google Scholar]
- 30.Diao D, Wang Z, Cheng Y, Zhang H, Guo Q, Song Y, et al. D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS ONE. 2014;9:e101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diao D, Cheng Y, Song Y, Zhang H, Zhou Z, Dang C. D-dimer is an essential accompaniment of circulating tumor cells in gastric cancer. BMC Cancer. 2017;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2015;8:805–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi X-W, Wang L, Zhang W-W, Sun P, Yan S-M, Liu P-P, et al. High pretreatment D-dimer levels correlate with adverse clinical features and predict poor survival in patients with natural killer/T-cell lymphoma. PLoS ONE. 2016;11:e0152842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignata S, Califano D, Lorusso D, Arenare L, Bartoletti M, De Giorgi U, et al. MITO END-3: efficacy of avelumab immunotherapy according to molecular profiling in first-line endometrial cancer therapy. Ann Oncol. 2024;35:667–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.