Abstract
Purpose
This review explores the role of pigment epithelium-derived factor (PEDF) in retinal degenerative and vascular disorders and assesses its potential both as an adjunct to established vascular endothelial growth factor inhibiting treatments for retinal vascular diseases and as a neuroprotective therapeutic agent.
Methods
A comprehensive literature review was conducted, focusing on the neuroprotective and anti-angiogenic properties of PEDF. The review evaluated its effects on retinal health, its dysregulation in ocular disorders, and its therapeutic application in preclinical models. Advances in drug delivery, including gene therapy, were also examined.
Results
PEDF, initially identified for promoting neuronal differentiation, is also a potent endogenous angiogenesis inhibitor. Strong anti-angiogenic and neuroprotective effects are observed in preclinical studies. It has pro-apoptotic and antiproliferative effects on endothelial cells thereby reducing neovascularization. Although promising, clinical development is limited with only a single conducted phase I clinical trial for macular neovascularization. Development of PEDF-derived peptides enhances potency and specificity, and emerging gene therapy approaches offer sustained PEDF expression for long-term treatment. However, questions regarding dosage, durability, and efficacy remain, particularly in large animal models.
Conclusions
PEDF shows significant therapeutic potential in preclinical models of retinal degeneration and vascular disorders. Despite inconclusive evidence on PEDF downregulation as a primary disease driver, many studies highlight its therapeutic benefits and favorable safety profile. Advances in gene therapy could enable long-acting PEDF-based treatments, but further research is needed to optimize dosage and durability, potentially leading to clinical trials and expanding treatment options for retinal disorders.
Keywords: pigment epithelium-derived factor (PEDF), neovascularization, neurodegeneration
There is an unmet need for improved ocular therapeutics for both vascular and degenerative chorioretinal disorders. Vascular endothelial growth factor (VEGF)-inhibiting therapies have proven effective treatments for the exudative and neovascular complications of chorioretinal vascular disorders. However, in particular, for macular neovascularization (MNV), functional and anatomic outcomes remain suboptimal. Visual gains tend to be lost in the years following treatment initiation with the development of subretinal fibrosis and extensive atrophy causing permanent blindness.1 Additionally, therapies for inherited and acquired retinal degenerations are almost non-existing except for disease-specific gene therapies as for RPE65-associated retinal degenerations.2
Among potential therapeutic candidates are a range of endogenous growth factors, including pigment epithelium-derived factor (PEDF). PEDF is not only a potent neurotrophic factor but also one of the most potent endogenous angiogenesis inhibitors.3 PEDF was first identified as a secreted factor from fetal retinal pigment epithelium (RPE) cells driving neuronal differentiation of human Y79 retinoblastoma cells4,5 and later established as an anti-angiogenic factor by its ability to inhibit proliferation of cultured endothelial cells (ECs) and attenuate corneal neovascularization.3 Unsurprisingly, PEDF has been extensively investigated in preclinical studies as a therapeutic agent for diverse ocular disorders both on its own and as a part of multitargeting ocular therapeutics. However, the promising reports have not been readily translated into the clinic. In this review, we survey the literature regarding the retinal effects of PEDF, emphasizing its neuroprotective and anti-angiogenic properties. In particular, evidence of PEDF dysregulation from human ocular samples, and the evaluation of PEDF delivery as a therapeutic strategy in humans and preclinical animal models is summarized in detail.
PEDF Structure, Functional Domains, and Binding Partners
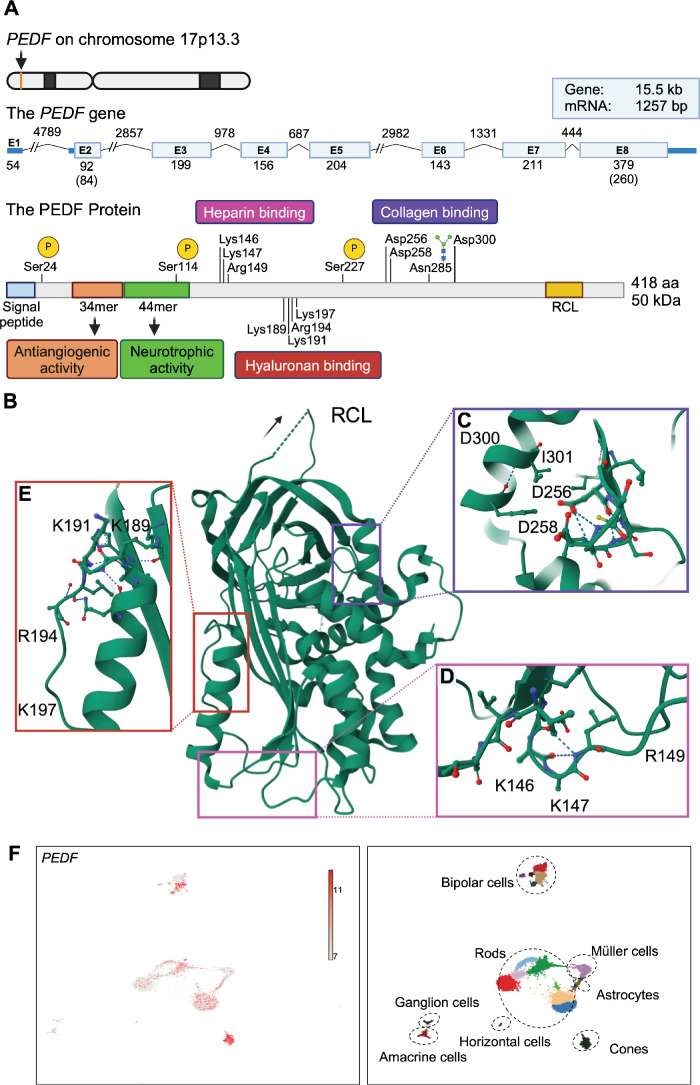
PEDF is a 50 kDa secreted glycoprotein, with neurotrophic, neuroprotective, and anti-angiogenic activities.6 The protein belongs to the non-inhibitory serine protease inhibitor (serpin) superfamily,4,6 which opposite most serpins does not inhibit serine proteases. The human PEDF gene (also known as SERPINF1; ENST00000254722.9) is located on chromosome 17p13.3 and spans approximately 15.5 kb, consisting of 8 exons and 7 introns (Fig. 1A).7 The protein precursor consists of 418 amino acids (aa) with an amino-terminal secretion signal peptide (see Fig. 1A).8 Amino acid sequence determination of secreted purified protein has revealed that the mature PEDF starts at position 21 of the precursor protein.6,8 However, according to the reference sequences (P36955 PEDF_HUMAN and UniProt) the secretion signal peptide of human PEDF is predicted as aa 1 to 19. PEDF has a tertiary structure (Fig. 1B) like other serpins with 10 α-helices and 3 β-sheets, and an exposed peptide loop known as the reactive center loop (RCL), which determines the protease specificity of inhibitory serpins.9,10 However, unlike other serpins, PEDF has an asymmetric charge distribution with an opposing basic and acidic region.10,11 As a non-inhibitory serpin without demonstrated serine protease inhibitory activity, the RCL of PEDF is not functional for inhibiting serine proteases and PEDF does not undergo a conformational change in response to RCL cleavage. Indeed, the neurotrophic12 and anti-angiogenic activites13,14 are unaffected by removal of the RCL, as evidenced by the ability of different small derived peptides to mediate these functions. Although the specific role of the RCL for PEDF function remains unclear, mutagenesis studies have revealed RCL mutations to block PEDF secretion. This effect seemed dependent on intermolecular interactions although a binding partner influencing secretion is currently unidentified.15 Furthermore, PEDF also localizes to the nucleus. The helix A motif YxxYRVRS constitutes a nuclear translocation signal interacting with transportin-SR2.16 The 50 kDa secreted glycoprotein is glycosylated on Asn285 and contains phosphorylation sites on Ser24, Ser114, and Ser227 (NCBI RefSeq sequence: NM_002615.7) influencing its neurotrophic and anti-angiogenic activities.17
Figure 1.
Schematic diagram of the genomic localization and structure of PEDF, the PEDF polypeptide, and PEDF expression in scRNA-seq data from human retina. (A) PEDF is encoded by the PEDF gene located on the forward strand of chromosome 17p13.3 (ID ENST00000254722.9). Exons 1 to 8 are denoted by blue boxes. UTRs (exon 1, and part of exon 2 and 8) are represented by dark blue boxes. The size (in bp) of each element (drawn to scale unless otherwise indicated by backslashes) is indicated by numbers. Numbers in parenthesis indicate translated bases of exons 2 and 8. The size of the mRNA without UTRs is 1257 bp. Introns are indicated by lines. The PEDF protein can be phosphorylated on serine 24, 114, and 227 (indicated by yellow circles), and glycosylated on asparagine 285 (sugar molecule indicated by blue squares and green circles). Two peptide regions contain specific activities: a 34 aa sequence (Asp44-Asn77) responsible for the antiangiogenic activities, and a 44 sequence (Val78-Thr121) responsible for the neurotrophic activities. The RCL as well as the three regions (marked with magenta, red, and purple squares) important for binding of heparin, hyaluronan, or collagen are indicated. (B) Ribbon diagram of human PEDF based on the 2.85 A crystal structure of PEDF (PDB DOI:https://doi.org/10.2210/pdb1IMV/pdb). The folded protein conformation is globular with the RCL (orientation indicated by an arrow) being exposed. It contains 3 β-sheets and 10 α-helices, and an asymmetrical charge distribution, resulting in binding to different components of the ECM. (C) Enlargement of the region containing the acidic amino acids, aspartic acid 256, aspartic acid 258, and aspartic acid 300 (D256, D258, and D300) involved in the collagen binding. The region is rotated approximately 180 degrees compared to B. Isolysine 301 (I301) is shown for orientation purposes. (D) Magnification of the heparin-binding region showing the involved basic amino acids, lysine 146, lysine 147, and arginine 149 (K146, K147, and R149). (E) Expansion of the region holding the basic amino acids, lysine 189, lysine 191, arginine 194, and lysine 197 (K189, K191, R194, and K197) involved in binding to hyaluronan. (F) Normalized PEDF expression in human retinal scRNA-seq data from human retinal tissue (left) with annotated subclusters (right). Expression is evident in a range of cell types particularly prominent in photoreceptor subclusters. (Fig. 1A was created using Biorender.com. Figs. 1B to 1E were prepared by using the 3D structure viewer provided by P.D.B. Fig. 1F processed scRNA-seq data from Lukowski et al.204 was obtained through the Human Cell Atlas Data Portal [WongAdultRetina]. Analyses were performed using Automated Single Cell Analysis Platform VI. The data were normalized using Seurat and dimensional reduction performed with UMAP. Clusters of cells were identified using Seurat and manually annotated using cell-type marker genes from Lukowski et al.204) PEDF, pigment epithelium-derived factor; aa, amino acids; RCL, reactive center loop; bp, base pairs; kb, kilobases; kDa, kilodaltons; UTR, untranslated region; ECM, extracellular matrix; E, exon.
PEDF has different non-overlapping regions with distinct binding affinities (see Fig. 1A).18 Notably, the functional effects have been mapped to specific regions. The anti-angiogenic effects are primarily mediated within a 34 aa sequence (Asp44-Asn77), and the neurotrophic effects within a 44 aa sequence (Val78-Thr121). The major PEDF receptors bind within these regions and the corresponding 34mer and 44mer peptides (or smaller derived peptides) can themselves induce the anti-angiogenic and neurotrophic effects, respectively. Specifically, a potent anti-angiogenic effect is retained in the 7 aa Asp64-Ser70 sequence (DLYRVRS).19 Likewise, the neurotrophic effect is dependent on the 17 aa Gln98-Ser114 sequence (QRTESIIHRALYYDLIS).20 The surface 37/67 kDa laminin receptor (LR)21 and the PEDF receptor (PEDF-R)22 are mainly responsible for the anti-angiogenic and neurotrophic respectively, but known receptors also include LRP6,23 VEGF receptor 1 (VEGF-R1), and 2 (VEGF-R2),24 cell surface F1-ATP synthase,25 and plexin domain containing 1 (PLXDC1) and 2 (PLXDC2).26 Additionally, PEDF binds to the extracellular matrix (ECM), which influences its biological activity.27 The negatively charged amino acids in the acidic region bind collagen (Fig. 1C),18 whereas the basic region contains binding sites for glycosaminoglycans, such as heparin (Fig. 1D) and hyaluronan (Fig. 1E)28 and proteoglycans.29 A particular high binding affinity for collagen I is observed,30 and the collagen I binding motif is important for the anti-angiogenic activity of PEDF in tumor xenografts,31 and collagen I enhances the anti-angiogenic efficacy of the PEDF-derived 34mer.32 Furthermore, collagen interaction is affected by collagen cross-linking spatiotemporally controlling PEDF signaling.33 However, the specific role of ECM binding in the eye remains uncharacterized. This includes a potential effect of vitreous syneresis. The binding sites to ECM constituents are non-overlapping with the neurotrophic and anti-angiogenic sites and the RCL.18
PEDF Expression in the Healthy Retina and During Development
PEDF is mainly expressed in the RPE in the adult and fetal retina and secreted from the apical surface into the interphotoreceptor matrix (IPM).34–36 The IPM extracts show PEDF protein concentrations several times higher than the vitreous and aqueous humour36,37 and high gene expression is found in the RPE cells compared with the retinal tissue.36,38 A difference in post-translational modification of the N-terminal residue of PEDF in the vitreous and IPM has been observed.37 The exact nature of this modification has not been determined, but indicates differences in cellular origin. PEDF is also expressed in human neuroretinal cells including photoreceptors, and cells in the ganglion cell layer (GCL) and the inner nuclear layer (INL). This is evidenced by single cell RNA sequencing (scRNA-seq) of human retinal samples (Fig. 1F) as well as RNA in situ hybridization studies.39 Presence of PEDF protein in human neuroretinal and choroidal cells in addition to the RPE has been demonstrated by immunolabeling39–42 although reported findings are somewhat variable likely due to the dependence of immunohistochemical methods on tissue processing and antibody specificity. However, the observations are similar to findings in the rat,43,44 mouse,45 and monkey36 eye, and PEDF is also prominently expressed in certain cultured retinal and choroidal cells compared with RPE cells.38 The exact contribution of neuroretinal and choroidal cells to the pool of secreted PEDF is unknown. This is also the case for potential cell-autonomous functions. PEDF is expressed in non-retinal cells. This includes prominent expression in the ciliary body epithelium39,45: PEDF secretion from the ciliary body contributes to the vitreous and aqueous pool,45 which likely promote the avascularity of the vitreous as well as the posterior and anterior chamber.
During human embryonic development, PEDF localizes to the RPE, developing cones, some neuroblasts, and several cells in the GCL.39 In mice, the onset of Pedf expression has been reported to occur late in gestation with maturation during the postnatal stage; PEDF protein localizes to the RPE, different neuroretinal cells, and prominently in the choroid.45 The increase during the early postnatal stage in mice is compatible with its role as an angiogenic inhibitor as vascular development is completed at this stage.3,45
PEDF downregulation during aging could promote pathological angiogenesis or neuronal loss in the context of age-related pathologies, such as age-related macular degeneration (AMD). Indeed, PEDF downregulation has been associated with cultured cell senescence including RPE cells.35 Reciprocally, Pedf deletion in mice induces RPE senescence.46 However, the evidence of a general downregulation in the aging eye is limited. Aqueous levels have been reported to decrease with age,47 and a negative correlation with age was recently reported in a cohort of patients with advanced proliferative diabetic retinopathy (PDR) and vitreomacular interface disorders.48 Direct comparison between young and aged retinal samples is lacking, but prominent immunostaining has been observed in RPE and choroid of aged eyes without MNV.49
Associations Between PEDF and Retinal Disease
PEDF Dysregulation in Patients With Chorioretinal Disease
Neovascularization development likely depends on the upregulation of pro-angiogenic factors with concomitant downregulation or unbalanced expression of anti-angiogenic factors.50 Several studies have investigated changes in intraocular and systemic PEDF levels associated with retinal vasculopathy and pathological angiogenesis. This includes focused studies using immunoassays, and studies evaluating global alterations in respective proteomes. Studies regarding systemic and ocular PEDF levels in the major retinal vascular disease diabetic retinopathy (DR) and retinal vein occlusion (RVO), and AMD/MNV were systematically retrieved using comprehensive search strings (Supplementary Material).
Diabetic Retinopathy
Several studies have investigated PEDF dysregulation in vitreous samples from patients with diabetes mellitus (DM) with varying degrees of DR (Supplementary Table S1). Vitreous levels are the closest approximation to retinal PEDF levels, although secretion from the ciliary body also contributes to the vitreous pool. Vitreous samples are typically acquired during planned vitreoretinal procedures with other indications for pars plan vitrectomy used as the control group. Initial studies suggested PEDF downregulation in patients with DR, but subsequent reports have been more conflicting in particularly regarding different DR stages. These contrasting findings may be related to methodological differences. For example, barrier disruption causes consistently increased vitreous protein levels in DR. Thus, normalization to the amount of protein or sample volume48 and strategies for the depletion of abundant protein may influence results. In addition, alterations in PEDF levels may be modulated by disease features, such as macular edema, and interventions, such as intravitreal drug delivery or photocoagulation. The angiogenic drive is probably controlled by the balance of pro- and anti-angiogenic factors. Thus, measures such as the VEGF/PEDF ratio could be more appropriate. In addition, the functional activity of measured PEDF could be explored as it depends on, for example, phosphorylation.17 Vitreous PEDF levels have not been robustly associated with visual outcomes or physiological parameters in patients with diabetes, and the cross-sectional design typically applied challenges for the evaluation of the etiological importance of PEDF dysregulation in DR.
The aqueous proteome in general correlate well with the vitreous proteome51 and is easily accessible to sampling. However, few studies have explored PEDF dysregulation in aqueous samples. They all suggest a negative correlation between the PEDF levels and the presence or severity of DR. Interestingly, an early study demonstrated an anti-angiogenic effect of aqueous from non-diabetic patients, which was blocked by an anti-PEDF antibody.52
Evaluation of retinal PEDF levels is mostly limited to analyses on excised pre-retinal neovascular membranes using very variable methodologies. Thus, conclusions about disease associations are difficult to draw. However, the only study including retinal sections showed an obvious reduction in retinal PEDF signal in patients with PDR compared with patients without diabetes and with nonproliferative disease.41
The retrieved studies (see Supplementary Table S1) suggest a positive correlation between systemic PEDF levels and advancing stages of DR. However, the PEDF does not seem to be an independent predictor of DR progression in multivariate analysis53 and the etiological implications of increased systemic PEDF levels are uncertain. In addition, there is no established correlation between vitreous and systemic levels54 further questioning the potential of systemic levels as a biomarker candidate.
Retinal Vein Occlusion
Low vitreous levels have been consistently associated with the presence and severity of RVO (Supplementary Table S2). In addition, it has been associated with features such as visual acuity, non-perfusion area, and macular blood flow. However, the studies are derived from only two research groups, and it is somewhat uncertain whether the different publications report on different patient cohorts. Two reports suggest higher aqueous levels in RVO and no association with systemic levels has been reported.
Age-Related Macular Degeneration and Choroidal Neovascularization
In the first investigation, vitreous PEDF downregulation was observed in patients with neovascular AMD compared with age-matched controls.55 One study has supported this finding,56 but a larger proteomics study suggested PEDF upregulation in the vitreous of patients with neovascular AMD compared with patients with idiopathic floaters.57 In addition, increased aqueous levels have generally been reported in patients with AMD/MNV (Supplementary Table S3).
Decreased PEDF immunoreactivity has been observed in the RPE, Bruch's membrane (BM), and choroid of patients with AMD without a simultaneous increase in VEGF.49 However, in contrast to other endogenous angiogenesis inhibitors, the decrease was not evident in the choriocapillaris.58 In contrast, high VEGF and PEDF levels have been observed in clinically active MNV membranes, whereas they were low in clinically quiescent membranes with fibrosis.59 Similarly, increased PEDF secretion from cultured RPE cells derived from patients with AMD compared with healthy donors have been reported.60 This may represent a compensatory mechanism and it would be interesting if future studies could provide cell-specific expression profiles at different disease stages.
PEDF Levels in Animal Models of Chorioretinal Diseases
Retinal pathology mimicking those observed in chorioretinal disorders can be experimentally induced in animals, which allows a more standardized evaluation of changes in angiogenic factors. In experimental diabetes, retinal Vegf overexpression is typically accompanied by downregulated Pedf expression. Thus, PEDF levels and/or expression are relatively lower in the vitreous of db/db mice serving as a type 2 DM model,61 the retina of streptozotocin (STZ)-induced62 and Ins2Akita63 mice serving as type 1 DM models, and in the vitreous64 and retina65 of STZ-induced diabetic rats. Few studies report PEDF upregulation in diabetic rats. In one case, limited PEDF upregulation was associated with a significantly increased VEGF/PEDF ratio.66 Higher PEDF levels are also seen in certain diabetic rat models.67,68
Following choroidal neovascularization (CNV) laser-induction in rats, prominent Pedf expression evaluated by in situ hybridization and PEDF immunoreactivity has been described in primarily RPE cells but also macrophages and fibroblasts of the neovascular membrane after 3 days. Expression and immunoreactivity were limited after 14 days and primarily seen in spindle-shaped RPE cells covering the lesion.43 In contrast, decreased retinal PEDF immunoreactivity was observed in proximity to the lasered area in other studies.44,69 No significant change was reported in the very low density lipoprotein receptor knockout (Vldlr−/−) model.70
PEDF Gene Variants and Risk of Chorioretinal Disorders
Loss of endogenous Pedf expression in Pedf knockout (Pedf−/−) mice was associated with elevated inflammatory markers, glial activation, loss of photoreceptors, and decline in visual functions.71 Increased microvascular density corresponding to increased vessel expansion during development and greater susceptibility to neovascularization during oxygen induced retinopathy (OIR) has also been observed.72 An increase in acellular capillaries is observed in Pedf−/− Ins2Akita mice compared with Ins2Akita mice without knockout.73 Knockout also worsened the degenerative phenotype in rd10 mice but did not affect sodium-iodate-induced retinal degeneration in the same study.74 Knockout itself caused minor morphological changes, but not any differences in ERG wave amplitudes. In contrast, another study found retinal thinning, ERG wave attenuation, and AMD-like lesions in Pedf−/− animals.75 Furthermore, knockout cause senescent gene expression profiles and deficient phagocytosis in RPE cells.46
The importance of endogenous PEDF expression for retinal morphology and disease susceptibility in humans is poorly characterized. Lack of PEDF due to genetic mutations causes osteogenesis imperfecta type VI.76 To our knowledge, a retinal phenotype has not been described in subjects with such mutations. Associations between PEDF polymorphisms and retinal disease have been explored. Positive associations have been reported between different PEDF variants and AMD development77 and response to therapy78 in certain populations. However, a meta-analysis of studies with patients with AMD/PCV did not suggest any association.79 Similarly, associations have been reported between PEDF and PEDF promotor variants and the development of DR in patients with type 2 DM,80 but have not been consistently replicated. For example, no associations between PEDF and DR were identified in an Indian population with type 2 DM.81 Notably, no significant associations with retinal disease have emerged in genome-wide association studies (https://www.ebi.ac.uk/gwas/).
PEDF Supplementation in Preclinical Models of Ischemic Retinopathies and Retinal Degeneration
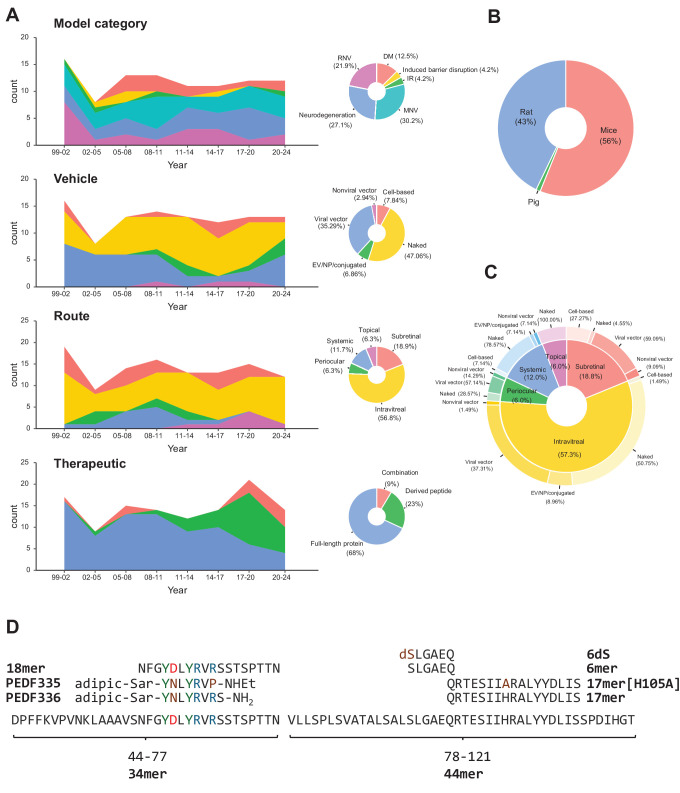
Although caveats exist, animal models remain the basis for preclinical assessment of therapeutic potential. Thus, comprehensive search strings were used to survey the literature regarding the protective effect of PEDF supplementation in different ocular disease models. The search strings (Supplementary Material) used to identify preclinical animal models were adapted from Hooijmans et al.82 and have been used to systematically review preclinical CNV models.83 Additionally, references in the included literature were screened for relevant studies. A total of 85 studies representing 99 individual models were retrieved. The included studies are summarized in Supplementary Table S4 and a graphical overview of the field is provided in Figure 2. The number of studies has been relatively stable, exploring primarily vascular disease models, but also models of neurodegenerative retinal disease (see Fig. 2A). All studies except one have been performed in rodents (see Fig. 2B). Different vehicles and delivery routes have been explored and recent years have seen increased interest in in vivo testing of derived peptides (see Figs. 2A, 2D). The used delivery vehicles vary with the delivery route (see Fig. 2C).
Figure 2.
Graphical summaries of PEDF in vivo studies. (A) Trends and distribution of used retinal model categories, delivery vehicles, delivery route, and therapeutic (top to bottom). The color is defined on the corresponding pie chart. For the trend plots the number of studies were binned into 3-year intervals. Model categories include diabetes mellitus, induced barrier disruption, ischemia-reperfusion injury, macular neovascularization, and retinal neovascularization. Vehicles include naked protein/peptides, EV/NP/conjugation, cell-based delivery, and viral and non-viral gene delivery vectors. Delivery routes include topical, subretinal, intravitreal, periocular (includes subconjunctival and retroorbital), and systemic. Therapeutics have been summarized as delivery or expression of full-length PEDF protein, PEDF-derived peptides, and PEDF combination therapy (combination), that is, co-delivery with or co-expression of another therapeutic compound. A study only evaluating transgenic PEDF overexpression and a study using zinc finger protein to activate PEDF expression is excluded from the summaries. (B) Pie chart with a summary of animal species used for in vivo experiments of chorioretinal disease. (C) Pie and donut chart summarizing the distribution of delivery vehicles for the different administration routes. (D) PEDF-derived peptides used for in vivo experiments of chorioretinal disease. The 6dS denotes substituting the first 6mer aa residue with the d form of serine (dS). Positive charged aa in blue; negative charged aa in red; tyrosine in green; and modified aa in brown. (Data analysis and graphical representations were made in R version R-4.4.1). aa, amino acid; adipic. adipic acid; DM, diabetes mellitus; EV, extracellular vesicle; IR, ischemia-reperfusion injury; MNV, macular neovascularization; NP, nanoparticle; PEDF, pigment epithelium-derived factor; RNV, retinal neovascularization; Sar, sarcosine.
Macular and/or Choroidal Neovascularization
Subretinal neovascularization occurs spontaneously in certain genetically modified mouse models but is typically induced in experimental animals by focal laser disruption of BM resulting in CNV formation. The laser-induced rodent CNV model dominates the current field.83 This is also true for studies evaluating PEDF therapy with only three studies using the Vldlr−/− mouse model. In these models, PEDF delivered as protein or overexpressed using different vector systems consistently demonstrates a reduction in leakage and CNV lesion area (see Supplementary Table S4). However, one study delivering recombinant PEDF using subcutaneous mini-osmotic pumps showed a dose-dependent effect with lower PEDF levels inhibiting CNV formation and higher levels promoting CNV formation.84 Reduced CNV inhibiting effect with higher doses of daily subconjunctival full-length PEDF or 34mer injections has also been described although the outcome measure was associated with high variation.69 Similarly, reduced CNV inhibition with higher doses has also been observed following intravitreal delivery of the 34mer.32
Anti-angiogenic therapeutics should suppress the formation of neovessels without adversely affecting the established vasculature. Importantly, the anti-angiogenic action of PEDF is specific for proliferating ECs in which PEDF induces apoptosis85 without affecting choroidal vascularity or neuroretinal integrity as observed with VEGF targeting comparator.86
Diabetic Retinopathy, Ischemic Retinopathies, and Retinal Neovascularization
Although experimental animals rarely develop pathology akin to advanced DR phenotypes, certain features can be replicated on reasonable timeframes using animal models.87 Both genetic models (e.g. Ins2Akita and db/db mice) or models induced by chemical ablation of the insulin-producing pancreatic β-cells develop hallmarks of diabetic eye disease, such as inner retinal neurodegeneration,88 vascular barrier disturbances, and pericyte and EC loss.89 Many studies (see Supplementary Table S4) have evaluated the effect of PEDF administration on such functional and anatomic outcomes. Intravenous,65 intravitreal,90,91 and topical92 delivery of recombinant PEDF or PEDF-derived peptides reduce retinal vessel leakage. A similar reduction has been observed by PEDF overexpression from an adeno-associated viral vector (AAV)-delivered transgene.93 Vascular barrier function restoration has been associated with decreased levels of pro-angiogenic mediators, such as VEGF91,93 and increased barrier protein levels including occludin.90 Similarly, ICAM-1 expression90,91 and leukostasis94 are attenuated. The effect on vascular cell density and vascular morphology has apparently not been systematically investigated in diabetic models.
The retinal neovascularizations (RNVs) characterizing PDR (and the advanced stages of other ischemic retinopathies) can be modeled in rodents by disturbing developmental angiogenesis by altering ambient oxygen levels. This OIR model has been widely used to study both the anti-angiogenetic and vasoprotective as well as anti-inflammatory and neuroprotective functions of PEDF (see Supplementary Table S4). PEDF therapy demonstrates a consistent reduction in the RNV area (or number of vascular cells anterior to the inner limiting membrane) using different formulations and delivery strategies. Several studies report an associated reduction in vaso-obliterative area91,95,96 and/or vascular leakage.90,91 The protective effects of PEDF are associated with the downregulation of pro-angiogenic and inflammatory factors.90,91 The limited reports on neuroretinal structure and function in these models suggest a protective effect.96
Severe ischemia with subsequent reperfusion injury can be induced by acute elevation of intraocular pressure (IOP). Topical delivery of PEDF-derived peptides protects microvascular structure with a reduction in the number of acellular capillaries in this high IOP-induced ischemia-reperfusion injury model.97 In addition, a neuroprotective effect is evident in this model as discussed below.
Retinal Degeneration Models
A protective effect of PEDF has also been investigated in spontaneous genetic and induced models of inner and outer retinal degeneration (see Supplementary Table S4). PEDF delivery has shown structural and functional protection in several genetic models of inherited retinal degenerations. Intravitreal injection of recombination PEDF protects against photoreceptor loss and ERG wave attenuation in the Royal College of Surgeons (RCS) rat, and the rd1, rd10, and rds mice.20,98–101 Similarly, protection is observed in the RCS rat, and the rd1 and rds mouse following subretinal SIV-hPEDF injection.102,103 The Ccl2 and Cx3cr1 double knockout with an rd8 background show focal retinal lesions somewhat akin to AMD, and PEDF reduce these along with the amount of the lipofuscin component A2E. Furthermore, PEDF had a significant neuroprotective effect on photoreceptors.104
Intravitreal PEDF and derived peptides also protects against phototoxicity used to model photoreceptor degeneration.105–107 As expected, this effect is PEDF-R-dependent.108 Systemic administration of sodium-iodate induces specific RPE toxicity with subsequent outer retinal degeneration. In this model, adenoviral delivery of PEDF significantly protected against retinal thinning and ERG attenuation. Furthermore, a reduction in drusen-like lesions was observed.75
PEDF is also neuroprotective in models of retinal ganglion cell (RGC) degeneration including the DBA/2J spontaneous glaucoma model,109 RGC degeneration induced by optic nerve lesions,110–114 transient ocular hypertension,115,116 and NMDA-induced excitotoxicity.115 The protective effect of intravitreal AAV-CMV-PEDF has been investigated in the DBA/2J mouse, which shows a phenotype similar to congenital glaucoma. Considerable protection against RGC loss and visual acuity reduction was observed although no statistical comparisons were reported.109 PEDF delivered topically or intravitreally protects against RGC loss following optic nerve crush (ONC).110,111,113,114 In the majority of cases, this was associated with increased RGC axon regeneration.110,111,113 Interestingly, the neuroprotective effect of PEDF in the ONC model has been related to the anti-angiogenic 34mer peptide.110,112
Molecular Mechanisms of PEDF
As evident from the summary of in vivo studies exploring the therapeutic effects of PEDF delivery to the eye, PEDF modulates common disease features across animal models of retinal disease. The reviewed in vivo studies as well as numerous in vitro studies of PEDF have revealed downstream molecular mechanisms of its action. The interaction with cell surface receptors inducing different biological activities is best characterized (Fig. 3).117 Thus, delineated receptor interactions and downstream pathways are emphasized in the following section. However, it should be noted that the observed PEDF binding to cytoskeletal structures35 and nuclear localization16 suggest that intracellular actions could be important for its neurotropic and anti-angiogenic actions as well as a regulatory effect on the cell-cycle118 and stem cell maintenance.119 Unfortunately, the putative mechanisms remain uncharacterized with the exception of a study in hepatocellular carcinoma cells.120
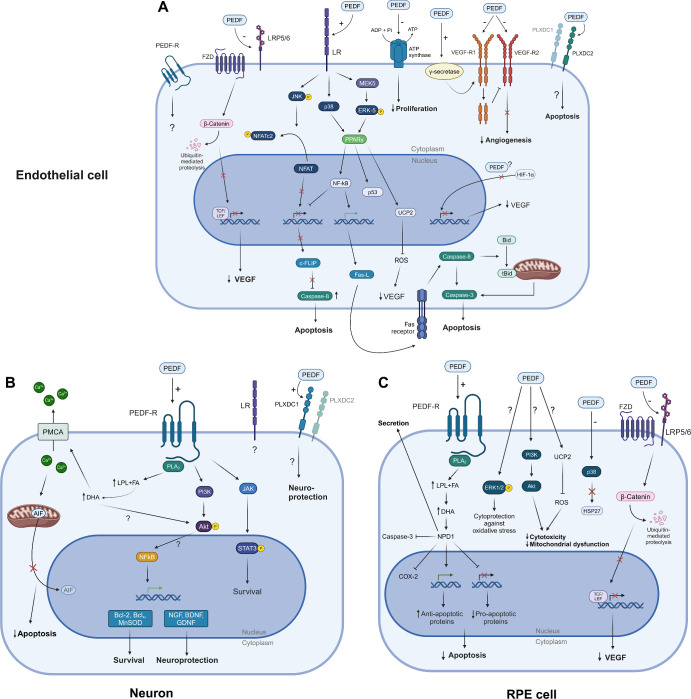
Figure 3.
PEDF signaling in endothelial, neuronal, and RPE cells. (A) PEDF exerts its anti-angiogenic effects on proliferating endothelial cells, by binding several receptors, including LR, LRP5/6, ATP synthase, VEGFR, and PLXDC2. It generally results in endothelial cell apoptosis, downregulation of VEGF, and inhibition of angiogenesis. Apoptosis is primarily caused by PEDF binding to LR, resulting in activation of several downstream pathways and gene expression alterations, which entails activation of caspases. (B) PEDF exerts neurotrophic and neuroprotective effects in retinal neurons, mainly through binding to PEDF-R. Several downstream pathways are activated, and intracellular Ca2+ levels are decreased, which inhibits apoptosis and entails neuroprotection and survival. (C) PEDF is mainly produced in and secreted from RPE cells. However, PEDF binding to RPE cell receptors also cause downstream effects, including NPD1 production, gene expression alterations favoring apoptosis inhibition, as well as other mechanisms entailing cytoprotection. See text for details. Created with BioRender.com. PEDF, pigment epithelium-derived factor; PEDF-R, PEDF receptor; FZD. frizzled; LRP, lipoprotein receptor-related protein; LR, laminin receptor; VEGF-R, VEGF receptor; PLXDC, plexin domain containing; HIF, hypoxia-inducible factor; JNK, c-Jun N-terminal kinase; NFAT, nuclear factor of activated T-cells; Ucp-2, uncoupling protein 2; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; PPAR-y, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor; c-FLIP, FLICE-like inhibitory protein; Fas-L, Fas ligand; MEK5-ERK5, mitogen-activated protein kinase 5-extracellular signal-regulated kinase 5; PMCA, plasma membrane Ca2+ ATPase; AIF, apoptosis-inducing factor; COX-2, cyclooxygenase-2; JAK, Janus kinase; STAT, signal transducers and activators of transcription; PLA2, phospholipase A2; LPL, lysophospholipids; FA, fatty acids; DHA, docosahexaenoic acid; NPD1, neuroprotection D1; PI3K, phosphoinositide 3-kinase; Akt, Akt kinase (protein kinase B); NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; GDNF, glial cell line-derived neurotrophic factor; MnSOD, manganese superoxide dismutase; Bcl, B cell lymphoma; TCF/LEF, T-cell factor/lymphoid enhancer factor.
Angiostatic Functions
PEDF was early identified as a potent anti-angiogenic compound.3 Numerous studies have since established its stabilizing effect on vascular barrier function, inhibitory effect on EC proliferation and migration, and ability to induce apoptosis of activated ECs. These actions depend on both direct effects and inhibition of VEGF signaling with complex and context dependent perturbation of downstream signaling pathways. The in situ effects will likely also depend on autocrine and paracrine signaling. In addition to the extensive in vivo evidence summarized above (see Supplementary Table S4), picomolar concentrations of PEDF or its anti-angiogenic derivates prominently inhibit EC migration, tube formation, and sprouting in different in vivo and ex vivo angiogenesis assays.3,96,121–123 However, a reduction or even reversal of the anti-angiogenic effect has been described in the nanomolar range.32,69,84 Notably, PEDF has a specific apoptosis-inducing effect on activated, proliferating ECs: animal studies demonstrate specific apoptosis-induction within neovascular lesions.85,124 LR, the major anti-angiogenic receptor, is primarily expressed in proliferating ECs125 and is upregulated by VEGF in retinal and choroidal ECs.95,126 VEGF also upregulate Fas on ECs, which makes the ECs susceptible to apoptosis following PEDF-induced FasL upregulation.127 In addition to inhibition of neovascularization, PEDF stabilizes endothelial barrier function in several retinal disease models, as described above (see Supplementary Table S4), but also in extraocular tissues.128 These findings are supported by a limited number of in vitro assays of endothelial permeability.128 Notably, PEDF inhibits VEGF-induced barrier disruption in bovine retinal ECs.129,130 Somewhat surprisingly, one study reported the neuroprotective 44mer to be responsible for inhibiting VEGF-induced barrier disruption in vivo.131 PEDF also attenuates AGE-induced barrier disruption by inhibiting ROS generation and subsequent induction of VEGF expression.132
The LR was the first identified anti-angiogenic PEDF receptor. It has particular high-affinity binding to the anti-angiogenic domain of PEDF and is essential for inhibition of EC proliferation and EC apoptosis-induction.21 Indeed, PEDF-derived peptides with increased anti-angiogenic activity (i.e. from the 34 aa region) also show more pronounced LR internalization,133 and phosphomimetic PEDF mutants with increased anti-angiogenic effect have increased LR affinity.134 Retinal cellular effects of PEDF-LR binding and downstream activities are best characterized in ECs (see Fig. 3A) Several mechanisms downstream of LR have been implicated in apoptosis induction135: JNK phosphorylation leads to cytoplasmic retention of NFATc2 and nuclear export of NFAT, resulting in decreased C-FLIP expression; C-FLIP is an endogenous inhibitor of caspase-8 and increased caspase-8-mediated apoptosis thus ensues.136 LR also activates p38134 and the MEK5-ERK5 pathway, which activates PPAR-γ137 resulting in the activation of NFκB, p53, and UCP-2. NFκB inhibits C-FLIP gene expression thereby increasing FasL expression.138 FasL binding to the Fas receptor results in apoptosis through caspase-8/3, and mitochondrial t-Bid/caspase-3 activation. UCP-2 decreases mitochondrial-derived reactive oxygen species (ROS) production,139 which in other studies have been shown to confer EC protection potentially by suppression of NAPDH oxidase induction.94,140
The ability of PEDF to modulate VEGF signaling has been of particular interest. PEDF binds VEGF-R1 and VEGF-R2 blocking the angiogenic effect of VEGF-A,24,121,129 for example, by blocking VEGF binding to VEGF-R2 (see Fig. 3A).141 The binding to VEGF-R2 blocks VEGF-A-induced VEGF-R2 kinase activity and autophosphorylation of tyrosine residues121 modulating VEGF signaling without altering VEGF-R2 levels. The binding to VEGF-R1 also inhibits the VEGF-induced barrier disruption by γ-secretase dependent cleavage and internalization.129 Similarly, PEDF protects RPE barrier functions in the presence of VEGF-R2 activation through a γ-secretase-dependent mechanism.142 Whether the observed binding of PEDF to Caveolin-1143 affects this internalization is unknown. In addition, the modulation on the receptor level, PEDF also regulates VEGF expression, as shown in different ischemic retinopathy and retinal angiogenesis animal models (see Supplementary Table S4), but also specifically in ECs,91 RPE cells,91 and glial cells.141 Involved mechanisms include regulation of HIF-1α activation.141,144
The effect on VEGF secretion is also dependent on the Wnt-inhibiting effect of PEDF (see Fig. 3A): PEDF binds the low-density lipoprotein receptor-related protein 6 (LRP6) and inhibits activation of the Wnt/β-catenin pathway in human retinal ECs and Müller cells, and ARPE19 cells with associated reduction in VEGF secretion.23 However, the role of the Wnt/β-catenin pathway in the developing vasculature and retinal vascular disease is quite complex.145 Thus, Wnt-inhibition attenuates barrier disturbances in DR146 and subretinal neovascularization147 but is also fundamental to vascular development and maintenance of its barrier integrity.148
The importance of other receptor interactions is less well-characterized (see Fig. 3A). PEDF binds the β-subunit of cell-surface F1-ATP synthase on ECs. This results in reduced F1-ATP synthase activity with subsequent reduction in extracellular ATP synthesis.25 The mechanisms by which cell surface F1-ATP synthase regulate EC function is uncertain but inhibition of F1-ATP synthase has anti-proliferative effects in HUVECs.149,150 PEDF binds to PLXDC1 on the cell surface and receptor activation promotes EC death.26 Interestingly, increased PLXDC1 expression is observed in ECs of fibrovascular membranes from patients with PDR151 and is associated with an angiogenic state.152
The potential for PEDF to support pericyte function and viability is less characterized. Pericytes are essential for vascular integrity and the retinal vasculature is characterized by a particularly high pericyte to EC ratio. Furthermore, the loss and dysfunction of pericytes are early and defining features of diabetic retinopathy.153,154 PEDF is an in vitro EC-derived pericyte mitogen155 and to provide pericyte cytoprotection through autocrine platelet-derived growth factor-B stimulation.156 PEDF also modulates the pericyte response to oxidative stress. This includes a cytoprotective effect and inhibition of pericyte release of angiogenic mediators.157,158 The signaling mechanisms including responsible surface receptors have not been characterized, and specific reduction of pericyte loss has not been established in diabetic animal models.
Neuroprotective Effects
Since the identification of PEDF as a stimulator of neuronal differentiation,4,5 it has been shown to influence the survival and maturation of different retinal and non-retinal cell types119 with a possible general role in cell cycle regulation and protection against proliferative senescence.118 PEDF secretion from the RPE is essential for retinal maturation during development, and PEDF plays central roles in neuronal stem cell maintenance.119 From a therapeutic perspective, the ability to protect neurons from death following different insults is of particular interest. In addition to the extensive in vivo studies (see Supplementary Table S4), PEDF protects different cultured retinal neurons159 from, for example, oxidative stress,160 light damage,161 serum starvation,162 and hypoxia.163
The PEDF-R is a major determinant of the neuroprotective action of PEDF (see Fig. 3B). It was the first identified PEDF receptor and has important cell signaling functions through the release of lipid mediators.117,164 It is also known as adipose triglyceride lipase (ATGL) or patatin-like phospholipase domain-containing protein 2 (PNPLA2).22 PEDF-R is expressed in the neural retina and RPE cells.22,117 Ablation of PEDF-R causes photoreceptor degeneration165 and PEDF-R is necessary for photoreceptor outer segment degradation by the RPE.166 Notably, the importance of the PEDF-R for the neuroprotective effect of PEDF on retinal neurons is evident.22,97,167–169
The exact downstream signaling mechanism is not fully delineated, but PEDF binding is known to stimulate the phospholipase A2 activity of PEDF-R resulting in fatty acid and lysophospholipid release.22,164,167 Potential bioactive lipids include docosahexaenoic acid (DHA). Indeed, the DHA derivate neuroprotectin D1 (NPD1) is released apically from RPE cells following PEDF stimulation mediating neuroprotective and anti-oxidative functions; additionally, DHA and NPD1 Both stimulate antiapoptotic gene expression (e.g. BCL2 and BFL1) and inhibit proapoptotic gene expression (e.g. BID, BAX, and BAD), and this mechanism is potentiated by PEDF.170 The importance of DHA is also shown by the ability to potentiate the protective effects of PEDF on corneal nerve regeneration through PEDF-R dependent mechanisms.168,169 In degenerating retinal neurons increased intracellular Ca2+ is observed due to, for example, glutamate-induced cytotoxicity. DHA release by PEDF-stimulated PEDF-R activity reduces Ca2+ overload by stimulating Ca2+ efflux through the plasma membrane Ca2+ ATPase (PMCA) pump. This in turn inactivates calpain and cathepsin D, which blocks mitochondrial translocation of BAX and nuclear translocation of apoptosis-inducing factor (AIF) promoting cell survival.100
Neuroprotection and survival of retinal neurons are also mediated through other pathways downstream PEDF-R. These include the PI3K-Akt pathway, which protects mice cone photoreceptor-derived 661W cells from light damage,161 and JAK-STAT3 activation involved in mouse RGC protection.162 Downstream effects necessary for PEDF neuroprotection also include NFκΒ activation, which entails prosurvival and neuroprotective gene expression signatures.171,172 The neuroprotective effect has been linked to other receptors as well (see Fig. 3B). Thus, the neurotrophic effect on RGC has surprisingly been reported to be mediated by the anti-angiogenic 34mer sequence.110,112 The responsible mechanisms including receptors are currently unknown, but both direct and indirect glia-mediated effects were demonstrated.112 Both RGCs and R28 retinal progenitor cells express PEDF-R as well as LR, and siRNA-mediated knockdown of both receptors reduces the in vitro neuroprotective effect of PEDF on R28 cells.173 In addition, PLXDC2 activation by PEDF protect the 661W photoreceptor cell line against oxidative stress.26
Support of Retinal Pigment Epithelium Survival and Function
Besides the broad supportive role for retinal neurons, PEDF is important in RPE health and function (see Fig. 3C). As noted above, constitutional PEDF knockdown results in senescent RPE changes and impaired phagocytosis.46 PEDF promotes the stability of the outer blood-retina barrier in response to VEGF stimulation.142 PEDF also protects RPE cells from oxidative stress-induced cell death and barrier dysfunction, through several only partly elucidated mechanisms. For example, PEDF induces phosphorylation of ERK1/2, which activates CREB and thereby promotes survival,174 and the protective effect on oxidant-mediated barrier dysfunction depend on prevention of p38/27-kDa heat shock protein signaling, as well as actin stabilization and junctional protein maintenance.175 PEDF is also RPE cytoprotective through NPD1 signaling downstream PEDF-R.170 Furthermore, PEDF can improve mitochondrial function in oxidative stress conditions through activation of PI3K-Akt signaling and decreases the level of ROS through UCP-2 regulation.176 PEDF also protects against sodium iodate RPE toxicity in vitro177 and in vivo by ferroptosis inhibition.75
Fibrosis
The development of fibrosis is increasingly recognized as contributing to poor visual outcomes in, for example, MNV.178 However, the anti-fibrotic actions of PEDF have been scarcely explored in the eye. PEDF reduces levels of pro-fibrotic mediators in different retinal disease models, such as LI-CNV179, IGF-1 overexpressing mice,180 and STZ-induced DM.91,181 However, in vivo evidence showing fibrosis reduction in retinal disease models is currently lacking. On the other hand, PEDF attenuates fibrosis in other organs.182 Examples include reduction of myocardial fibrosis by limiting endothelial-to-mesenchymal transition through inhibition of the Wnt-signaling pathway183 and bleomycin-induced pulmonary fibrosis by TGF-β1/smad pathway inhibition through upregulation of PPAR-γ.184
Inflammation and Glial Reactivity
PEDF also modulates inflammatory responses. Its anti-inflammatory action is evident in ischemic retinopathy models by downregulation of inflammatory mediators (e.g. ICAM, MCP-1, TNFα, and IL-1β) and inhibition of leukostasis (see Supplementary Table S4) although the effects may be secondary. However, the findings in DR models are supported by, for example, the ability of PEDF to block high glucose-induced inflammatory reactions in cultured ECs through its anti-oxidative properties with a reduction in NFκΒ activation.185 PEDF specifically inhibit caveolin-1-induced EC inflammation,143 and PEDF-mediated inhibition of macrophage polarization contributed to the protective effect in the OIR model.186 Furthermore, PEDF also downregulates IL-6 expression in ARPE-19 cells187 and may blunt RPE inflammatory reactions by its anti-oxidant properties in these cells.
Retinal glial cells are important for homeostasis and dysregulation of glial functions promotes diverse retinal pathologies.188 Release of PEDF from Müller cells and other retinal glia may provide trophic support for inner retinal neurons such as RGCs.162,163 The protective effects of PEDF in animal models is associated with a reduction of glial activation evaluated by GFAP expression. Metabolic activation but inhibition of microglia proliferation as wells as astrocyte proliferation inhibition through a paracrine mechanism has also been described.189 On the other hand, PEDF stimulate the release of pro-inflammatory mediators from microglia190,191 and neonatal astrocytes192 in cell culture experiments dependent on NFκΒ activation. Thus, as with other aspects of PEDF signaling the effects seem highly complex and context dependent.
Toward Clinical Application
As summarized above, ample preclinical evidence demonstrates an anti-angiogenic and neuroprotective effect of PEDF and derived compounds. Thus, the challenges for clinical translation seem surmountable.193 However, only a single phase I clinical trial evaluating intravitreal delivery of an adenoviral vector expressing PEDF has been conducted.194 In this cohort of patients with quite advanced neovascular AMD (best corrected visual acuity ≤ 20/200), adenoviral gene therapy was deemed safe, and the data were suggestive of a stabilizing effect on lesion size in the high-dose group. We are not aware of continued development of this approach, which may relate to the dominating position of AAVs for ocular gene therapy and intravitreal anti-VEGF therapy becoming standard therapy in the subsequent years.
The clinical evaluation of PEDF therapy has been limited by inherent challenges to the therapeutic use of proteins and polypeptides in retinal disease. Notably, such strategies are limited by pharmacokinetic properties. The physiological half-life of PEDF is short123 suggesting that therapy must rely on novel methods for sustained delivery. Encouragingly, sustained delivery strategies to the posterior segment are rapidly developing as extensively reviewed by others.195–197 Strategies range from structural modifications over nanocarriers to gene transfer using viral and non-viral vector systems (Fig. 4). As an example, encapsulated cell technology has enabled ciliary neurotropic factor expression for more than a decade198 with protection against patients with outer retinal degeneration in macular telangiectasia type 2 in phase III studies. Several PEDF therapy extension approaches have been explored in preclinical animal models (see Supplementary Table S4, Fig. 2). Notably, gene transfer using different viral and nonviral vector systems has demonstrated efficacy. Gene therapy has established itself as a treatment modality for inherited retinal degenerations2 and is intensively investigated as a platform for long-term expression of anti-angiogenic factors.199 Indeed, PEDF seems particularly suited for this gene-therapeutic biofactory approach to treat pathological angiogenesis and neurodegeneration. As an alternative, PEDF-derived peptides may allow better ocular penetration. Indeed, topical administration of different derived peptides (see Fig. 2D) has demonstrated efficacy in several in vivo disease models. These findings await confirmation in animals with ocular pharmacokinetic properties more comparable to humans, but the strategy is promising as it offers increased specificity and safety, and more cost-effective synthesis. Derived peptides can also be injected peri- or intraocularly with, for example, chemical modifications or conjugation to nanoparticles promoting ocular retention,14,95 and modification such as phosphomimetic mutants may further increase potency and stability (see Fig. 4).134 However, the question of treatment durability does not only relate to optimized delivery. In particular, the ability of PEDF to durably protect retinal neurons cannot easily be extrapolated from rodent studies with weeks of follow-up compared with the life-long treatment necessary for inherited retinal degenerations in human patients. Thus, long-term follow-up should be a focus for further studies especially as some animal studies have described loss of treatment effect with time.102 Furthermore, it will be important to compare the potency as a therapeutic agent for different retinal pathologies with other neurotrophic and anti-angiogenic factors. This will focus clinical development on the most relevant applications in the competitive landscape of ocular drug development.
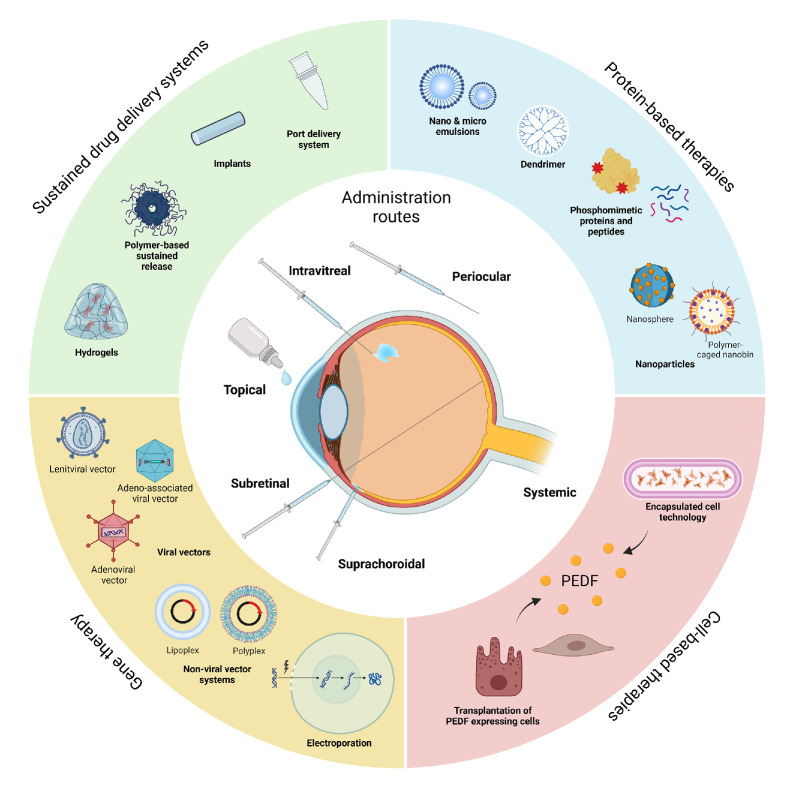
Figure 4.
Strategies for optimized delivery and efficacy of PEDF-based therapies: future perspectives for the treatment of vascular and neurodegenerative retinal disease. A major challenge for clinical application of PEDF therapeutics is efficient and durable drug delivery. PEDF therapeutics can be delivered using intraocular and periocular injections and potentially by topical application using small, derived peptides or nanocarriers. The limited ocular retention of PEDF makes direct injection clinically impractical, but advances in sustained drug delivery systems using, for example, polymer-based complexes would allow extended release with acceptable injection intervals. Truly extended therapies can be achieved using cell- and gene-based therapies. These strategies aim to achieve prolonged release of PEDF therapeutics by delivering PEDF transgenes to resident retinal cells or by modification of cells to continuously express PEDF; these cells can then be delivered to the posterior segment for engraftment or encapsulated for implantation into the vitreous. Created with BioRender.com. PEDF, pigment epithelium-derived factor.
Attainment of improved treatment outcomes for retinal disease will most likely depend on targeting several pathogenic pathways. Interestingly, PEDF expression from different vector systems has been explored as part of multitargeting therapeutics. This includes combined expression with a soluble VEGF and complement inhibitor in models of subretinal neovascularization,200,201 combination with RNAi-mediated VEGF knockdown in the laser-induced CNV model,202 and combination with Plgf knockdown in experimental DM.203 However, the specific nature of the additive effects cannot be readily discerned from these studies. Thus, further studies should identify how combination therapies potentiate the amelioration of specific disease features – or enable the attenuation of additional features. Importantly, the additive effect on VEGF targeting therapies should be characterized as the effects of PEDF partly result from modulation of VEGF signaling and the apoptosis-inducing effects on ECs is dependent on VEGF priming.95 Indeed, a lack14,95 or insignificant202 additive effect by combining PEDF with VEGF inhibitors has been observed. Global interrogation using omics-based methods could support a detailed understanding of these interactions.
Safety is a major concern for clinical translation. This is particularly true for compounds with pleiotropic effects like PEDF. Thus, although PEDF seems able to attenuate multiple pathological features of retinal degeneration and vascular disease, the specificity of action may be a concern. This provides a rationale for developing derived peptides with more specific and potent action. In addition, small peptides lower immunogenicity risk.95 However, the combination of anti-angiogenic and neurotrophic effects seems attractive from a therapeutic standpoint and mounting preclinical evidence demonstrate that PEDF therapy is safe. Transgenic overexpression of PEDF is not associated with adverse retinal effects,179 and the different strategies for PEDF delivery have not been associated with adverse events in preclinical studies. Notably, the anti-angiogenic effect relies on specific apoptosis induction in proliferating ECs. Indeed, PEDF does not adversely affect retinal or choroidal vascularity, which would be a major concern.
PEDF acts through several receptors with the PEDF-R as a major mediator of the neurotrophic properties, whereas the anti-angiogenic effects seem mainly mediated by the LR and regulation of VEGF signaling. However, the detailed mechanism remains unclear, and uncertainties exist regarding the context- and dose-dependent effects of PEDF in the retina. A few studies have reported U-shaped dose-responses on, for example, CNV formation.69 Thus, dose-titration experiments are needed preferably in large animal models. Large animal studies would also be useful to confirm the translational potential of PEDF-based therapies. In addition, conflicting evidence exists regarding the contribution of PEDF-regulated pathways to retinal pathology. For example, PEDF has emerged as an inhibitor of the canonical Wnt-pathway.23 Inhibition of this pathway has been suggested as a treatment strategy for, as an example, pathological angiogenesis and subretinal fibrosis, but agonists are also being explored to protect against retinal barrier disruption.148 Such observations underscore the complex molecular basis of retinal disease and the need to understand the cell-specific and context-dependent retinal effects of PEDF providing insights necessary for clinical translation.
Concluding Remarks
The non-inhibitory serpin PEDF has pleiotropic functions influencing diverse cellular processes dysregulated in retinal disease. Important features of PEDF signaling have been characterized with PEDF-R and the LR emerging as the major receptors. Even though the field has presented a growing body of evidence for the beneficial effects, much is yet to be learned about the complex and context-dependent effects of PEDF.
The evidence for PEDF downregulation as an etiological step in degenerative and neovascular ocular disorders is less convincing than initially suggested. Nonetheless, a remarkable number of preclinical animal studies demonstrate potent therapeutic effects of PEDF in neurodegenerative and vascular retinal pathologies with excellent safety profile. However, a paucity of studies in large animal models exists and questions of dose and durability need to be addressed.
Functional effects and receptor interactions have been mapped to specific sequence fragments enabling the development of PEDF-derived peptides with improved potency and delivery potential. Recent developments in ocular drug delivery including gene therapy have also been widely applied in a preclinical setting. Thus, the avenue toward clinical trials is open, hopefully enabling PEDF-based therapies to be added to the toolbox of the retinal physician.
Supplementary Material
Acknowledgments
The scRNA-seq analysis was performed by the Bioinformatics Core Facility at the Department of Biomedicine, Aarhus University, and the authors thank the staff at the core facility for their excellent support.
Supported by the Faculty of Health Sciences, Aarhus University (T.S.J.), Fight for Sight, Denmark (T.S.J.), Ophthalmologist Else Bruntses’ Foundation (T.S.J.), Synoptik Foundation (T.S.J.), the Danish Eye Research Foundation (A.L.A.), APTaps (T.J.C.), the VELUX Foundation (T.J.C.), and the Novo Nordisk Foundation (T.J.C.).
Author Contributions: Conceptualization, T.S.J.; methodology, T.S.J.; literature search, T.S.J.; article screening, T.S.J. and R.L.A.; data extraction, T.S.J. and R.L.A.; writing—original draft preparation, T.S.J. and R.L.A.; writing—review and editing, T.S.J., R.L.A., A.L.A., and T.J.C.; visualization, T.S.J., R.L.A., A.L.A., and T.J.C.; supervision, T.S.J., A.L.A., and T.J.C.; project administration, T.S.J., A.L.A., and T.J.C. All authors have read and agreed to the published version of the manuscript.
Disclosure: T.S. Jakobsen, None; R.L. Adsersen, None; A.L. Askou, None; T.J. Corydon, None
References
- 1. Ciulla TA, Hussain RM, Taraborelli D, Pollack JS, Williams DF.. Longer-term anti-VEGF therapy outcomes in neovascular age-related macular degeneration, diabetic macular edema, and vein occlusion-related macular edema: clinical outcomes in 130 247 eyes. Ophthalmol Retina. 2022; 6: 796–806. [DOI] [PubMed] [Google Scholar]
- 2. Askou AL, Jakobsen TS, Corydon TJ.. Retinal gene therapy: an eye-opener of the 21st century. Gene Ther. 2021; 28: 209–216. [DOI] [PubMed] [Google Scholar]
- 3. Dawson DW, Volpert OV, Gillis P, et al.. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999; 285: 245–248. [DOI] [PubMed] [Google Scholar]
- 4. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J.. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci USA. 1993; 90: 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tombran-Tink J, Chader GG, Johnson LV.. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991; 53: 411–414. [DOI] [PubMed] [Google Scholar]
- 6. Becerra SP. Focus on molecules: pigment epithelium-derived factor (PEDF). Exp Eye Res. 2006; 82: 739–740. [DOI] [PubMed] [Google Scholar]
- 7. Xu X, Zhang SS-M, Barnstable CJ, Tombran-Tink J.. Molecular phylogeny of the antiangiogenic and neurotrophic serpin, pigment epithelium derived factor in vertebrates. BMC Genomics. 2006; 7: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becerra SP. Structure-Function Studies on PEDF. New York, NY: Springer US; 1997: 223–237. [PubMed] [Google Scholar]
- 9. Belkacemi L, Zhang SX.. Anti-tumor effects of pigment epithelium-derived factor (PEDF): implication for cancer therapy. A mini-review. J Exp Clin Cancer Res. 2016; 35: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simonovic M, Gettins PGW, Volz K.. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc Natl Acad Sci USA. 2001; 98: 11131–11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gettins PGW, Simonovic M, Volz K.. Pigment epithelium-derived factor (PEDF), a serpin with potent anti-angiogenic and neurite outgrowth-promoting properties. Biol Chem. 2002; 383: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 12. Becerra SP, Sagasti A, Spinella P, Notario V.. Pigment epithelium-derived factor behaves like a noninhibitory serpin. J Biol Chem. 1995; 270: 25992–25999. [DOI] [PubMed] [Google Scholar]
- 13. Filleur S, Volz K, Nelius T, et al.. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005; 65: 5144–5152. [DOI] [PubMed] [Google Scholar]
- 14. Sheibani N, Wang S, Darjatmoko SR, et al.. Novel anti-angiogenic PEDF-derived small peptides mitigate choroidal neovascularization. Exp Eye Res. 2019; 188: 107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shao H, Schvartz I, Shaltiel S.. Secretion of pigment epithelium-derived factor. Eur J Biochem. 2003; 270: 822–831. [DOI] [PubMed] [Google Scholar]
- 16. Anguissola S, McCormack WJ, Morrin MA, Higgins WJ, Fox DM, Worrall DM.. Pigment epithelium-derived factor (PEDF) interacts with transportin SR2, and active nuclear import is facilitated by a novel nuclear localization motif. PLoS One. 2011; 6: e26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maik-Rachline G, Shaltiel S, Seger R.. Extracellular phosphorylation converts pigment epithelium–derived factor from a neurotrophic to an antiangiogenic factor. Blood. 2005; 105: 670–678. [DOI] [PubMed] [Google Scholar]
- 18. Meyer C, Notari L, Becerra SP.. Mapping the type I collagen-binding site on pigment epithelium-derived factor. J Biol Chem. 2002; 277: 45400–45407. [DOI] [PubMed] [Google Scholar]
- 19. Ho TC, Yeh SI, Chen SL, Chu TW, Tsao YP.. A short peptide derived from pigment epithelial-derived factor exhibits an angioinhibitory effect. BMC Ophthalmol. 2022; 22: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kenealey J, Subramanian P, Comitato A, et al.. Small retinoprotective peptides reveal a receptor-binding region on pigment epithelium-derived factor. J Biol Chem. 2015; 290: 25241–25253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernard A, Gao-Li J, Franco C-A, Bouceba T, Huet A, Li Z.. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J Biol Chem. 2009; 284: 10480–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Notari L, Baladron V, Aroca-Aguilar JD, et al.. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006; 281: 38022–38037. [DOI] [PubMed] [Google Scholar]
- 23. Park K, Lee K, Zhang B, et al.. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011; 31: 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnston EK, Francis MK, Knepper JE.. Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim. 2015; 51: 730–738. [DOI] [PubMed] [Google Scholar]
- 25. Notari L, Arakaki N, Mueller D, Meier S, Amaral J, Becerra SP.. Pigment epithelium-derived factor binds to cell-surface F1-ATP synthase. FEBS J. 2010; 277: 2192–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng G, Zhong M, Kawaguchi R, et al.. Identification of PLXDC1 and PLXDC2 as the transmembrane receptors for the multifunctional factor PEDF. Elife. 2014; 3: e05401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yasui N, Mori T, Morito D, et al.. Dual-site recognition of different extracellular matrix components by anti-angiogenic/neurotrophic serpin, PEDF. Biochemistry. 2003; 42: 3160–3167. [DOI] [PubMed] [Google Scholar]
- 28. Becerra SP, Perez-Mediavilla LA, Weldon JE, et al.. Pigment epithelium-derived factor binds to hyaluronan. Mapping of a hyaluronan binding site. J Biol Chem. 2008; 283: 33310–33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alberdi E, Hyde CC, Becerra SP.. Pigment epithelium-derived factor (PEDF) binds to glycosaminoglycans: analysis of the binding site. Biochemistry. 1998; 37: 10643–10652. [DOI] [PubMed] [Google Scholar]
- 30. Sekiya A, Okano-Kosugi H, Yamazaki CM, Koide T.. Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J Biol Chem. 2011; 286: 26364–26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosomichi J, Yasui N, Koide T, Soma K, Morita I.. Involvement of the collagen I-binding motif in the anti-angiogenic activity of pigment epithelium-derived factor. Biochem Biophys Res Commun. 2005; 335: 756–761. [DOI] [PubMed] [Google Scholar]
- 32. Kim HW, Roh K-H, Kim SW, et al.. Type I pig collagen enhances the efficacy of PEDF 34-mer peptide in a mouse model of laser-induced choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2019; 257: 1709–1717. [DOI] [PubMed] [Google Scholar]
- 33. Kawahara K, Yoshida T, Maruno T, et al.. Spatiotemporal regulation of PEDF signaling by type I collagen remodeling. Proc Natl Acad Sci USA. 2020; 117: 11450–11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnstable CJ, Tombran-Tink J.. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004; 23: 561–577. [DOI] [PubMed] [Google Scholar]
- 35. Tombran-Tink J, Shivaram S, Chader G, Johnson L, Bok D.. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995; 15: 4992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Becerra SP, Fariss RN, Wu YQ, Montuenga LM, Wong P, Pfeffer BA.. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res. 2004; 78: 223–234. [DOI] [PubMed] [Google Scholar]
- 37. Wu YQ, Becerra SP.. Proteolytic activity directed toward pigment epithelium-derived factor in vitreous of bovine eyes. Implications of proteolytic processing. Invest Ophthalmol Vis Sci. 1996; 37: 1984–1993. [PubMed] [Google Scholar]
- 38. Sorenson CM, Song Y-S, Sheibani N.. Pigment epithelium derived factor in ocular vascular development, neovascularization and function. Reference Module in Neuroscience and Biobehavioral Psychology. New York, NY: Elsevier; 2024. [Google Scholar]
- 39. Karakousis PC, John SK, Behling KC, et al.. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001; 7: 154–163. [PubMed] [Google Scholar]
- 40. Kim SY, Mocanu C, McLeod DS, et al.. Expression of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp Eye Res. 2003; 77: 433–445. [DOI] [PubMed] [Google Scholar]
- 41. Spranger J, Osterhoff M, Reimann M, et al.. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001; 50: 2641–2645. [DOI] [PubMed] [Google Scholar]
- 42. Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A.. PEDF derived from glial Müller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004; 299: 68–78. [DOI] [PubMed] [Google Scholar]
- 43. Ogata N, Wada M, Otsuji T, Jo N, Tombran-Tink J, Matsumura M.. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002; 43: 1168–1175. [PubMed] [Google Scholar]
- 44. Renno RZ, Youssri AI, Michaud N, Gragoudas ES, Miller JW.. Expression of pigment epithelium-derived factor in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002; 43: 1574–1580. [PubMed] [Google Scholar]
- 45. Behling KC, Surace EM, Bennett J.. Pigment epithelium-derived factor expression in the developing mouse eye. Mol Vis. 2002; 8: 449–454. [PubMed] [Google Scholar]
- 46. Rebustini IT, Crawford SE, Becerra SP.. PEDF deletion induces senescence and defects in phagocytosis in the RPE. Int J Mol Sci. 2022; 23: 7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogata N, Matsuoka M, Imaizumi M, Arichi M, Matsumura M.. Decrease of pigment epithelium-derived factor in aqueous humor with increasing age. Am J Ophthalmol. 2004; 137: 935–936. [DOI] [PubMed] [Google Scholar]
- 48. Al-Dwairi R, El-Elimat T, Aleshawi A, et al.. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic and non-diabetic retinopathy: associated factors and anatomical correlation. Int J Retina Vitreous. 2024; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhutto IA, McLeod DS, Hasegawa T, et al.. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006; 82: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tong JP, Yao YF.. Contribution of VEGF and PEDF to choroidal angiogenesis: a need for balanced expressions. Clin Biochem. 2006; 39: 267–276. [DOI] [PubMed] [Google Scholar]
- 51. Wilson S, Siebourg-Polster J, Titz B, et al.. Correlation of aqueous, vitreous, and serum protein levels in patients with retinal diseases. Transl Vis Sci Technol. 2023; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boehm BO, Lang G, Volpert O, et al.. Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia. 2003; 46: 394–400. [DOI] [PubMed] [Google Scholar]
- 53. Lee C-H, Lui DT-W, Cheung CY-Y, et al.. Circulating AFABP, FGF21, and PEDF levels as prognostic biomarkers of sight-threatening diabetic retinopathy. J Clin Endocrinol Metab. 2023; 108: e799–e806. [DOI] [PubMed] [Google Scholar]
- 54. Noma H, Funatsu H, Mimura T, Harino S, Eguchi S, Hori S.. Pigment epithelium-derived factor and vascular endothelial growth factor in branch retinal vein occlusion with macular edema. Graefes Arch Clin Exp Ophthalmol. 2010; 248: 1559–1565. [DOI] [PubMed] [Google Scholar]
- 55. Holekamp NM, Bouck N, Volpert O.. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol. 2002; 134: 220–227. [DOI] [PubMed] [Google Scholar]
- 56. Huber M, Wachtlin J.. Vitreous levels of proteins implicated in angiogenesis are modulated in patients with retinal or choroidal neovascularization. Ophthalmologica. 2012; 228: 188–193. [DOI] [PubMed] [Google Scholar]
- 57. Nobl M, Reich M, Dacheva I, et al.. Proteomics of vitreous in neovascular age-related macular degeneration. Exp Eye Res. 2016; 146: 107–117. [DOI] [PubMed] [Google Scholar]
- 58. Bhutto IA, Koichi U, Merges C, Zhang L, McLeod DS, Lutty GA.. Reduction of endogenous angiogenesis inhibitors in Bruch's membrane of the submacular region in eyes with age-related macular degeneration. Arch Ophthalmol. 2008; 126: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M.. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004; 88: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. An E, Lu X, Flippin J, et al.. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006; 5: 2599–2610. [DOI] [PubMed] [Google Scholar]
- 61. Cohen MP, Hud E, Shea E, Shearman CW.. Vitreous fluid of db/db mice exhibits alterations in angiogenic and metabolic factors consistent with early diabetic retinopathy. Ophthalmic Res. 2008; 40: 5–9. [DOI] [PubMed] [Google Scholar]
- 62. Zhang P, Tan Y, Gao L.. Protective effects of piperine on the retina of mice with streptozotocin-induced diabetes by suppressing HIF-1/VEGFA pathway and promoting PEDF expression. Int J Ophthalmol. 2021; 14: 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Araújo RS, Silva MS, Santos DF, Silva GA.. Dysregulation of trophic factors contributes to diabetic retinopathy in the Ins2(Akita) mouse. Exp Eye Res. 2020; 194: 108027. [DOI] [PubMed] [Google Scholar]
- 64. Cohen MP, Hud E, Wu V-Y, Shearman CW.. Amelioration of diabetes-associated abnormalities in the vitreous fluid by an inhibitor of albumin glycation. Invest Opthalmol Vis Sci. 2008; 49: 5089. [DOI] [PubMed] [Google Scholar]
- 65. Yoshida Y, Yamagishi SI, Matsui T, et al.. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009; 25: 678–686. [DOI] [PubMed] [Google Scholar]
- 66. Zheng Z, Chen H, Ke G, et al.. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor–to–pigment epithelium–derived factor ratio. Diabetes. 2009; 58: 954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matsuoka M, Ogata N, Minamino K, Higuchi A, Matsumura M.. High levels of pigment epithelium-derived factor in the retina of a rat model of type 2 diabetes. Exp Eye Res. 2006; 82: 172–178. [DOI] [PubMed] [Google Scholar]
- 68. Maeda S, Yamagishi S-I, Matsui T, et al.. Beneficial effects of vildagliptin on retinal injury in obese type 2 diabetic rats. Ophthalmic Res. 2013; 50: 221–226. [DOI] [PubMed] [Google Scholar]
- 69. Amaral J, Becerra SP.. Effects of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization. Invest Opthalmol Vis Sci. 2010; 51: 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li C. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice. Arch Ophthalmol. 2007; 125: 795. [DOI] [PubMed] [Google Scholar]
- 71. Chen X, Xu M, Zhang X, Barnstable CJ, Li X, Tombran-Tink J.. Deletion of the Pedf gene leads to inflammation, photoreceptor loss and vascular disturbances in the retina. Exp Eye Res. 2022; 222: 109171. [DOI] [PubMed] [Google Scholar]
- 72. Huang Q, Wang S, Sorenson CM, Sheibani N.. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res. 2008; 87: 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sorenson C, Wang S, Gendron R, Paradis H, Sheibani N.. Thrombospondin-1 deficiency exacerbates the pathogenesis of diabetic retinopathy. J Diabetes Metab. 2013;Suppl 12: 10.4172/2155-6156.S12-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dixit S, Polato F, Samardzija M, et al.. PEDF deficiency increases the susceptibility of rd10 mice to retinal degeneration. Exp Eye Res. 2020; 198: 108121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xiang W, Li L, Zhao Q, et al.. PEDF protects retinal pigment epithelium from ferroptosis and ameliorates dry AMD-like pathology in a murine model. GeroScience. 2023; 46: 2697–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Venturi G, Gandini A, Monti E, et al.. Lack of expression of SERPINF1, the gene coding for pigment epithelium-derived factor, causes progressively deforming osteogenesis imperfecta with normal type I collagen. J Bone Miner Res. 2012; 27: 723–728. [DOI] [PubMed] [Google Scholar]
- 77. Lin JM, Wan L, Tsai YY, et al.. Pigment epithelium-derived factor gene Met72Thr polymorphism is associated with increased risk of wet age-related macular degeneration. Am J Ophthalmol. 2008; 145: 716–721. [DOI] [PubMed] [Google Scholar]
- 78. Cobos E, Recalde S, Anter J, et al.. Association between CFH, CFB, ARMS2, SERPINF1, VEGFR1 and VEGF polymorphisms and anatomical and functional response to ranibizumab treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2018; 96: e201–e212. [DOI] [PubMed] [Google Scholar]
- 79. Ma L, Tang SM, Rong SS, et al.. Association of PEDF polymorphisms with age-related macular degeneration and polypoidal choroidal vasculopathy: a systematic review and meta-analysis. Sci Rep. 2015; 5: 9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Iizuka H, Awata T, Osaki M, et al.. Promoter polymorphisms of the pigment epithelium-derived factor gene are associated with diabetic retinopathy. Biochem Biophys Res Commun. 2007; 361: 421–426. [DOI] [PubMed] [Google Scholar]
- 81. Balasubbu S, Sundaresan P, Rajendran A, et al.. Association analysis of nine candidate gene polymorphisms in Indian patients with type 2 diabetic retinopathy. BMC Med Genet. 2010; 11: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M.. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim. 2010; 44: 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fabian-Jessing BK, Jakobsen TS, Jensen EG, et al.. Animal models of choroidal neovascularization: a systematic review. Invest Ophthalmol Vis Sci. 2022; 63: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Apte RS, Barreiro RA, Duh E, Volpert O, Ferguson TA.. Stimulation of neovascularization by the anti-angiogenic factor PEDF. Invest Opthalmol Vis Sci. 2004; 45: 4491. [DOI] [PubMed] [Google Scholar]
- 85. Mori K, Gehlbach P, Ando A, McVey D, Wei L, Campochiaro PA.. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002; 43: 2428–2434. [PubMed] [Google Scholar]
- 86. Murakami Y, Ikeda Y, Yonemitsu Y, et al.. Inhibition of choroidal neovascularization via brief subretinal exposure to a newly developed lentiviral vector pseudotyped with Sendai viral envelope proteins. Hum Gene Ther. 2010; 21: 199–209. [DOI] [PubMed] [Google Scholar]
- 87. Kern TS, David AA, Smith LEH.. Pathophysiology of diabetic retinopathy: contribution and limitations of laboratory research. Ophthalmic Res. 2019; 62: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sergeys J, Etienne I, Van Hove I, et al.. Longitudinal in vivo characterization of the streptozotocin-induced diabetic mouse model: focus on early inner retinal responses. Invest Opthalmol Vis Sci. 2019; 60: 807. [DOI] [PubMed] [Google Scholar]
- 89. Sadikan MZ, Abdul Nasir NA, Lambuk L, et al.. Diabetic retinopathy: a comprehensive update on in vivo, in vitro and ex vivo experimental models. BMC Ophthalmol. 2023; 23: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX.. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006; 20: 323–325. [DOI] [PubMed] [Google Scholar]
- 91. Qu Q, Park K, Zhou K, et al.. Sustained therapeutic effect of an anti-inflammatory peptide encapsulated in nanoparticles on ocular vascular leakage in diabetic retinopathy. Front Cell Dev Biol. 2022; 10: 1049678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu Y, Leo LF, McGregor C, Grivitishvili A, Barnstable CJ, Tombran-Tink J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol Med. 2012; 18: 1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yu H, Chen L, Jiang J.. Administration of pigment epithelium-derived factor delivered by adeno-associated virus inhibits blood-retinal barrier breakdown in diabetic rats. Mol Vis. 2010; 16: 2384–2394. [PMC free article] [PubMed] [Google Scholar]
- 94. Yamagishi S, Matsui T, Nakamura K, Takeuchi M, Imaizumi T.. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res. 2006; 72: 86–90. [DOI] [PubMed] [Google Scholar]
- 95. Sheibani N, Zaitoun IS, Wang S, et al.. Inhibition of retinal neovascularization by a PEDF-derived nonapeptide in newborn mice subjected to oxygen-induced ischemic retinopathy. Exp Eye Res. 2020; 195: 108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fan R, Su L, Zhang H, et al.. Enhanced therapeutic effect of PEDF-loaded mesenchymal stem cell-derived small extracellular vesicles against oxygen-induced retinopathy through increased stability and penetrability of PEDF. J Nanobiotechnology. 2023; 21: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sun MH, Ho TC, Yeh SI, Chen SL, Tsao YP.. Short peptides derived from pigment epithelium-derived factor attenuate retinal ischemia reperfusion injury through inhibition of apoptosis and inflammatory response in rats. Exp Eye Res. 2024; 238: 109743. [DOI] [PubMed] [Google Scholar]
- 98. Cayouette M, Smith SB, Becerra SP, Gravel C.. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis. 1999; 6: 523–532. [DOI] [PubMed] [Google Scholar]
- 99. Akiyama G, Sakai T, Kuno N, et al.. Photoreceptor rescue of pigment epithelium-derived factor-impregnated nanoparticles in Royal College of Surgeons rats. Mol Vis. 2012; 18: 3079–3086. [PMC free article] [PubMed] [Google Scholar]
- 100. Comitato A, Subramanian P, Turchiano G, Montanari M, Becerra SP, Marigo V.. Pigment epithelium-derived factor hinders photoreceptor cell death by reducing intracellular calcium in the degenerating retina. Cell Death Dis. 2018; 9: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hernández-Pinto A, Polato F, Subramanian P, et al.. PEDF peptides promote photoreceptor survival in rd10 retina models. Exp Eye Res. 2019; 184: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Miyazaki M, Ikeda Y, Yonemitsu Y, et al.. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003; 10: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 103. Miyazaki M, Ikeda Y, Yonemitsu Y, et al.. Synergistic neuroprotective effect via simian lentiviral vector-mediated simultaneous gene transfer of human pigment epithelium-derived factor and human fibroblast growth factor-2 in rodent models of retinitis pigmentosa. J Gene Med. 2008; 10: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 104. Wang Y, Subramanian P, Shen D, Tuo J, Becerra SP, Chan CC.. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro. 2013; 5: e00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Imai D, Yoneya S, Gehlbach PL, Wei LL, Mori K.. Intraocular gene transfer of pigment epithelium-derived factor rescues photoreceptors from light-induced cell death. J Cell Physiol. 2005; 202: 570–578. [DOI] [PubMed] [Google Scholar]
- 106. Cao W, Tombran-Tink J, Elias R, Sezate S, Mrazek D, McGinnis JF.. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2001; 42: 1646–1652. [PubMed] [Google Scholar]
- 107. Ortín-Martínez A, Valiente-Soriano FJ, García-Ayuso D, et al.. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS One. 2014; 9: e113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Valiente-Soriano FJ, Di Pierdomenico J, García-Ayuso D, et al.. Pigment epithelium-derived factor (PEDF) fragments prevent mouse cone photoreceptor cell loss induced by focal phototoxicity in vivo. Int J Mol Sci. 2020; 21: 7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhou X, Li F, Kong L, Chodosh J, Cao W.. Anti-inflammatory effect of pigment epithelium-derived factor in DBA/2J mice. Mol Vis. 2009; 15: 438–450. [PMC free article] [PubMed] [Google Scholar]
- 110. Vigneswara V, Esmaeili M, Deer L, Berry M, Logan A, Ahmed Z.. Eye drop delivery of pigment epithelium-derived factor-34 promotes retinal ganglion cell neuroprotection and axon regeneration. Mol Cell Neurosci. 2015; 68: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vigneswara V, Berry M, Logan A, Ahmed Z.. Pigment epithelium-derived factor is retinal ganglion cell neuroprotective and axogenic after optic nerve crush injury. Invest Opthalmol Vis Sci. 2013; 54: 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vigneswara V, Ahmed Z.. Pigment epithelium-derived factor mediates retinal ganglion cell neuroprotection by suppression of caspase-2. Cell Death Dis. 2019; 10: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang WMM, Zhang ZRM, Zhang YGM, Gao YSM.. Neural stem cell-based intraocular administration of pigment epithelium-derived factor promotes retinal ganglion cell survival and axon regeneration after optic nerve crush injury in rat: an experimental study. Iran J Med Sci. 2016; 41: 382–390. [PMC free article] [PubMed] [Google Scholar]
- 114. Nascimento-Dos-Santos G, Teixeira-Pinheiro LC, Da Silva-Júnior AJ, et al.. Effects of a combinatorial treatment with gene and cell therapy on retinal ganglion cell survival and axonal outgrowth after optic nerve injury. Gene Ther. 2020; 27: 27–39. [DOI] [PubMed] [Google Scholar]
- 115. Miyazaki M, Ikeda Y, Yonemitsu Y, et al.. Pigment epithelium-derived factor gene therapy targeting retinal ganglion cell injuries: neuroprotection against loss of function in two animal models. Hum Gene Ther. 2011; 22: 559–565. [DOI] [PubMed] [Google Scholar]
- 116. Ogata N, Wang L, Jo N, et al.. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr Eye Res. 2001; 22: 245–252. [DOI] [PubMed] [Google Scholar]
- 117. Xu M, Chen X, Yu Z, Li X.. Receptors that bind to PEDF and their therapeutic roles in retinal diseases. Front Endocrinol (Lausanne). 2023; 14: 1116136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pignolo RJ, Francis MK, Rotenberg MO, Cristofalo VJ.. Putative role for EPC-1/PEDF in the G0 growth arrest of human diploid fibroblasts. J Cell Physiol. 2003; 195: 12–20. [DOI] [PubMed] [Google Scholar]
- 119. Craword SE, Fitchev P, Veliceasa D, Volpert OV.. The many facets of PEDF in drug discovery and disease: a diamond in the rough or split personality disorder? Expert Opin Drug Discov. 2013; 8: 769–792. [DOI] [PubMed] [Google Scholar]
- 120. Li C, Huang Z, Zhu L, et al.. The contrary intracellular and extracellular functions of PEDF in HCC development. Cell Death Dis. 2019; 10: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang M, Tombran-Tink J, Yang S, Zhang X, Li X, Barnstable CJ.. PEDF is an endogenous inhibitor of VEGF-R2 angiogenesis signaling in endothelial cells. Exp Eye Res. 2021; 213: 108828. [DOI] [PubMed] [Google Scholar]
- 122. Johnen S, Djalali-Talab Y, Kazanskaya O, et al.. Antiangiogenic and neurogenic activities of sleeping beauty-mediated PEDF-transfected RPE cells in vitro and in vivo. Biomed Res Int. 2015; 2015: 863845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bai Y-J, Huang L-Z, Xu X-L, et al.. Polyethylene glycol-modified pigment epithelial-derived factor: new prospects for treatment of retinal neovascularization. J Pharmacol Exp Ther. 2012; 342: 131–139. [DOI] [PubMed] [Google Scholar]
- 124. Stellmach V, Crawford SE, Zhou W, Bouck N.. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci USA. 2001; 98: 2593–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stitt AW, McKenna D, Simpson DA, et al.. The 67-kd laminin receptor is preferentially expressed by proliferating retinal vessels in a murine model of ischemic retinopathy. Am J Pathol. 1998; 152: 1359–1365. [PMC free article] [PubMed] [Google Scholar]
- 126. Wang FH, Sun XD, Zhang X, et al.. Role of pigment epithelium-derived factor on proliferation and migration of choroidal capillary endothelium induced by vascular endothelial growth factor in vitro. Chin Med J (Engl). 2007; 120: 1534–1538. [PubMed] [Google Scholar]
- 127. Volpert OV, Zaichuk T, Zhou W, et al.. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium–derived factor. Nat Med. 2002; 8: 349–357. [DOI] [PubMed] [Google Scholar]
- 128. Liang J, Luo Q, Shen N, et al.. PEDF protects endothelial barrier integrity during acute myocardial infarction via 67LR. Int J Mol Sci. 2023; 24: 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cai J, Wu L, Qi X, et al.. PEDF regulates vascular permeability by a γ-secretase-mediated pathway. PLoS One. 2011; 6: e21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yang J, Duh EJ, Caldwell RB, Behzadian MA.. Antipermeability function of PEDF involves blockade of the MAP kinase/GSK/β-catenin signaling pathway and uPAR expression. Invest Opthalmol Vis Sci. 2010; 51: 3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu H, Ren J-G, Cooper WL, Hawkins CE, Cowan MR, Tong PY.. Identification of the antivasopermeability effect of pigment epithelium-derived factor and its active site. Proc Natl Acad Sci USA. 2004; 101: 6605–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yamagishi S-I, Nakamura K, Matsui T, et al.. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem. 2006; 281: 20213–20220. [DOI] [PubMed] [Google Scholar]
- 133. Shi W, Li J, Zhou J, et al.. Exploration of novel anti-angiogenic PEDF-derived peptides with improved activitives by inhibiting proliferation, suppressing migration, and inducing 67LR internalization. Bioorg Chem. 2021; 116: 105323. [DOI] [PubMed] [Google Scholar]
- 134. Konson A, Pradeep S, D'Acunto CW Seger R. Pigment epithelium-derived factor and its phosphomimetic mutant induce JNK-dependent apoptosis and P38-mediated migration arrest. Cell Physiol Biochem. 2018; 49: 512–529. [DOI] [PubMed] [Google Scholar]
- 135. He X, Cheng R, Benyajati S, Ma JX.. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond). 2015; 128: 805–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV.. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004; 199: 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Biyashev D, Veliceasa D, Kwiatek A, Sutanto MM, Cohen RN, Volpert OV.. Natural angiogenesis inhibitor signals through Erk5 activation of peroxisome proliferator-activated receptor gamma (PPARgamma). J Biol Chem. 2010; 285: 13517–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aurora AB, Biyashev D, Mirochnik Y, et al.. NF-kappaB balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2010; 116: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zheng Z, Chen H, Zhao H, et al.. Inhibition of JAK2/STAT3-mediated VEGF upregulation under high glucose conditions by PEDF through a mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci. 2010; 51: 64–71. [DOI] [PubMed] [Google Scholar]
- 140. Yamagishi S, Inagaki Y, Nakamura K, et al.. Pigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generation. J Mol Cell Cardiol. 2004; 37: 497–506. [DOI] [PubMed] [Google Scholar]
- 141. Zhang SX, Wang JJ, Gao G, Parke K, Ma J-X. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF–VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006; 37: 1–12. [DOI] [PubMed] [Google Scholar]
- 142. Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K.. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through γ-secretase. J Biol Chem. 2009; 284: 30177–30186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Matsui T, Higashimoto Y, Taira J, Yamagishi S.. Pigment epithelium-derived factor (PEDF) binds to caveolin-1 and inhibits the pro-inflammatory effects of caveolin-1 in endothelial cells. Biochem Biophys Res Commun. 2013; 441: 405–410. [DOI] [PubMed] [Google Scholar]
- 144. Zhang Y, Han J, Yang X, et al.. Pigment epithelium-derived factor inhibits angiogenesis and growth of gastric carcinoma by down-regulation of VEGF. Oncol Rep. 2011; 26: 681–686. [DOI] [PubMed] [Google Scholar]
- 145. Dejana E, Kühl M.. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010; 107: 943–952. [DOI] [PubMed] [Google Scholar]
- 146. Chen Y, Hu Y, Zhou T, et al.. Activation of the Wnt pathway plays a pathogenic role in diabetic retinopathy in humans and animal models. Am J Pathol. 2009; 175: 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma J-X.. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest Opthalmol Vis Sci. 2013; 54: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yemanyi F, Bora K, Blomfield AK, Wang Z, Chen J.. Wnt signaling in inner blood–retinal barrier maintenance. Int J Mol Sci. 2021; 22: 11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Moser TL, Kenan DJ, Ashley TA, et al.. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001; 98: 6656–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Arakaki N, Nagao T, Niki R, et al.. Possible role of cell surface H+ -ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003; 1: 931–939. [PubMed] [Google Scholar]
- 151. Yamaji Y, Yoshida S, Ishikawa K, et al.. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Opthalmol Vis Sci. 2008; 49: 3151. [DOI] [PubMed] [Google Scholar]
- 152. Bagley RG, Rouleau C, Weber W, et al.. Tumor endothelial marker 7 (TEM-7): a novel target for antiangiogenic therapy. Microvasc Res. 2011; 82: 253–262. [DOI] [PubMed] [Google Scholar]
- 153. Mizutani M, Kern TS, Lorenzi M.. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996; 97: 2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Antonetti DA, Silva PS, Stitt AW.. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021; 17: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yamagishi S, Matsui T, Nakamura K, Inoue H.. Pigment epithelium-derived factor is a pericyte mitogen secreted by microvascular endothelial cells: possible participation of angiotensin II-elicited PEDF downregulation in diabetic retinopathy. Int J Tissue React. 2005; 27: 197–202. [PubMed] [Google Scholar]
- 156. Yamagishi S, Nakamura K, Takenaka K, Matsui T, Jinnouchi Y, Imaizumi T.. Pigment epithelium-derived factor (PEDF) promotes growth of pericytes through autocrine production of platelet-derived growth factor-B. Microvasc Res. 2005; 69: 128–134. [DOI] [PubMed] [Google Scholar]
- 157. Amano S, Yamagishi S, Inagaki Y, et al.. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res. 2005; 69: 45–55. [DOI] [PubMed] [Google Scholar]
- 158. Sheikpranbabu S, Haribalaganesh R, Gurunathan S.. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab. 2011; 37: 505–511. [DOI] [PubMed] [Google Scholar]
- 159. Pagan-Mercado G, Becerra SP.. Signaling Mechanisms Involved in PEDF-Mediated Retinoprotection. New York, NY: Springer International Publishing; 2019: 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res. 1999; 57: 789–800. [PubMed] [Google Scholar]
- 161. Rapp M, Woo G, Al-Ubaidi MR, Becerra SP, Subramanian P.. Pigment Epithelium-Derived Factor Protects Cone Photoreceptor-Derived 661W Cells from Light Damage Through Akt Activation. New York, NY: Springer; 2014: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Eichler W, Savković-Cvijić H, Bürger S, et al.. Müller cell-derived PEDF mediates neuroprotection via STAT3 activation. Cell Physiol Biochem. 2017; 44: 1411–1424. [DOI] [PubMed] [Google Scholar]
- 163. Unterlauft JD, Eichler W, Kuhne K, et al.. Pigment epithelium-derived factor released by Müller glial cells exerts neuroprotective effects on retinal ganglion cells. Neurochem Res. 2012; 37: 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Subramanian P, Notario PM, Becerra SP.. Pigment Epithelium-derived Factor Receptor (PEDF-R): A Plasma Membrane-linked Phospholipase with PEDF Binding Affinity. New York, NY: Springer; 2010: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bernardo-Colón A, Dong L, Abu-Asab M, Brush RS, Agbaga MP, Becerra SP.. Ablation of pigment epithelium-derived factor receptor (PEDF-R/Pnpla2) causes photoreceptor degeneration. J Lipid Res. 2023; 64: 100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bullock J, Polato F, Abu-Asab M, et al.. Degradation of photoreceptor outer segments by the retinal pigment epithelium requires pigment epithelium-derived factor receptor (PEDF-R). Invest Opthalmol Vis Sci. 2021; 62: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Subramanian P, Locatelli-Hoops S, Kenealey J, Desjardin J, Notari L, Becerra SP.. Pigment epithelium-derived factor (PEDF) prevents retinal cell death via PEDF receptor (PEDF-R). J Biol Chem. 2013; 288: 23928–23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pham TL, He J, Kakazu AH, Jun B, Bazan NG, Bazan HEP.. Defining a mechanistic link between pigment epithelium–derived factor, docosahexaenoic acid, and corneal nerve regeneration. J Biol Chem. 2017; 292: 18486–18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. He J, Cortina MS, Kakazu A, Bazan HEP.. The PEDF neuroprotective domain plus DHA induces corneal nerve regeneration after experimental surgery. Invest Opthalmol Vis Sci. 2015; 56: 3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG.. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci. 2007; 104: 13152–13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yabe T, Wilson D, Schwartz JP.. NFκB activation is required for the neuroprotective effects of pigment epithelium-derived factor (PEDF) on cerebellar granule neurons. J Biol Chem. 2001; 276: 43313–43319. [DOI] [PubMed] [Google Scholar]
- 172. Yabe T, Kanemitsu K, Sanagi T, Schwartz JP, Yamada H.. Pigment epithelium-derived factor induces pro-survival genes through cyclic AMP-responsive element binding protein and nuclear factor kappa B activation in rat cultured cerebellar granule cells: implication for its neuroprotective effect. Neuroscience. 2005; 133: 691–700. [DOI] [PubMed] [Google Scholar]
- 173. Bürger S, Meng J, Zwanzig A, et al.. Pigment epithelium-derived factor (PEDF) receptors are involved in survival of retinal neurons. Int J Mol Sci. 2020; 22: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tsao YP, Ho TC, Chen SL, Cheng HC.. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006; 79: 545–550. [DOI] [PubMed] [Google Scholar]
- 175. Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP.. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun. 2006; 342: 372–378. [DOI] [PubMed] [Google Scholar]
- 176. He Y, Leung KW, Ren Y, Pei J, Ge J, Tombran-Tink J. PEDF. improves mitochondrial function in RPE cells during oxidative stress. Invest Ophthalmol Vis Sci. 2014; 55: 6742–6755. [DOI] [PubMed] [Google Scholar]
- 177. Nadal-Nicolas FM, Becerra SP.. Pigment Epithelium-derived Factor Protects Retinal Pigment Epithelial Cells Against Cytotoxicity “In Vitro”. New York, NY: Springer International Publishing; 2018: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tenbrock L, Wolf J, Boneva S, et al.. Subretinal fibrosis in neovascular age-related macular degeneration: current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022; 387: 361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Park K, Jin J, Hu Y, Zhou K, Ma J-X.. Overexpression of pigment epithelium–derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011; 178: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Haurigot V, Villacampa P, Ribera A, et al.. Long-term retinal PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS One. 2012; 7: e41511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Xiang W, Li L, Hong F, et al.. N-cadherin cleavage: a critical function that induces diabetic retinopathy fibrosis via regulation of β-catenin translocation. FASEB J. 2023; 37: e22878. [DOI] [PubMed] [Google Scholar]
- 182. Xi L. Pigment epithelium-derived factor as a possible treatment agent for choroidal neovascularization. Oxid Med Cell Longev. 2020; 2020: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Zhang H, Hui H, Li Z, et al.. Pigment epithelium-derived factor attenuates myocardial fibrosis via inhibiting endothelial-to-mesenchymal transition in rats with acute myocardial infarction. Sci Rep. 2017; 7: 41932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Qin X, Jia C, Liang J, et al.. PEDF is an antifibrosis factor that inhibits the activation of fibroblasts in a bleomycin-induced pulmonary fibrosis rat model. Respir Res. 2022; 23: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nakashima S, Matsui T, Yamagishi S.. Pigment epithelium-derived factor (PEDF) blocks high glucose-induced inflammatory reactions in endothelial cells through its anti-oxidative properties. Int J Cardiol. 2013; 168: 3004–3006. [DOI] [PubMed] [Google Scholar]
- 186. Gao S, Li C, Zhu Y, et al.. PEDF mediates pathological neovascularization by regulating macrophage recruitment and polarization in the mouse model of oxygen-induced retinopathy. Sci Rep. 2017; 7: 42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bernardo-Colón A, Lerner M, Becerra SP.. Pigment epithelium-derived factor is an interleukin-6 antagonist in the RPE: Insight of structure-function relationships. Front Physiol. 2022; 13: 1045613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Reichenbach A, Bringmann A.. Glia of the human retina. Glia. 2020; 68: 768–796. [DOI] [PubMed] [Google Scholar]
- 189. Sugita Y, Becerra SP, Chader GJ, Schwartz JP.. Pigment epithelium-derived factor (PEDF) has direct effects on the metabolism and proliferation of microglia and indirect effects on astrocytes. J Neurosci Res. 1997; 49: 710–718. [DOI] [PubMed] [Google Scholar]
- 190. Sanagi T, Yabe T, Yamada H.. The regulation of pro-inflammatory gene expression induced by pigment epithelium-derived factor in rat cultured microglial cells. Neurosci Lett. 2005; 380: 105–110. [DOI] [PubMed] [Google Scholar]
- 191. Takanohashi A, Yabe T, Schwartz JP.. Pigment epithelium-derived factor induces the production of chemokines by rat microglia. Glia. 2005; 51: 266–278. [DOI] [PubMed] [Google Scholar]
- 192. Yabe T, Sanagi T, Schwartz JP, Yamada H.. Pigment epithelium-derived factor induces pro-inflammatory genes in neonatal astrocytes through activation of NF-κB and CREB. Glia. 2005; 50: 223–234. [DOI] [PubMed] [Google Scholar]
- 193. Chader GJ. Surmountable challenges in translating pigment epithelium-derived factor (PEDF) therapy from animal models to clinical trials for retinal degenerations. Retina. 2005; 25: S29–S30. [DOI] [PubMed] [Google Scholar]
- 194. Campochiaro PA, Nguyen QD, Shah SM, et al.. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006; 17: 167–176. [DOI] [PubMed] [Google Scholar]
- 195. Garkal A, Bangar P, Rajput A, et al.. Long-acting formulation strategies for protein and peptide delivery in the treatment of PSED. J Control Release. 2022; 350: 538–568. [DOI] [PubMed] [Google Scholar]
- 196. Rebustini IT, Bernardo-Colón A, Nalvarte AI, Becerra SP.. Delivery systems of retinoprotective proteins in the retina. Int J Mol Sci. 2021; 22: 5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Biswas A, Choudhury AD, Bisen AC, et al.. Trends in formulation approaches for sustained drug delivery to the posterior segment of the eye. AAPS PharmSciTech. 2023; 24: 217. [DOI] [PubMed] [Google Scholar]
- 198. Kauper K, Orecchio L, Nystuen A, et al.. Continuous intraocular drug delivery lasting over a decade: ciliary neurotrophic factor (CNTF) secreted from Neurotech's NT-501 implanted in subjects with retinal degenerative disorders. Invest Ophthalmol Vis Sci. 2023; 64: 3680. [Google Scholar]
- 199. Askou AL, Jakobsen TS, Corydon TJ.. Toward lentiviral vectors for antiangiogenic ocular gene therapy. Mol Ther Methods Clin Dev. 2023; 30: 443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wei-Zhang S, Cui B, Xing M, et al.. Chimpanzee adenovirus-mediated multiple gene therapy for age-related macular degeneration. iScience. 2023; 26: 107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Bai T, Cui B, Xing M, et al.. Stable inhibition of choroidal neovascularization by adeno-associated virus 2/8-vectored bispecific molecules. Gene Ther. 2024; 31: 511–523. [DOI] [PubMed] [Google Scholar]
- 202. Askou AL, Alsing S, Benckendorff JNE, et al.. Suppression of choroidal neovascularization by AAV-based dual-acting antiangiogenic gene therapy. Mol Ther Nucleic Acids. 2019; 16: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Araújo RS, Bitoque DB, Silva GA.. Dual-acting antiangiogenic gene therapy reduces inflammation and regresses neovascularization in diabetic mouse retina. Mol Ther Nucleic Acids. 2020; 22: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lukowski SW, Lo CY, Sharov AA, et al.. A single-cell transcriptome atlas of the adult human retina. Embo J. 2019; 38: e100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.