Abstract
Background
The high-sensitivity C-reactive protein (hs-CRP) to high-density lipoprotein cholesterol (HDL-C) ratio, a composite marker of low-grade inflammation and lipid metabolism, is reportedly associated with the occurrence of new cardiovascular diseases (CVDs) in certain people. However, the predictive value of the hs-CRP/HDL-C ratio for long-term mortality in the general population remains unclear.
Methods
This retrospective cohort study included data from 9,492 adults obtained from the National Health and Nutrition Examination Survey (NHANES) (2015–2018) in the United States. Multivariate Cox regression, two-piecewise linear regression, restricted cubic spline (RCS) models and subgroup analysis by age, sex, smoking status and drinking status were applied to evaluate the associations of the hs-CRP/HDL-C ratio with long-term all-cause and cardiovascular mortality.
Results
The overall median age of the cohort was 47.0 years (interquartile range (IQR) 32.0–62.0), and 4,585 (48.30%) patients were male. During a median follow-up period of 37.0 months, 239 (2.52%) all-cause deaths occurred, 59 (0.62%) of which were attributed to cardiovascular events. Participants with all-cause and cardiovascular mortality presented a higher hs-CRP/HDL-C ratio than did those without events [0.56 (0.24–1.38) vs. 0.37 (0.14–0.94) and 0.60 (0.23–1.60) vs. 0.37 (0.14–0.95), P < 0.001 and P = 0.002]. According to multivariate Cox regression models, the hs-CRP/HDL-C ratio was found to be an independent risk factor for both long-term all-cause mortality [hazard ratio (HR) = 1.09, 95% confidence interval (CI): 1.05–1.13] and cardiovascular mortality (HR = 1.11, 95% CI: 1.05–1.19). A two-piecewise linear regression model indicated that the risk of all-cause mortality increased more prominently when the hs-CRP/HDL-C ratio was less than 1.21. In addition, a significant interaction effect with smoking status was discovered (P = 0.006), indicating that the association of the hs-CRP/HDL-C ratio with all-cause mortality was stronger in nonsmokers. The RCS curve revealed a positive linear association of the hs-CRP/HDL-C ratio with long-term mortality after adjustment for potential confounders.
Conclusions
The hs-CRP/HDL-C ratio is a crucial predictor of long-term mortality in the general population, independent of potential confounding factors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04446-1.
Keywords: hs-CRP, HDL-C, Long-term mortality, NHANES, Correlational analysis
Introduction
As a composite marker of low-grade inflammation and lipid metabolism, the high-sensitivity C-reactive protein (hs-CRP) to high-density lipoprotein cholesterol (HDL-C) ratio enables the simultaneous investigation of distinct effects and a comprehensive understanding of the interaction between hs-CRP and HDL-C [1]. The two haematological parameters are well standardized, simple and inexpensive to measure. These features make the hs-CRP/HDL-C ratio a promising clinical indicator. Recently, it has been reported that this indicator is associated with the occurrence of new cardiovascular diseases (CVDs) in middle-aged and elderly individuals in China [2]. However, its predictive value lacks a population-wide evaluation. Furthermore, there is limited research on its role in long-term all-cause and cardiovascular mortality.
CVDs are responsible for approximately 30% of the global annual mortality rate (approximately 17.6 million individuals) and almost 10% of the global burden of diseases [3, 4]. Dyslipidaemia and low-grade inflammation play crucial roles in the development of atherosclerosis, a pathological condition that contributes to the onset and progression of the majority of CVDs [5]. Low-grade inflammation is defined as a mild and chronic increase in inflammatory markers that does not reach the extent of acute inflammation [6]. Hs-CRP, a reliable marker for low-grade inflammation [7], is an acute-phase protein that is formed by hepatic aortic endothelial cells and coronary artery smooth muscle cells in response to proinflammatory cytokines triggered by oxidative stress or inflammatory activation [8, 9]. Previous research has shown that increased hs-CRP levels are associated with the occurrence [10–12] and recurrence [13] of CVDs. According to the American Heart Association, hs-CRP levels of 1 mg and 3 mg are considered diagnostic thresholds for individuals at medium and high risk of CVDs [14]. In contrast, HDL-C is commonly recognized as a protective factor against atherosclerosis. The primary cardioprotective mechanisms are reverse cholesterol transport, antioxidative ability, anti-inflammatory effects and the protection of vascular endothelial cells [15]. In epidemiological studies, HDL-C is a significant predictor of mortality and cardiovascular mortality in specific individuals [16, 17]. Nevertheless, owing to the heterogeneity of the population and the presence of several confounding or residual risk factors, it is challenging to use hs-CRP or lipids alone to assess the prognosis of CVD patients [18–20].
Increasing evidence has demonstrated that abnormal lipid levels are frequently associated with aberrant levels of inflammatory biomarkers, such as hs-CRP [21, 22]. However, the potential interaction between hs-CRP and dyslipidaemia is unclear. To date, the association of the hs-CRP/HDL-C ratio with long-term mortality in the general population remains uncertain. Hence, our aim was to evaluate the predictive value of the hs-CRP/HDL-C ratio for long-term all-cause and cardiovascular mortality in adults in the general population of the United States based on the clinical data obtained from the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population and ethics
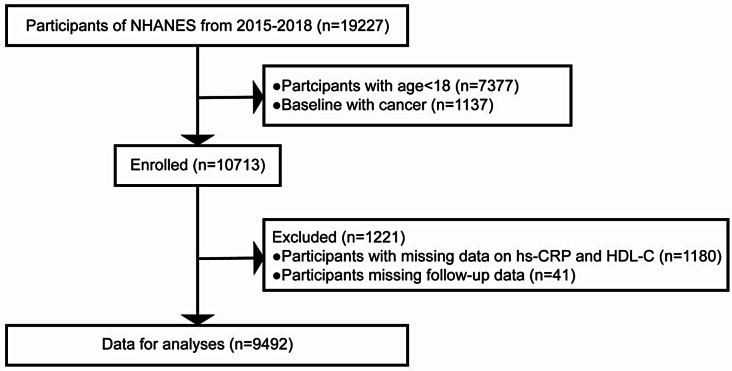
This study cohort was selected from the official website of the NHANES, a nationwide survey designed to collect data in an unprejudiced manner from the noninstitutionalized civilian population in the United States. The comprehensive protocol is available in the NHANES process manual (https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx). In total, 19,227 participants across 2 consecutive cycles of the NHANES (2015–2018) were initially included in this study. The exclusion criteria were as follows: [1] age < 18 years (n = 7,377) [2], baseline cancer status (n = 1,137) [3], missing data on hs-CRP and HDL-C (n = 1,180), and [4] missing follow-up data (n = 41). After manual data filtration, a final selection of 9,492 individuals remained for subsequent analyses. Figure 1 shows a detailed flowchart for study cohort recruitment. All participants (or their proxies/legal guardians) provided written informed consent to participate in the study. This study was reviewed and approved by the National Center for Health Statistics (NCHS) Ethics Review Board, with the approval number for each phase available at https://www.cdc.gov/nchs/nhanes/irba98.htm. The approaches were conducted in compliance with ethical principles and regulations.
Fig. 1.
Flow diagram of study selection. NHANES, National Health and Nutrition Examination Survey
Assessment of exposure
Fasting blood samples were collected in accordance with established protocols and procedures for venipuncture. The quantification of hs-CRP and HDL-C in the serum was performed via standard enzyme colorimetric assays (see Supplementary material 1). All samples were processed according to the standardized and uniform NHANES protocol. The hs-CRP/HDL-C ratio was calculated by dividing the hs-CRP concentration (mg/L) by the HDL-C concentration (mg/L).
Covariates
In accordance with previous studies, we collected as many covariates as possible that were clearly associated with all-cause and cardiovascular mortality. The study cohort underwent in-person or computer-assisted personal interviews. The participants responded to a questionnaire that requested demographic and health details. The participants provided demographic data, including their date of birth, sex, race and health-related characteristics (such as medical history, smoking behaviours, alcohol consumption patterns and concomitant medication), at baseline. The formula for calculating body mass index (BMI) was weight (kg)/height squared (m2). The following data pertaining to medical history were collected from self-reported information: hypertension, diabetes, heart failure, coronary artery disease (CAD), and stroke. Subjects were classified as smokers if they had smoked for a duration exceeding 6 months or consumed more than 100 cigarettes. Additionally, subjects who had consumed a minimum of 12 drinks within the previous year were classified as drinkers [23].
Ascertainment of outcomes
The causes of mortality were classified in accordance with the 10th edition of the International Classification of Diseases (ICD-10). Endpoints included all-cause and cardiovascular mortality (defined as deaths attributable to cardiovascular disease (codes I00-I99)). The mortality data were acquired by cross-referencing the National Death Index (https://www.cdc.gov/nchs/data-linkage/mortality.htm) with the NHANES datasets. The follow-up period commenced on the date of survey participation and ended on December 31, 2018, when mortality, dropout, or the date specified in the survey occurred.
Statistical analysis
Owing to the utilization of a series of intricate sampling designs in the NHANES survey, our analytical methods incorporated the sample weights associated with distinct research periods to derive precise estimates of statistics related to health [24]. The Shapiro–Wilk test was conducted to assess the normality of the distribution of continuous variables. Nonnormally distributed variables are presented as medians (interquartile ranges, IQRs), whereas categorical variables are presented as proportions. The Mann–Whitney U test or χ2 test was applied for group comparisons. A two-piecewise linear regression model was applied to determine the inflection points. The log-rank test was used to evaluate the difference in fitting capability between regression models.
Multiple Cox regression models were employed to explore the independent association of the hs-CRP/HDL-C ratio with long-term mortality. Model 1 was a rudimentary model excluding adjustment for confounders. Model 2 included covariates such as age, sex, race, BMI, alcohol consumption and smoking status. Model 3 further incorporated lipid-lowering drug use and conventional cardiovascular risk factors such as hypertension, diabetes, heart failure, CAD, and stroke, in addition to the covariates already included in Model 2. Subgroup analysis was performed by examining the results of the fully adjusted models that were stratified by sex, age, smoking status, and alcohol consumption status. Moreover, the interactions between these variables were assessed. Furthermore, we utilized restricted cubic spline (RCS) models to visualize the relationship between the hs-CRP/HDL-C ratio and mortality risk. The optimal cut-off value was determined for risk stratification based on the Youden index, and Kaplan–Meier (K–M) survival curves and log-rank tests were used to evaluate differences in survival between groups.
Missing values were replaced by multiple imputation. All the statistical analyses were conducted using R software version 4.2.0 (http://www.R-project.org, R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The overall median age of this study cohort (n = 9,492) was 47.0 years (IQR 32.0–62.0), and 4,585 (48.30%) were male. The median hs-CRP/HDL-C ratio was 0.38 (IQR 0.14–0.95), with a percentile range of 0.04 to 3.05 (5th to 95th percentile) and 0.01 to 8.41 (1st to 99th percentile). During a median follow-up period of 37.0 months, 239 (2.52%) all-cause deaths occurred, 59 (0.62%) of which were attributed to cardiovascular events.
The study population was divided into two groups based on long-term all-cause mortality: subjects without events (n = 9,253) and those with events (n = 239). Table 1 provides a thorough comparison of the fundamental characteristics of the two groups. The participants in the group with events were older and mostly male, and this group had a greater proportion of smokers (all P values < 0.001). Cerebrovascular diseases, including hypertension, diabetes, heart failure, CAD and stroke, as well as the use of lipid-lowering medications, were more prevalent in the group with all-cause mortality (all P values < 0.001). Importantly, the participants with events had significantly higher hs-CRP (P < 0.001) and hs-CRP/HDL-C ratios [0.56 (0.24–1.38) vs. 0.37 (0.14–0.94), P < 0.001].
Table 1.
Demographic and baseline characteristics of participants by long-term all-cause mortality
| No events (n = 9,253) | Events (n = 239) | P value | |
|---|---|---|---|
| Age (years) | 46.00 (31.00–61.00) | 73.00 (62.00–80.00) | < 0.001 |
| Age group | |||
| < 65 years | 7533 (81.41%) | 74 (30.96%) | < 0.001 |
| ≥ 65 years | 1720 (18.59%) | 165 (69.04%) | |
| Sex | |||
| Male | 4437 (47.95%) | 148 (61.92%) | < 0.001 |
| Female | 4816 (52.05%) | 91 (38.08%) | |
| Race | |||
| Mexican American | 1575 (17.02%) | 40 (16.74%) | < 0.001 |
| Non-Hispanic white | 1093 (11.81%) | 21 (8.79%) | |
| Non-Hispanic black | 2844 (30.74%) | 107 (44.77%) | |
| Other Hispanic | 2045 (22.10%) | 50 (20.92%) | |
| Other races | 1696 (18.33%) | 21 (8.79%) | |
| Drinking | 4392 (47.47%) | 81 (33.89%) | < 0.001 |
| Smoking | 3598 (38.88%) | 149 (62.34%) | < 0.001 |
| Hypertension | 3055 (33.02%) | 147 (61.51%) | < 0.001 |
| Diabetes | 1252 (13.53%) | 80 (33.47%) | < 0.001 |
| Heart failure | 237 (2.56%) | 49 (20.50%) | < 0.001 |
| CAD | 291 (3.14%) | 40 (16.74%) | < 0.001 |
| Stroke | 301 (3.25%) | 35 (14.64%) | < 0.001 |
| Lipid-lowering drugs | 1597 (17.26%) | 89 (37.24%) | < 0.001 |
| BMI (kg/m2) | 28.40 (24.50–33.40) | 28.55 (24.60–32.20) | 0.629 |
| Hs-CRP (mg/L) | 1.88 (0.80–4.43) | 3.01 (1.30–6.59) | < 0.001 |
| HDL-C (mmol/L) | 1.32 (1.09–1.60) | 1.29 (1.03–1.58) | 0.144 |
| HDL-C (mg/L) | 5.10 (4.22–6.19) | 4.99 (3.98–6.11) | |
| Hs-CRP/HDL-C ratio | 0.37 (0.14–0.94) | 0.56 (0.24–1.38) | < 0.001 |
Data were presented as median (Q1-Q3) or N (%). CAD, coronary artery disease; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; Divide HDL-C (mmol/L) by 0.2586 to convert to mg/L
On the basis of long-term cardiovascular mortality, the study population was categorized into two groups: those with events (n = 59) and those without events (n = 9,433). The baseline characteristics of these two groups are presented in Table 2. The participants with cardiovascular events were older and predominantly male (both P values < 0.001). In the group with cardiovascular mortality, there was a higher incidence of hypertension, diabetes, heart failure, CAD and stroke, as well as an increased use of lipid-lowering drugs (all P values < 0.001). Additionally, these patients presented a considerably higher hs-CRP/HDL-C ratio [0.60 (0.23–1.60) vs. 0.37 (0.14–0.95), P = 0.002] as a result of their higher serum hs-CRP and lower HDL-C levels (both P values < 0.05).
Table 2.
Demographic and baseline characteristics of participants by long-term cardiovascular mortality
| No events (n = 9,433) | Events (n = 59) | P value | |
|---|---|---|---|
| Age (years) | 47.00 (32.00–61.00) | 74.00 (67.50–80.00) | < 0.001 |
| Age group | |||
| < 65 years | 7593 (80.49%) | 14 (23.73%) | < 0.001 |
| ≥ 65 years | 1840 (19.51%) | 45 (76.27%) | |
| Sex | |||
| Male | 4542 (48.15%) | 43 (72.88%) | < 0.001 |
| Female | 4891 (51.85%) | 16 (27.12%) | |
| Race | |||
| Mexican American | 1605 (17.01%) | 10 (16.95%) | 0.069 |
| Non-Hispanic white | 1105 (11.71%) | 9 (15.25%) | |
| Non-Hispanic black | 2926 (31.02%) | 25 (42.37%) | |
| Other Hispanic | 2083 (22.08%) | 12 (20.34%) | |
| Other races | 1714 (18.17%) | 3 (5.08%) | |
| Drinking | 4452 (47.20%) | 21 (35.59%) | 0.075 |
| Smoking | 3717 (39.40%) | 30 (50.85%) | 0.073 |
| Hypertension | 3162 (33.52%) | 40 (67.80%) | < 0.001 |
| Diabetes | 1305 (13.83%) | 27 (45.76%) | < 0.001 |
| Heart failure | 264 (2.80%) | 22 (37.29%) | < 0.001 |
| CAD | 314 (3.33%) | 17 (28.81%) | < 0.001 |
| Stroke | 329 (3.49%) | 7 (11.86%) | < 0.001 |
| Lipid-lowering drugs | 1659 (17.59%) | 27 (45.76%) | < 0.001 |
| BMI (kg/m2) | 28.40 (24.50–33.40) | 29.10 (25.30–32.20) | 0.796 |
| Hs-CRP (mg/L) | 1.90 (0.80–4.50) | 2.85 (1.25–6.06) | 0.010 |
| HDL-C (mmol/L) | 1.32 (1.09–1.60) | 1.22 (0.94–1.47) | 0.006 |
| HDL-C (mg/L) | 5.10 (4.22–6.19) | 4.72 (3.62–5.68) | |
| Hs-CRP/HDL-C ratio | 0.37 (0.14–0.95) | 0.60 (0.23–1.60) | 0.002 |
Data were presented as median (Q1-Q3) or N (%). CAD, coronary artery disease; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; Divide HDL-C (mmol/L) by 0.2586 to convert to mg/L
Baseline characteristics described in accordance with the quartiles of the hs-CRP/HDL-C ratio were shown in Supplementary Table 1. All baseline confounders had significant differences among quartiles of the hs-CRP/HDL-C ratio (all P values < 0.01).
Association of the hs-CRP/HDL-C ratio with long-term all-cause mortality
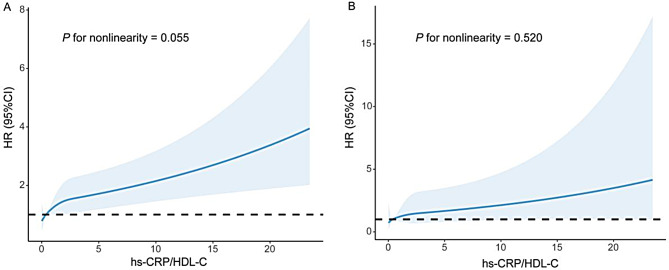
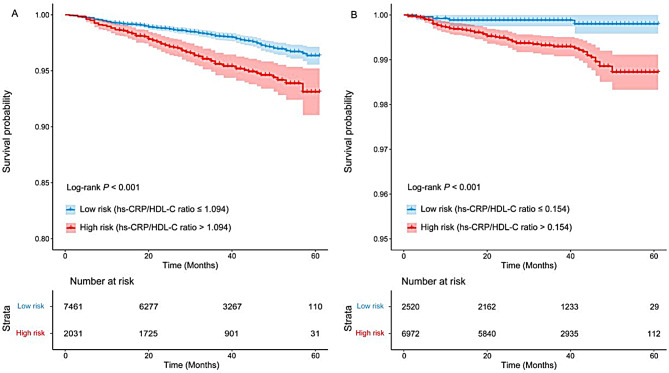
To address the effects of potential confounders, covariates such as age, sex, race, BMI, drinking, smoking, hypertension, diabetes, heart failure, CAD and stroke were included as adjustment factors in the multivariate regression. The final Cox regression model demonstrated a substantial association between an increase in the hs-CRP/HDL-C ratio and an elevated risk of long-term all-cause mortality (HR = 1.09, 95% CI: 1.05–1.13; Table 3). An increase in the ratio by 1 was associated with a 9% increase in the risk of all-cause mortality. The RCS model revealed a positive linear correlation between the hs-CRP/HDL-C ratio and long-term all-cause mortality (P for nonlinearity = 0.055, Fig. 2A). As shown in Table 4, the two-piecewise linear regression model provided superior fitting capability in comparison with the standard linear model (log likelihood ratio = 0.008). When the hs-CRP/HDL-C ratio was less than the inflection point of 1.21, the risk of all-cause mortality increased more substantially (HR = 1.92, 95% CI: 1.39–2.65, P < 0.001), and after 1.21, the mortality risk tended to flatten out (HR = 1.09, 95% CI: 1.05–1.13, P < 0.001). The participants were divided into a low-risk group (hs-CRP/HDL-C ratio ≤ 1.094) and a high-risk group (hs-CRP/HDL-C ratio > 1.094) based on the optimal cut-off value determined by the Youden index. K–M survival curves revealed a significant disparity in the risk of all-cause mortality between the groups (P for log-rank test < 0.001, Fig. 5A).
Table 3.
Multivariate Cox models for long-term mortality for the hs-CRP/HDL-C ratio in the pooled cohort
| Model 1 HR (95% CI) |
P value | Model 2 HR (95% CI) |
P value | Model 3 HR (95% CI) |
P value | |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| HDL-C (mg/L) | 0.98 (0.90, 1.05) | 0.528 | 0.94 (0.86, 1.03) | 0.208 | 0.98 (0.90, 1.08) | 0.737 |
| Hs-CRP (mg/L) | 1.02 (1.01, 1.03) | < 0.001 | 1.02 (1.01, 1.03) | < 0.001 | 1.02 (1.01, 1.03) | < 0.001 |
| Hs-CRP/HDL-C ratio | 1.10 (1.07, 1.13) | < 0.001 | 1.10 (1.07, 1.14) | < 0.001 | 1.09 (1.05, 1.13) | < 0.001 |
| Cardiovascular mortality | ||||||
| HDL-C (mg/L) | 0.75 (0.62, 0.91) | 0.003 | 0.74 (0.60, 0.91) | 0.005 | 0.82 (0.66, 1.02) | 0.071 |
| Hs-CRP (mg/L) | 1.02 (1.00, 1.03) | 0.013 | 1.02 (1.01, 1.04) | 0.002 | 1.02 (1.00, 1.04) | 0.014 |
| Hs-CRP/HDL-C ratio | 1.10 (1.05, 1.16) | < 0.001 | 1.14 (1.08, 1.21) | < 0.001 | 1.11 (1.05, 1.19) | 0.001 |
HR, hazard ratio; CI, confidence interval
Model 1: non-adjusted
Model 2: adjusted for age, sex, race, drinking, smoking and BMI
Model 3: adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs
Fig. 2.
Restricted cubic spline plots of the association between the hs-CRP/HDL-C ratio ratio with long-term all-cause mortality (A) and cardiovascular mortality (B) in the general population. Analysis was adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs. HR, hazard ratio
Table 4.
Threshold effect analysis of the hs-CRP/HDL-C ratio on long-term all-cause and cardiovascular mortality by using two-piecewise linear regression
| All-cause mortality | Cardiovascular mortality | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Fitting by standard linear model | 1.09 (1.05, 1.13) | < 0.001 | 1.11 (1.05, 1.19) | 0.001 |
| Fitting by two-piecewise linear model | ||||
| Inflection point | 1.21 | 1.53 | ||
| < Inflection point | 1.92 (1.39, 2.65) | < 0.001 | 2.09 (1.25, 3.49) | 0.005 |
| ≥ Inflection point | 1.09 (1.05, 1.13) | < 0.001 | 1.11 (1.04, 1.19) | 0.003 |
| Log likelihood ratio | 0.008 | 0.078 | ||
HR, hazard ratio; CI, confidence interval. Adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs
Association of the hs-CRP/HDL-C ratio with long-term cardiovascular mortality
The fully adjusted Cox model demonstrated a statistically significant positive association between the hs-CRP/HDL-C ratio and long-term cardiovascular mortality (HR = 1.11, 95% CI: 1.05–1.19; Table 3). An increase in the ratio of 1 was associated with an 11% increase in the risk of long-term mortality from cardiovascular causes. The RCS analysis presented in Fig. 2B indicated a progressive linear increase in HRs for long-term cardiovascular mortality as the hs-CRP/HDL-C ratio increased (P for nonlinearity = 0.520). As illustrated in Table 4, the fitting capability of the two-piecewise linear regression model was not statistically distinct from that of the standard linear model (log likelihood ratio = 0.078). Similarly, the optimal cut-off value for classifying participants into low-risk and high-risk groups was 0.154. The long-term risk of cardiovascular mortality varied significantly between the groups, according to the K–M survival curves (P for log-rank test < 0.001, Fig. 5B).
Subgroup analysis
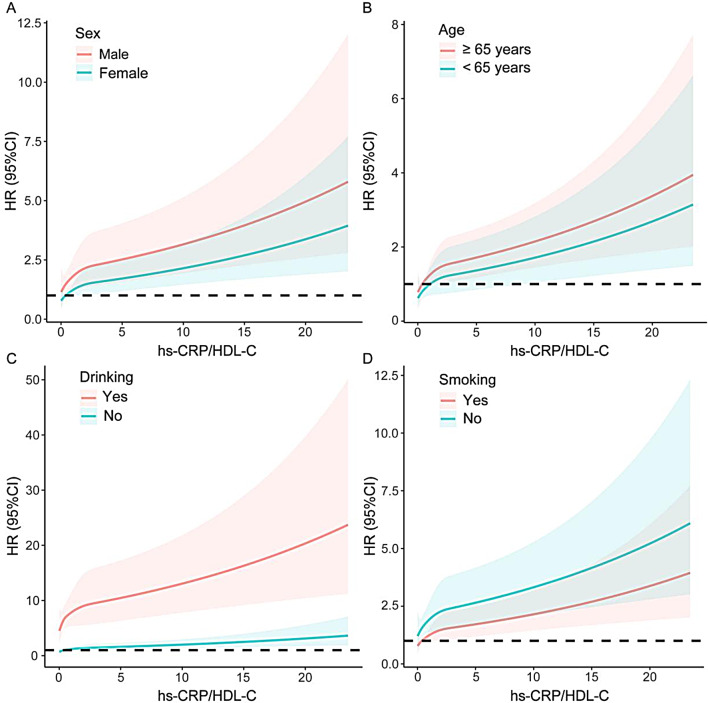
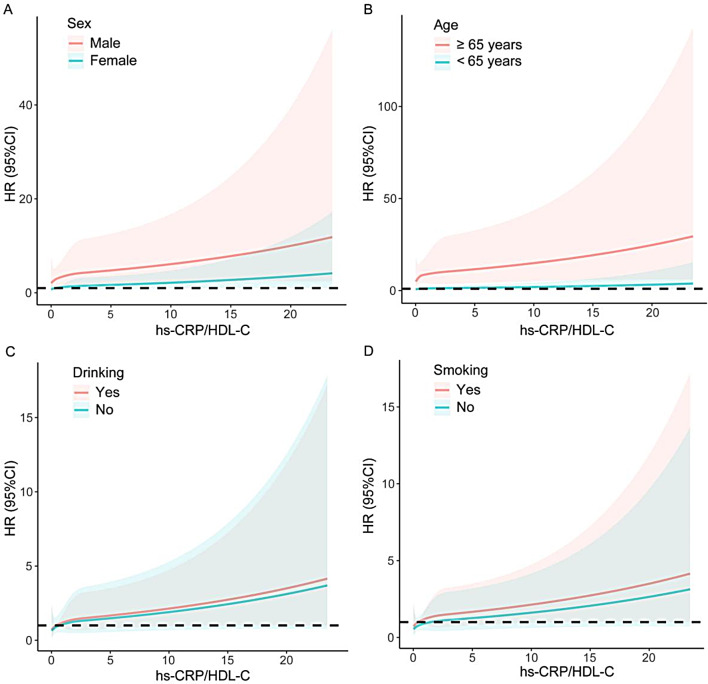
A stratified analysis by sex, age, drinking status and smoking status further examined the associations of the hs-CRP/HDL-C ratio with long-term mortality (Table 5). All the subgroups, except for the nondrinker subgroup, presented a positive association between the hs-CRP/HDL-C ratio and long-term all-cause mortality (all P values < 0.05). A positive association between the hs-CRP/HDL-C ratio and long-term cardiovascular mortality was also observed in all subgroups (all P values < 0.05), with the exception of elderly individuals, females, and drinkers. Additionally, a notable interaction existed between smoking status and long-term all-cause mortality (P for interaction = 0.006), indicating that the association of the hs-CRP/HDL-C ratio with all-cause mortality was more apparent in nonsmokers (P < 0.001). The remaining subgroups did not exhibit significant interactions (all P values for interaction > 0.05). The RCS curve showed in detail how the mortality risk increased with increasing hs-CRP/HDL-C ratios in each subgroup (Figs. 3 and 4).
Table 5.
Subgroup analysis for the association between the hs-CRP/HDL-C ratio and long-term mortality
| Subgroups | All-cause mortality | Cardiovascular mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Hs-CRP/HDL-C ratio HR (95% CI) | P value | P-int | N (%) | Hs-CRP/HDL-C ratio HR (95% CI) |
P value | P-int | |
| Age | 0.247 | 0.635 | ||||||
| < 65 years | 74 (0.97%) | 1.09 (1.04, 1.14) | 0.001 | 14 (0.18%) | 1.10 (1.02, 1.20) | 0.021 | ||
| ≥ 65 years | 165 (8.75%) | 1.08 (1.02, 1.13) | 0.005 | 45 (2.39%) | 1.09 (0.98, 1.21) | 0.111 | ||
| Sex | 0.499 | 0.278 | ||||||
| male | 148 (3.23%) | 1.10 (1.05, 1.15) | < 0.001 | 43 (0.94%) | 1.13 (1.05, 1.21) | 0.001 | ||
| female | 91 (1.85%) | 1.07 (1.01, 1.14) | 0.025 | 16 (0.33%) | 1.03 (0.83, 1.28) | 0.802 | ||
| Drinking | 0.105 | 0.618 | ||||||
| Yes | 81 (1.81%) | 1.14 (1.08, 1.19) | < 0.001 | 21 (0.47%) | 1.11 (0.96, 1.28) | 0.161 | ||
| No | 158 (3.15%) | 1.05 (0.99, 1.11) | 0.079 | 38 (0.76%) | 1.13 (1.05, 1.21) | 0.001 | ||
| Smoking | 0.006 | 0.368 | ||||||
| Yes | 149 (3.98%) | 1.07 (1.02, 1.12) | 0.007 | 30 (0.80%) | 1.10 (1.02, 1.19) | 0.017 | ||
| No | 90 (1.57%) | 1.18 (1.12, 1.25) | < 0.001 | 29 (0.50%) | 1.19 (1.05, 1.34) | 0.006 | ||
HR, hazard ratio; CI, confidence interval; P-int, P for interaction. Adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs except the subgroup variable
Fig. 3.
Subgroup analysis of restricted cubic spline plots for the association between the hs-CRP/HDL-C ratio and long-term all-cause mortality by sex (A), age (B), drinking (C) and smoking (D). Adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs except the subgroup variable
Fig. 4.
Subgroup analysis of restricted cubic spline plots for the association between the hs-CRP/HDL-C ratio and long-term cardiovascular mortality by sex (A), age (B), drinking (C) and smoking (D). Adjusted for age, sex, race, drinking, smoking, BMI, hypertension, diabetes, heart failure, coronary artery disease, stroke and lipid-lowering drugs except the subgroup variable
Fig. 5.
K-M survival analysis for long-term all-cause mortality (hs-CRP/HDL-C ratio ≤ 1.094 vs. > 1.094) (A) and cardiovascular mortality (hs-CRP/HDL-C ratio ≤ 0.154 vs. > 0.154) (B)
Discussion
The innovative significance of this study lies in the first evaluation exploring the predictive value of a composite marker of low-grade inflammation and lipid metabolism, the hs-CRP/HDL-C ratio, in adults the general population. This finding indicated that an elevated hs-CRP/HDL-C ratio was associated with increased long-term mortality, independent of other established risk factors, and the same results were also observed in different subgroups. Compared with that in smokers, the hs-CRP/HDL-C ratio in nonsmokers was more strongly associated with the risk of all-cause mortality. Subsequent analysis revealed a positive linear relationship between the hs-CRP/HDL-C ratio and long-term mortality.
Recently, a growing number of studies have concentrated on potential risk markers of long-term clinical outcomes, particularly composite predictors of haematological parameters, as a single indicator ignores certain residual risk factors, hence decreasing the accuracy of predictions. Several composite inflammatory indicators (e.g., ratios of neutrophils to lymphocytes, platelets to lymphocytes, monocytes to lymphocytes) and composite lipid indices [e.g., ratios of triglycerides (TGs) to HDL-C, low-density lipoprotein cholesterol (LDL-C) to HDL-C, and non-HDL-C to apolipoprotein B] are well-studied predictors of mortality and specific-cause mortality [25–30], but it is clear that they are unable to reflect the interaction between the inflammatory state and lipid metabolism. In fact, both inflammatory reactions and abnormal lipid metabolism are often involved in most pathological processes [5]. Composite markers of inflammatory indicators in routine blood tests and lipid parameters (e.g., ratios of monocyte to HDL-C and lymphocyte to HDL-C) have also been found to be associated with mortality in certain individuals [31, 32]. However, the general applicability of these findings is limited by the fact that leukocyte parameters have a wide range of values, lag behind the inflammatory response, and are affected by underlying diseases, physiological conditions, and drug use [33]. Low-grade inflammation has been proven to be involved in a variety of pathological mechanisms, including atherosclerosis [34]. Consequently, a more sensitive inflammatory indicator with a stronger anti-interference ability is needed to replace leukocytes. Owing to the use of a more sensitive detection method, hs-CRP can be measured to a minimum detection limit of 0.11 mg/L, which is significantly lower than the physiological CRP level of 3 mg/L [35].
However, the clinical application of hs-CRP or HDL-C alone to evaluate long-term outcomes has many limitations. First, there are distinct racial and population differences in hs-CRP, with a significantly higher level in Caucasians [36], making it difficult to set appropriate clinical decision points. Furthermore, not all studies support hs-CRP as an independent predictor of mortality in the general population [37], and conflicting results have been reported in people dying from cancer [37–39], which may be related to the protective effects of endogenous reproductive hormones, such as oestrogen [40]. Moreover, statin therapy can significantly reduce the level of hs-CRP, and this effect is time- and dose-dependent [41, 42]; as a consequence, the use of composite indicators can account for the modification effect of statins on lipoproteins such as HDL-C. In regard to HDL-C, the failure of targeted therapy to increase HDL-C in recent years has raised doubts about its cardiovascular protective effect [20], as it cannot account for cardiovascular residual risk [43, 44]. Recent epidemiological studies have demonstrated that HDL-C is associated with all-cause mortality in a U-shaped pattern [45–47], and similar evidence has also emerged in Mendelian randomization studies [48, 49]. The possible mechanism is that moderate to high concentrations of HDL-C cause activation of the Rho-associated kinase pathway, which paradoxically impairs human endothelial progenitor cells and associated angiogenesis [50]. Second, variants of certain genes, such as CETP, SCARB1, ABCA1, and LIPC, could contribute to elevated HDL-C levels as well as an increased risk of adverse events [51–54]. Furthermore, at high HDL-C concentrations, extremely large HDL particles may cause particles such as LDL to be trapped in the arterial intima, which accelerates the development of atherosclerosis [47]. The U-shaped association of HDL-C with all-cause mortality may partly explain the novel finding in this study. When the hs-CRP/HDL-C ratio is less than 1.21, the risk of all-cause mortality increases more prominently and then gradually stabilizes because HDL-C, the denominator of the ratio, is positively correlated with the risk of all-cause mortality above a certain threshold.
Importantly, the ability of the hs-CRP/HDL-C ratio to predict long-term outcomes has not been fully explored. Similar studies have been restricted to specific areas and populations and have only examined associations with new CVDs. For example, in a 12-year prospective cohort study of 4,128 adults aged 19 to 96 years from Yixing city, China, negative effects of abnormal blood lipid levels and hs-CRP on the risk of CVDs were identified [1]. Similarly, a study utilizing data from the China Health and Retirement Longitudinal Study (CHARLS) revealed that a high hs-CRP/HDL-C ratio was a significant risk factor for CVDs [2]. These studies provide a factual basis for our conclusions.
There are complex interactions between inflammation and lipid metabolism during the progression of diseases. In addition to its anti-inflammatory ability, HDL-C has antioxidative, endothelial/vasodilatory, antithrombotic, and cytoprotective effects, as well as the ability to reverse cholesterol transport [55]. Specifically, the anti-inflammatory ability of HDL-C inhibits the oxidative modification of LDL-C by lowering the expression of adhesion molecules on the surface of endothelial cells [56]. HDL-C inhibits the adhesion of T lymphocytes and monocytes to the vascular endothelium and their migration to atherosclerotic regions [57, 58]. In contrast, inflammation causes a reduction in HDL-C levels and changes in HDL-C structure, as well as notable modifications in HDL-C-related proteins, enzymes, and transfer proteins involved in HDL-C metabolism and function [59]. However, the specific mechanism of the interaction between hs-CRP and HDL-C remains to be studied with exact methodology.
At present, several composite indicators that combine hs-CRP with other lipid markers, such as triglyceride-glucose (TyG) [60] and remnant lipoprotein cholesterol [61], exist in clinical practice, but they have many limitations. Primarily, there are insufficient studies to support the reliability of these indicators, and their predictive ability in the whole population is lacking. In addition, these studies were limited to certain areas and populations and were related only to CVDs. Furthermore, the determination of TyG requires fasting, whereas HDL-C is independent of TGs variability and can be reliably measured regardless of fasting status [62, 63]. Moreover, remnant cholesterol is an estimate that relies on accurate measurements of LDL-C, but LDL-C levels are also inaccurate, with TGs concentrations greater than 800 mg/L, regardless of the methods used [64].
In the future, cardiovascular-related inflammasomes [65] and more precise methods for measuring lipid profiles [66, 67] (e.g., nuclear magnetic resonance spectroscopy, vertical autoprofile and ion mobility analysis) will be a focus of interest. Although these measurements are expensive and require special equipment and strict storage and transport procedures, it is exciting that these methods are expected to be used to accurately predict the risks of long-term adverse events.
The current study has several key aspects and notable strengths. First, it is based on the NHANES database, which is characterized by its extensive sample size, comprehensive coverage of various demographic groups in the United States and reliable data quality. In addition, this retrospective cohort study offers substantial evidence regarding the ability of the hs-CRP/HDL-C ratio to predict long-term all-cause and cardiovascular mortality in the general population. No comparable research has been conducted previously. Finally, we initially employed RCS models to visualize the correlation between the hs-CRP/HDL-C ratio and the long-term risk of mortality.
Nevertheless, it is imperative to recognize that our study has certain limitations. Our conclusions are drawn primarily from individuals who reside in the United States, so surveys in different countries and regions are warranted to determine the global applicability of these findings. Additionally, data on hs-CRP in the NHANES are missing before 2015 and after 2018. This is because with the innovation of detection methods, hs-CRP replaced CRP as a common inflammatory indicator after 2015. Currently, the mortality data linked to the NHANES are updated only to 2018. Consequently, more databases can serve as external validation sets for this study. The assessment of hs-CRP, HDL-C and other covariates was limited to the baseline, thereby discounting possible changes that might have occurred during the follow-up period. There is also the possibility that some participants may have elevated hs-CRP levels due to an acute inflammation state at baseline. The long-term levels among adults may not be accurately represented by the ratio. Moreover, constraints regarding comprehensive monitoring and analysis of physical activity and medication adherence made it difficult to exclude the possibility that medication and physical activity influence long-term outcomes. Finally, the covariates pertaining to the medical history data were collected via self-reports, which inevitably introduced recall bias. Above all, although the hs-CRP/HDL-C ratio has good predictive power, its wide clinical application still needs to be verified by more external data and prospective studies.
Conclusions
In summary, our study demonstrated that the hs-CRP/HDL-C ratio is closely associated with long-term mortality in the general population, independent of potential confounding factors. These findings indicate that it may serve as a prospective predictor of long-term clinical outcomes. Owing to the limited study population, more cohort studies with larger sample sizes are needed to generalize our conclusions and provide reliable risk stratification for populations of different races/ethnicities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express gratitude to the researchers and participants of the NHANES study for their invaluable contributions.
Abbreviations
- BMI
Body mass index
- CAD
Coronary artery disease
- CHARLS
China Health and Retirement Longitudinal Study
- CI
Confidence interval
- CVDs
Cardiovascular diseases
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard ratio
- Hs-CRP
High-sensitivity C-reactive protein
- ICD
International Classification of Diseases
- IQR
Interquartile range
- K-M
Kaplan-Meier
- LDL-C
Low-density lipoprotein cholesterol
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- RCS
Restricted cubic spline
- TGs
Triglycerides
- TyG
Triglyceride-glucose
Author contributions
KZ designed the study and conceived the paper. YW performed statistical analysis and drafted the manuscript. LW and KZ was responsible for methodology and investigation. ZZ and SY arranged the data and performed visualization. XT contributed to validation. YW funded the study. KZ critically revised the manuscript, and all authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, 82103925) and Suzhou Key Laboratory of Cardiovascular Disease (SZS2024015).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bafei SEC, Zhao X, Chen C, Sun J, Zhuang Q, Lu X, et al. Interactive effect of increased high sensitive C-reactive protein and dyslipidemia on cardiovascular diseases: a 12-year prospective cohort study. Lipids Health Dis. 2023;22(1):95. 10.1186/s12944-023-01836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao Y, Wang M, Wang R, Jiang J, Hu Y, Wang W, et al. The predictive value of the hs-CRP/HDL-C ratio, an inflammation-lipid composite marker, for cardiovascular disease in middle-aged and elderly people: evidence from a large national cohort study. Lipids Health Dis. 2024;23(1):66. 10.1186/s12944-024-02055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 2020;141(9):e139–596. 10.1161/cir.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcena ML, Aslam M, Pozdniakova S, Norman K, Ladilov Y. Cardiovascular Inflammaging: mechanisms and translational aspects. Cells. 2022;11(6). 10.3390/cells11061010. [DOI] [PMC free article] [PubMed]
- 7.Banait T, Wanjari A, Danade V, Banait S, Jain J. Role of high-sensitivity C-reactive protein (Hs-CRP) in non-communicable diseases: a review. Cureus. 2022;14(10):e30225. 10.7759/cureus.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108(16):1930–2. 10.1161/01.Cir.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 9.Badimon L, Peña E, Arderiu G, Padró T, Slevin M, Vilahur G, et al. C-Reactive protein in Atherothrombosis and angiogenesis. Front Immunol. 2018;9:430. 10.3389/fimmu.2018.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Wang X, Zhang L, Chen Z, Zheng C, Wang J, et al. High-sensitivity C reactive protein and risk of cardiovascular disease in China-CVD study. J Epidemiol Community Health. 2019;73(2):188–92. 10.1136/jech-2018-211433. [DOI] [PubMed] [Google Scholar]
- 11.Koosha P, Roohafza H, Sarrafzadegan N, Vakhshoori M, Talaei M, Sheikhbahaei E, et al. High sensitivity C-Reactive protein Predictive Value for Cardiovascular Disease: a nested Case Control from Isfahan Cohort Study (ICS). Glob Heart. 2020;15(1):3. 10.5334/gh.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quispe R, Michos ED, Martin SS, Puri R, Toth PP, Al Suwaidi J, et al. High-sensitivity C-Reactive protein discordance with atherogenic lipid measures and incidence of atherosclerotic Cardiovascular Disease in Primary Prevention: the ARIC Study. J Am Heart Assoc. 2020;9(3):e013600. 10.1161/jaha.119.013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mengozzi M, Kirkham FA, Girdwood EER, Bunting E, Drazich E, Timeyin J, et al. C-Reactive protein predicts further ischemic events in patients with transient ischemic attack or Lacunar Stroke. Front Immunol. 2020;11:1403. 10.3389/fimmu.2020.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biasucci LM, CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease. Application to clinical and Public Health Practice: clinical use of inflammatory markers in patients with cardiovascular diseases: a background paper. Circulation. 2004;110(25):e560–567. 10.1161/01.Cir.0000148983.88334.80. [DOI] [PubMed] [Google Scholar]
- 15.Assmann G, Gotto AM. Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004; 109(23 Suppl 1): Iii8-14. 10.1161/01.Cir.0000131512.50667.46 [DOI] [PubMed]
- 16.Yang Y, Han K, Park SH, Kim MK, Yoon KH, Lee SH. High-density lipoprotein cholesterol and the risk of myocardial infarction, stroke, and cause-specific mortality: a Nationwide Cohort Study in Korea. J Lipid Atheroscler. 2021;10(1):74–87. 10.12997/jla.2021.10.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan SD, Schold JD, Walther CP, Arrigain S, Jolly SE, Virani SS, et al. High-density lipoprotein cholesterol and causes of death in chronic kidney disease. J Clin Lipidol. 2018;12(4):1061–e10711067. 10.1016/j.jacl.2018.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levinson SS. Brief review and critical examination of the use of hs-CRP for cardiac risk assessment with the conclusion that it is premature to use this test. Clin Chim Acta. 2005;356(1–2):1–8. 10.1016/j.cccn.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62(5):397–408. 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Schoch L, Alcover S, Padró T, Ben-Aicha S, Mendieta G, Badimon L, et al. Update of HDL in atherosclerotic cardiovascular disease. Clin Investig Arterioscler. 2023;35(6):297–314. 10.1016/j.arteri.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Tang L, Peng H, Xu T, Wang A, Wang G, Tong W, et al. Association of biomarkers of inflammation with dyslipidemia and its components among mongolians in China. PLoS ONE. 2014;9(2):e89023. 10.1371/journal.pone.0089023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin D, Zhu DM, Hu HL, Yao MN, Yin WJ, Tao RX, et al. Vitamin D status affects the relationship between lipid profile and high-sensitivity C-reactive protein. Nutr Metab (Lond). 2020;17:57. 10.1186/s12986-020-00455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noble N, Paul C, Turon H, Oldmeadow C. Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and physical activity (‘SNAP’) health risk factors. Prev Med. 2015;81:16–41. 10.1016/j.ypmed.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation procedures. Vital Health Stat 2. 2020;50(184): 1–35. [PubMed]
- 25.Fest J, Ruiter TR, Groot Koerkamp B, Rizopoulos D, Ikram MA, van Eijck CHJ, et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: the Rotterdam Study. Eur J Epidemiol. 2019;34(5):463–70. 10.1007/s10654-018-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang F, Chen R, Cao X, Shen B, Liu Z, Tan X, et al. Monocyte/lymphocyte ratio as a better predictor of cardiovascular and all-cause mortality in hemodialysis patients: a prospective cohort study. Hemodial Int. 2018;22(1):82–92. 10.1111/hdi.12549. [DOI] [PubMed] [Google Scholar]
- 27.Lin T, Xia X, Yu J, Qiu Y, Yi C, Lin J, et al. The predictive study of the relation between elevated low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and mortality in peritoneal dialysis. Lipids Health Dis. 2020;19(1):51. 10.1186/s12944-020-01240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, et al. Association of Serum Triglyceride to HDL cholesterol ratio with all-cause and Cardiovascular Mortality in Incident Hemodialysis patients. Clin J Am Soc Nephrol. 2017;12(4):591–602. 10.2215/cjn.08730816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong G, Huang A, Liu L. Platelet-to-lymphocyte ratio and prognosis in STEMI: a meta-analysis. Eur J Clin Invest. 2021;51(3):e13386. 10.1111/eci.13386. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Wei C, Shao Y, Wang L, Zhao Z, Yin S, et al. Association of non-HDL-C/apoB ratio with long-term mortality in the general population: a cohort study. Heliyon. 2024;10(6):e28155. 10.1016/j.heliyon.2024.e28155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Zhong Z, Shi D, Li J, Li B, Zhang R, et al. Association between monocyte count to high-density lipoprotein cholesterol ratio and mortality in patients undergoing peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2021;31(7):2081–8. 10.1016/j.numecd.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Xiong C, Shao X, Ning J, Gao P, Xiao H, et al. Lymphocyte to high-density lipoprotein ratio as a New Indicator of inflammation and metabolic syndrome. Diabetes Metab Syndr Obes. 2019;12:2117–23. 10.2147/dmso.S219363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–88. 10.4149/bll_2021_078. [DOI] [PubMed] [Google Scholar]
- 34.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–26. 10.1056/nejm199901143400207. [DOI] [PubMed] [Google Scholar]
- 35.Moutachakkir M, Lamrani Hanchi A, Baraou A, Boukhira A, Chellak S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann Biol Clin (Paris). 2017;75(2):225–9. 10.1684/abc.2017.1232. [DOI] [PubMed] [Google Scholar]
- 36.Anand SS, Razak F, Yi Q, Davis B, Jacobs R, Vuksan V, et al. C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol. 2004;24(8):1509–15. 10.1161/01.ATV.0000135845.95890.4e. [DOI] [PubMed] [Google Scholar]
- 37.Nisa H, Hirata A, Kohno M, Kiyohara C, Ohnaka K, High-Sensitivity C-R. Protein and risks of all-cause and cause-specific mortality in a Japanese Population. Asian Pac J Cancer Prev. 2016;17(5):2643–8. [PubMed] [Google Scholar]
- 38.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217–24. 10.1200/jco.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 39.Morrison L, Laukkanen JA, Ronkainen K, Kurl S, Kauhanen J, Toriola AT. Inflammatory biomarker score and cancer: a population-based prospective cohort study. BMC Cancer. 2016;16:80. 10.1186/s12885-016-2115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423–31. 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 42.Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, et al. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106(12):1447–52. 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 43.Varbo A, Nordestgaard BG. Remnant lipoproteins. Curr Opin Lipidol. 2017;28(4):300–7. 10.1097/mol.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 44.Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. 2016;62(4):593–604. 10.1373/clinchem.2015.253757. [DOI] [PubMed] [Google Scholar]
- 45.Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al-Aly Z. High density lipoprotein cholesterol and the risk of all-cause mortality among U.S. Veterans. Clin J Am Soc Nephrol. 2016;11(10):1784–93. 10.2215/cjn.00730116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CL, Liu XC, Liu L, Lo K, Yu YL, Huang JY, et al. U-Shaped Association of High-Density Lipoprotein Cholesterol with all-cause and Cardiovascular Mortality in Hypertensive Population. Risk Manag Healthc Policy. 2020;13:2013–25. 10.2147/rmhp.S272624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li ZH, Lv YB, Zhong WF, Gao X, Byers Kraus V, Zou MC, et al. High-density lipoprotein cholesterol and all-cause and cause-Specific Mortality among the Elderly. J Clin Endocrinol Metab. 2019;104(8):3370–8. 10.1210/jc.2018-02511. [DOI] [PubMed] [Google Scholar]
- 48.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. 10.1016/s0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen JX, Li Y, Zhang YB, Wang Y, Zhou YF, Geng T, et al. Nonlinear relationship between high-density lipoprotein cholesterol and cardiovascular disease: an observational and mendelian randomization analysis. Metabolism. 2024;154:155817. 10.1016/j.metabol.2024.155817. [DOI] [PubMed] [Google Scholar]
- 50.Huang CY, Lin FY, Shih CM, Au HK, Chang YJ, Nakagami H, et al. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating rho-associated kinase pathways. Arterioscler Thromb Vasc Biol. 2012;32(10):2405–17. 10.1161/atvbaha.112.248617. [DOI] [PubMed] [Google Scholar]
- 51.Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–86. 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 52.Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010;208(2):305–16. 10.1016/j.atherosclerosis.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Genga KR, Trinder M, Kong HJ, Li X, Leung AKK, Shimada T, et al. CETP genetic variant rs1800777 (allele A) is associated with abnormally low HDL-C levels and increased risk of AKI during sepsis. Sci Rep. 2018;8(1):16764. 10.1038/s41598-018-35261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351(6278):1166–71. 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kosmas CE, Martinez I, Sourlas A, Bouza KV, Campos FN, Torres V, et al. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context. 2018;7:212525. 10.7573/dic.212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lampka M, Olszewska-Słonina D, Hołyńska-Iwan I, Grąbczewska Z, Obońska K, Cwynar A, et al. Effect of low high-density lipoprotein level on endothelial activation and prothrombotic processes in coronary artery Disease-A Pilot Study. Int J Environ Res Public Health. 2022;19(14). 10.3390/ijerph19148637. [DOI] [PMC free article] [PubMed]
- 57.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(9):2532–44. 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomaraschi M, Calabresi L, Franceschini G. Protective effects of HDL Against Ischemia/Reperfusion Injury. Front Pharmacol. 2016;7:2. 10.3389/fphar.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–16. 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng G, Yang M, Xu L, Liu Y, Yu J, Zang Y, et al. Combined effects of high sensitivity C-reactive protein and triglyceride-glucose index on risk of cardiovascular disease among middle-aged and older Chinese: evidence from the China Health and Retirement Longitudinal Study. Nutr Metab Cardiovasc Dis. 2023;33(6):1245–53. 10.1016/j.numecd.2023.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Chevli PA, Islam T, Pokharel Y, Rodriguez F, Virani SS, Blaha MJ, et al. Association between remnant lipoprotein cholesterol, high-sensitivity C-reactive protein, and risk of atherosclerotic cardiovascular disease events in the multi-ethnic study of atherosclerosis (MESA). J Clin Lipidol. 2022;16(6):870–7. 10.1016/j.jacl.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Langlois MR, Nordestgaard BG, Langsted A, Chapman MJ, Aakre KM, Baum H, et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Clin Chem Lab Med. 2020;58(4):496–517. 10.1515/cclm-2019-1253. [DOI] [PubMed] [Google Scholar]
- 63.Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944–58. 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson PWF, Jacobson TA, Martin SS, Jackson EJ, Le NA, Davidson MH, et al. Lipid measurements in the management of cardiovascular diseases: practical recommendations a scientific statement from the national lipid association writing group. J Clin Lipidol. 2021;15(5):629–48. 10.1016/j.jacl.2021.09.046. [DOI] [PubMed] [Google Scholar]
- 65.Olsen MB, Gregersen I, Sandanger Ø, Yang K, Sokolova M, Halvorsen BE, et al. Targeting the Inflammasome in Cardiovascular Disease. JACC Basic Transl Sci. 2022;7(1):84–98. 10.1016/j.jacbts.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Vosegaard T, Guo Z. Applications of nuclear magnetic resonance in lipid analyses: an emerging powerful tool for lipidomics studies. Prog Lipid Res. 2017;68:37–56. 10.1016/j.plipres.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Kanonidou C. Small dense low-density lipoprotein: Analytical review. Clin Chim Acta. 2021;520:172–8. 10.1016/j.cca.2021.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.