Abstract
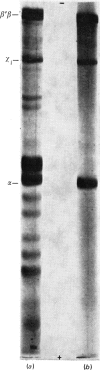
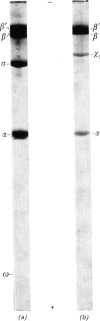
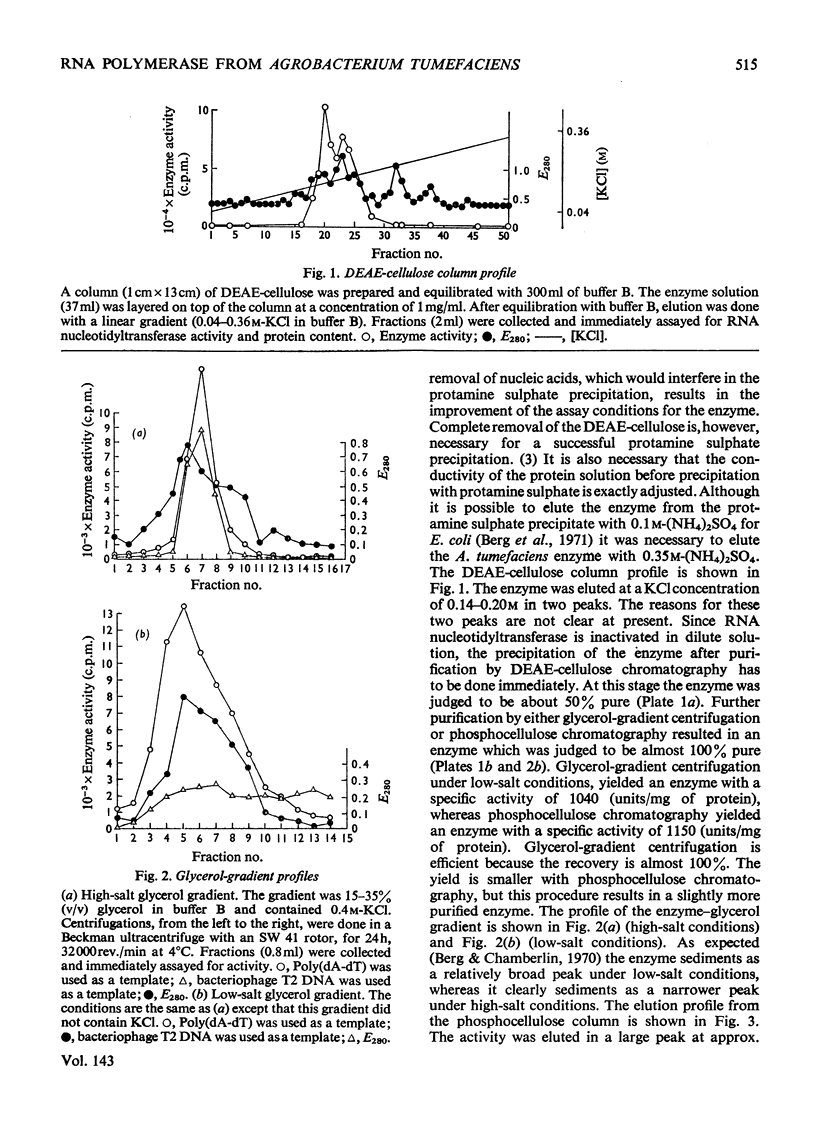
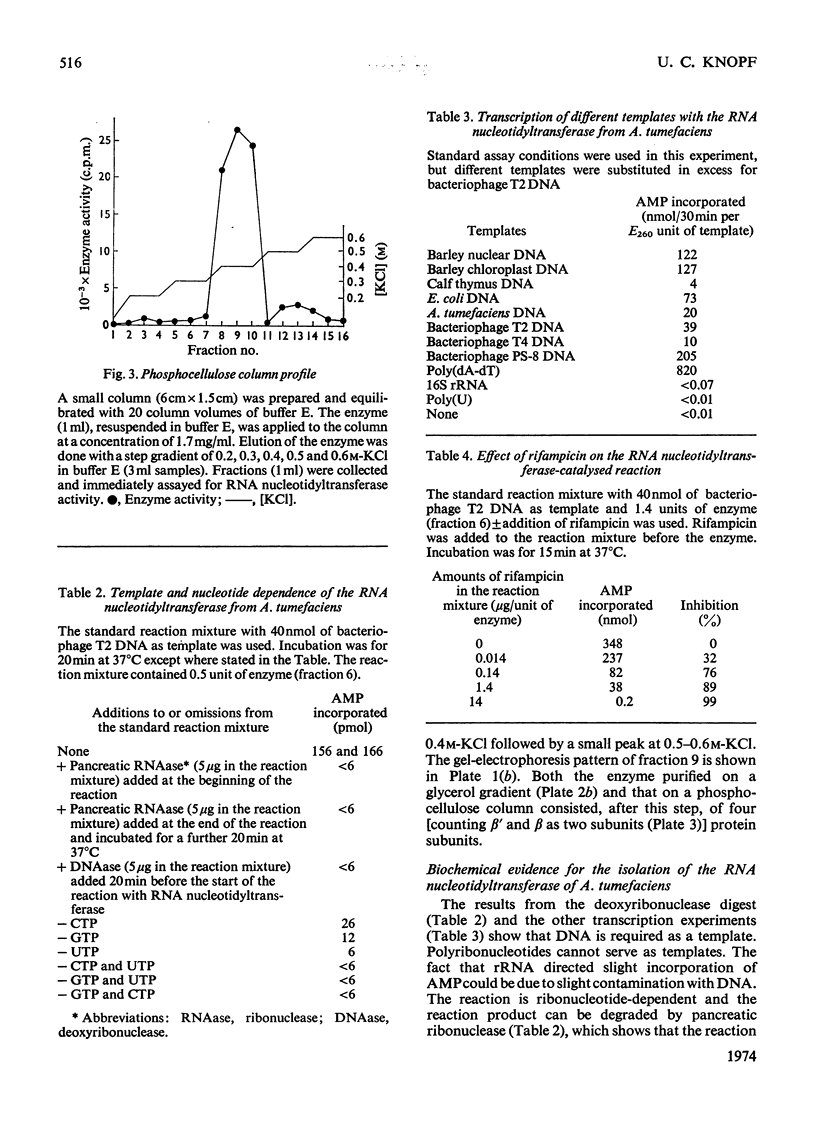
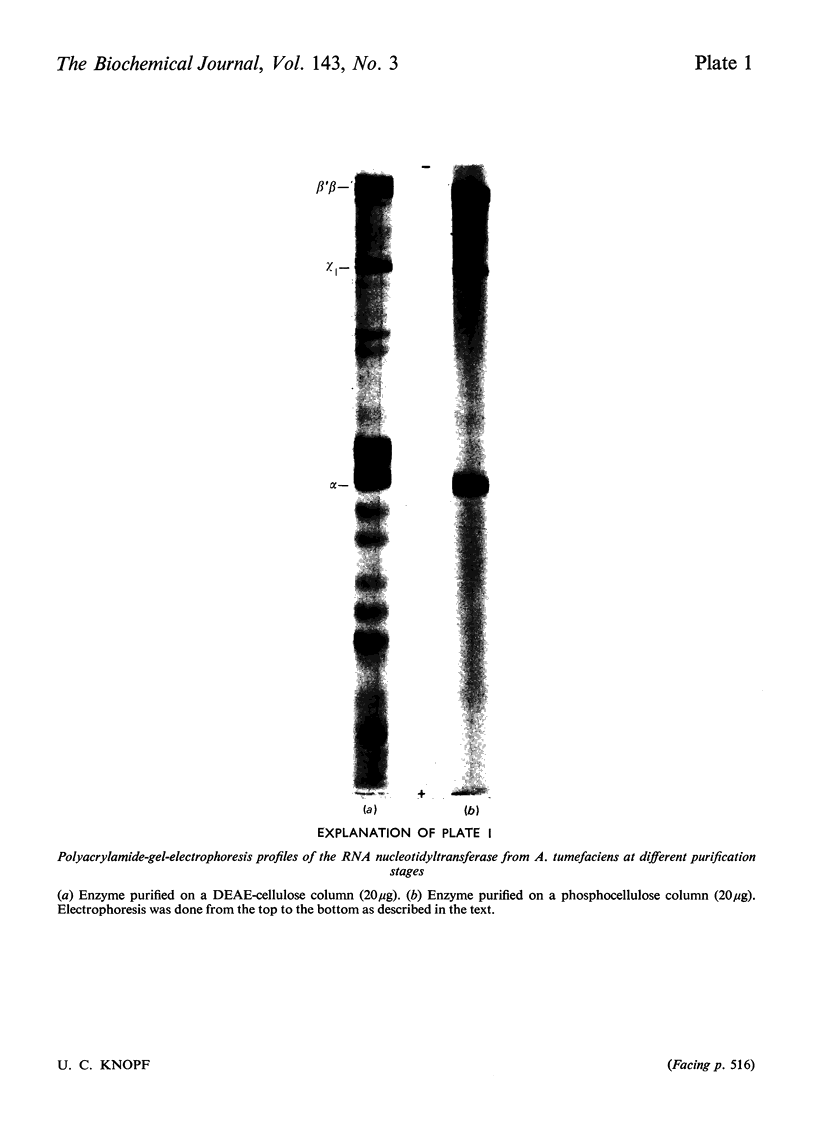
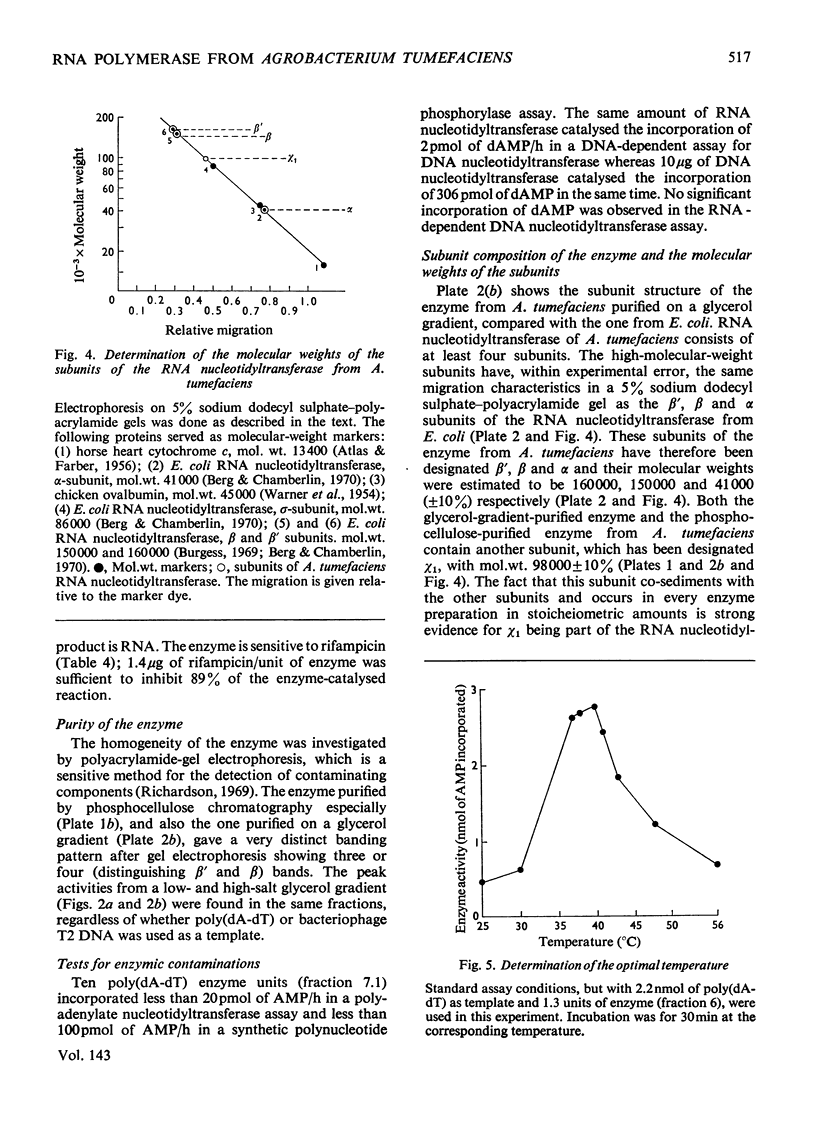
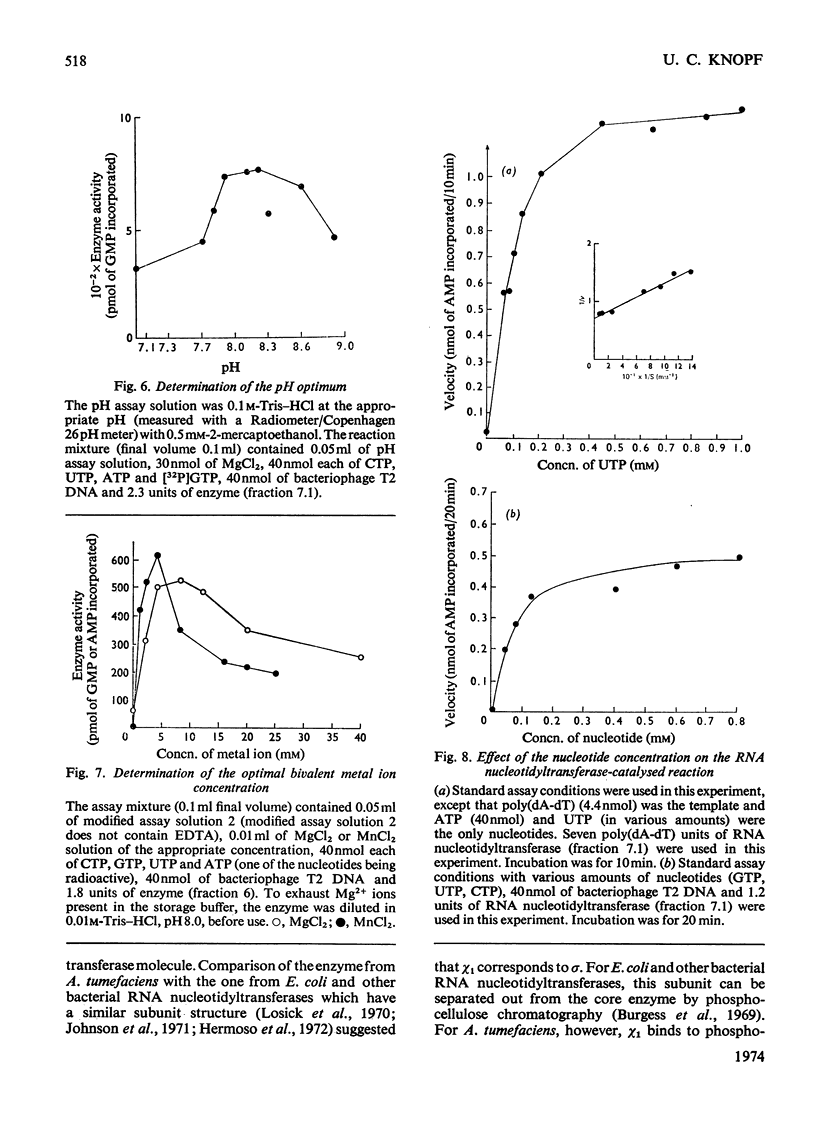
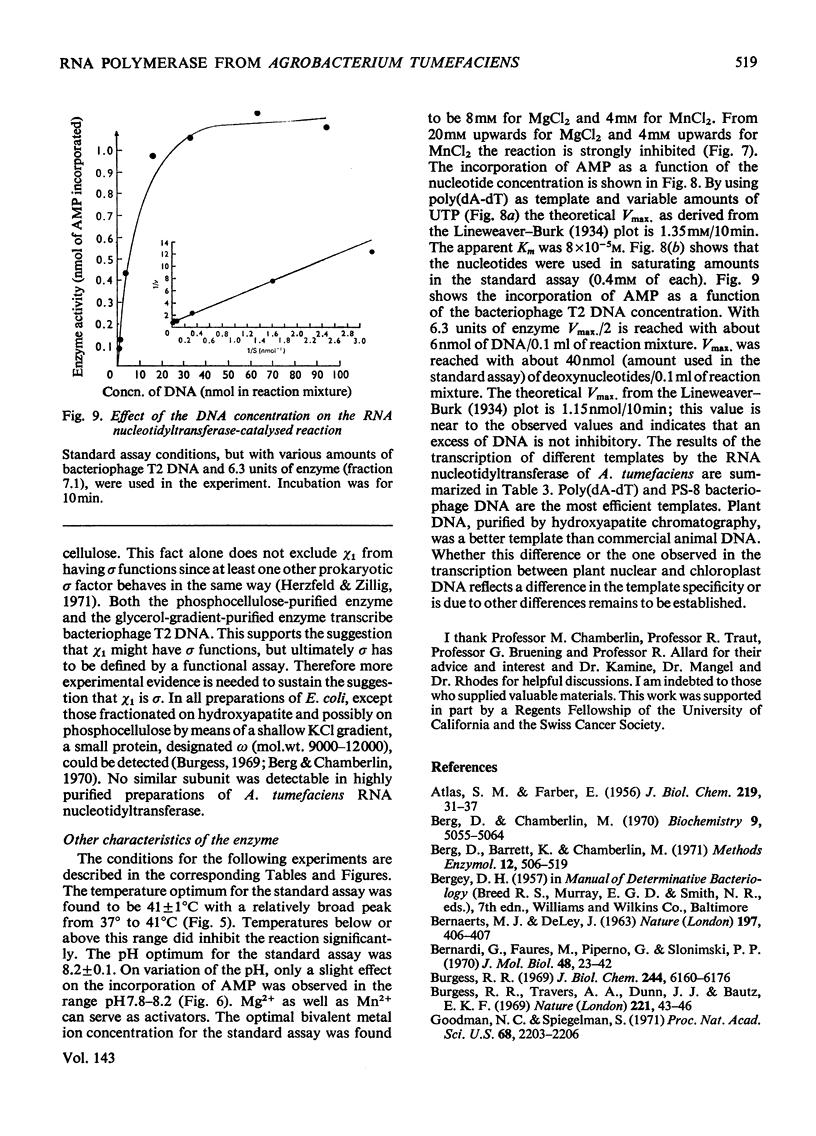
The RNA nucleotidyltransferase (RNA polymerase) of the plant-tumorigenic bacterium Agrobacterium tumefaciens was purified. The method involves the disruption of the bacterial cells with glass beads in a Waring Blendor, treatment with DEAE-cellulose, fractionation with (NH4)2SO4, protamine sulphate precipitation, DEAE-cellulose column chromatography and either glycerol-gradient centrifugation or phosphocellulose chromatography. The subunit structure of the highly purified enzyme is similar to, although not identical with, the RNA nucleotidyltransferase of Escherichia coli. It can be described as β′, β, χ1 and α (mol.wts. 160000, 150000, 98000, and 41000±10% respectively). χ1 is the temporary designation for a protein subunit, which might have the same functions as the σ subunit in E. coli. The enzyme of A. tumefaciens is rifampicin-sensitive, has a temperature optimum in vitro of 41±1°C and a pH optimum of 8.2±0.1. Mg2+ and Mn2+ are activators. The enzyme transcribes with different efficiencies artificial, viral, bacterial, plant and animal templates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATLAS S. M., FARBER E. On the molecular weight of cytochrome c from mammalian heart muscle. J Biol Chem. 1956 Mar;219(1):31–37. [PubMed] [Google Scholar]
- Berg D., Chamberlin M. Physical studies on ribonucleic acid polymerase from Escherichia coli B. Biochemistry. 1970 Dec 22;9(26):5055–5064. doi: 10.1021/bi00828a003. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Faures M., Piperno G., Slonimski P. P. Mitochondrial DNA's from respiratory-sufficient and cytoplasmic respiratory-deficient mutant yeast. J Mol Biol. 1970 Feb 28;48(1):23–42. doi: 10.1016/0022-2836(70)90216-0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Avila J., Jiménez F., Salas M. RNA polymerase from Bacillus amyloliquefaciens. Biochim Biophys Acta. 1972 Aug 25;277(2):280–283. doi: 10.1016/0005-2787(72)90409-1. [DOI] [PubMed] [Google Scholar]
- Herzfeld F., Zillig W. Subunit composition of DNA-dependent RNA polymerase of Anacystis nidulans. Eur J Biochem. 1971 Dec;24(2):242–248. doi: 10.1111/j.1432-1033.1971.tb19676.x. [DOI] [PubMed] [Google Scholar]
- Johnson J. C., DeBacker M., Boezi J. A. Deoxyribonucleic acid-dependent ribonucleic acid polymerase of Pseudomonas putida. J Biol Chem. 1971 Mar 10;246(5):1222–1232. [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Srivastava B. I. RNA-DNA hybridization studies with the crown gall bacteria and the tobacco tumor tissue. Biochem Biophys Res Commun. 1969 Jan 27;34(2):196–199. doi: 10.1016/0006-291x(69)90631-7. [DOI] [PubMed] [Google Scholar]
- Quétier F., Huguet T., Guillé E. Induction of Crown-gall: partial homology between tumor-cell DNA, bacterial DNA and the G+C--rich DNA of stressed normal cells. Biochem Biophys Res Commun. 1969 Jan 6;34(1):128–133. doi: 10.1016/0006-291x(69)90538-5. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol. 1969 Mar;97(3):1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. RNA polymerase and the control of RNA synthesis. Prog Nucleic Acid Res Mol Biol. 1969;9:75–116. doi: 10.1016/s0079-6603(08)60768-0. [DOI] [PubMed] [Google Scholar]
- SCHACHMAN H. K., ADLER J., RADDING C. M., LEHMAN I. R., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VII. Synthesis of a polymer of deoxyadenylate and deoxythymidylate. J Biol Chem. 1960 Nov;235:3242–3249. [PubMed] [Google Scholar]
- Schilperoort R. A., Van Sittert N. J., Schell J. The presence of both phage PS8 and Agrobacterium tumefaciens A 6 DNA base sequences in A 6 -induced sterile crown-gall tissue cultured in vitro. Eur J Biochem. 1973 Feb 15;33(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Veldstra H., Warnaar S. O., Mulder G., Cohen J. A. Formation of complexes between DNA isolated from tobacco crown gall tumours and RNA complementary to Agrobacterium tumefaciens DNA. Biochim Biophys Acta. 1967 Sep 26;145(2):523–525. doi: 10.1016/0005-2787(67)90075-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith E. F., Townsend C. O. A PLANT-TUMOR OF BACTERIAL ORIGIN. Science. 1907 Apr 26;25(643):671–673. doi: 10.1126/science.25.643.671. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Chadha K. C. Liberation of Agrobacterium tumefaciens DNA from the crown gall tumor cell DNA by shearing. Biochem Biophys Res Commun. 1970 Aug 24;40(4):968–972. doi: 10.1016/0006-291x(70)90998-8. [DOI] [PubMed] [Google Scholar]
- Stroun M., Anker P. Bacterial nucleic acid synthesis in plants following bacterial contact. Mol Gen Genet. 1971;113(1):92–98. doi: 10.1007/BF00335008. [DOI] [PubMed] [Google Scholar]
- Stroun M. On the nature of the polymerase responsible for the transcription of released bacterial DNA in plant cells. Biochem Biophys Res Commun. 1971 Aug 6;44(3):571–578. doi: 10.1016/s0006-291x(71)80121-3. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Hegeman G. D. Tumor induction by Agrobacterium tumefaciens: specific transfer of bacterial deoxyribonucleic acid to plant tissue. J Bacteriol. 1971 Dec;108(3):973–979. doi: 10.1128/jb.108.3.973-979.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]