Abstract
Background
Veno-arterial (V-A) extracorporeal membrane oxygenation (ECMO) is commonly used for patients with cardiac arrest, cardiogenic shock, or heart failure and is a life-saving technique. Computed tomography angiography (CTA) examination in patients on ECMO presents certain challenges. Due to the dual circulation characteristics of blood flow in ECMO patients, vascular imaging and interpretation can be difficult and may even present pitfalls.
Case presentation
A 59-year-old male was admitted with a diagnosis of cardiogenic shock due to “sudden onset of chest discomfort for 6 hours and altered mental status for 4 hours”. He underwent V-A ECMO treatment twice and had two aortic CTA examinations. The initial CTA mistakenly diagnosed an aortic dissection. Considering the dual circulation blood flow characteristic in ECMO patients, a second CTA was performed. Combined with echocardiography, the patient was accurately diagnosed with left ventricular rupture and underwent left ventricular rupture repair surgery. The patient was successfully weaned off ECMO, transferred out of the ICU, and eventually discharged in good condition.
Conclusion
The unique hemodynamics of V-A ECMO patients necessitate interpreting CTA examinations with an understanding of the dual circulation characteristic to avoid misdiagnosis.
Keywords: Extracorporeal membrane oxygenation, Computed tomography angiography, Watershed, Cardiac rupture, Pitfalls
Introduction
V-A ECMO is an extracorporeal life support technique commonly used in patients with heart and lung failure [1, 2]. Contraindications for ECMO include severe aortic regurgitation and aortic dissection [3, 4]. Echocardiography is the most common examination used to diagnose aortic dissection and aortic regurgitation; however, it also has certain limitations [5], and aortic CTA is the gold standard for diagnosing aortic dissection [6]. The dual circulation hemodynamics of V-A ECMO patients differ significantly from those of normal patients, posing substantial diagnostic and therapeutic challenges, including potential pitfalls [7]. We reviewed the literature on CTA examinations in ECMO patients and obtained informed consent from the patient’s family to report a case initially suspected of aortic dissection, but ultimately diagnosed as left ventricular rupture, to analyze the characteristics and pitfalls of aortic CTA in V-A ECMO patients.
Case report
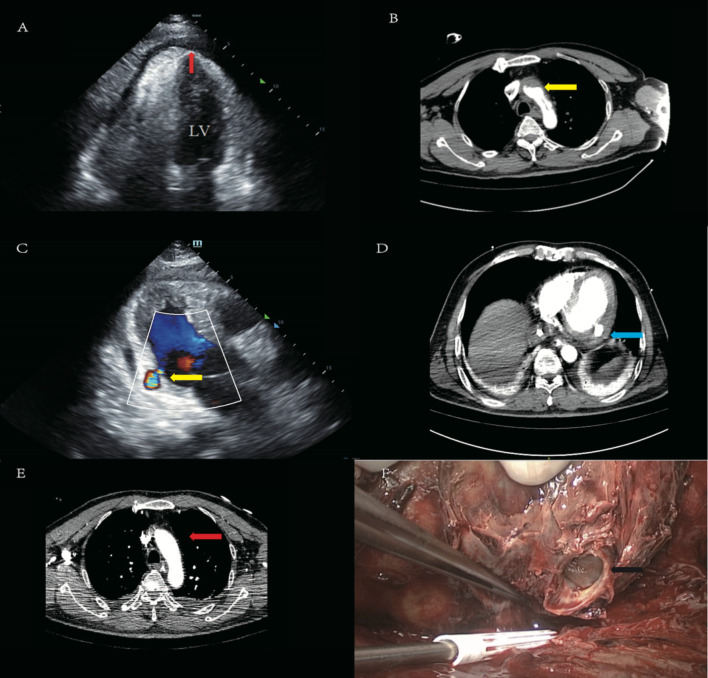
The patient is a 59-year-old male worker who was admitted due to “sudden onset of chest discomfort for 6 hours and altered mental status for 4 hours.” The patient had a history of hypertension and type 2 diabetes. The patient was taking oral antihypertensive medications and using insulin for glycemic control; however, his blood pressure and blood sugar levels remained poorly controlled. There is no family history of hereditary diseases. At the time of his arrival at the emergency department, his blood pressure was 80/40 mmHg (norepinephrine at 4 µg/kg/min)and SPO2 was not detectable. He was intubated and mechanically ventilated due to unexplained shock and admitted to the ICU. Upon initial assessment, the patient had a temperature of 35.2 °C, a heart rate of 108 beats per minute, a breathing rate of 15 breaths per minute, and a blood pressure of 95/76 mmHg (norepinephrine at 4 µg/kg/min). The patient presented with altered mental status, equal bilateral pupils with delayed light reflexes, coarse breath sounds in both lungs, regular heart rhythm with diminished heart sounds, and cool skin temperature in the extremities. Troponin-I was greater than 100 ng/mL, CK-MB was 34.2 ng/mL, and the ECG indicated sinus tachycardia with ST segment depression in leads V1-6, II, III, and aVF. Emergency ultrasound indicated a small amount of pericardial effusion and an EF of 40%, with no pleural effusion, leading to a diagnosis of cardiogenic shock and initiation of V-A ECMO (21 F/45 cm femoral venous cannulation: 96370-023; Medtronic, Inc. Minneapolis, MN, USA, and 17 F/20 cm femoral arterial cannulation: 96570-015; Medtronic, MN, USA) therapy with parameters of 3256 RPM, a blood flow rate of 3.15 L/min, and an oxygen flow rate of 3.0 L/min. A subsequent echocardiogram revealed left ventricular wall thickening, reduced left chamber size, decreased left ventricular function with an EF of 40%, and increased echogenicity in the pericardial cavity (possibly indicating a blood clot), along with increased pericardial effusion (Fig. 1A), raising suspicion of an aortic dissection, and an emergency aortic CTA was performed (Fig. 1B), suggesting aortic dissection with associated pericardial tamponade. Pericardial effusion was drained, and the patient was not immediately operated on for aortic dissection. As the patient’s hemodynamics gradually stabilized, a repeat aortic CTA revealed no aortic dissection. A follow-up echocardiogram indicated an EF of 58% and a reduction in pericardial effusion. The ECMO rotation speed was reduced, and the patient’s hemodynamics remained stable, and ECMO was weaned on the 7th day of admission. The day after ECMO weaning, the patient’s hemodynamics destabilized again with a heart rate of 48 bpm and blood pressure of 48/39 mmHg (norepinephrine at 1 µg/kg/min). Bedside echocardiography showed a large pericardial effusion with pericardial thrombus formation, suggesting tamponade. The patient experienced inadequate drainage of pericardial effusion. A follow-up echocardiogram, in conjunction with the second aortic CTA, suggested the possibility of cardiac rupture secondary to acute myocardial infarction (Fig. 1C, D). V-A ECMO (21 F/48 cm femoral venous cannulation: 96370-023; Medtronic, Inc. Minneapolis, MN, USA, and 17 F/23 cm femoral arterial cannulation: 96570-015; Medtronic, MN, USA) was reinitiated with parameters of 3250 RPM, a blood flow rate of 3.5 L/min, and an oxygen flow rate of 3.5 L/min. The patient underwent left ventricular rupture repair under extracorporeal circulation, with intraoperative findings suggesting left ventricular aneurysm rupture (Fig. 1F). Postoperatively, the patient’s condition gradually improved and stabilized. He was transferred out of the ICU on the 41st day (21 days post-surgery) and discharged in good condition on the 54th day (34 days post-surgery). It is recommended that the patient undergo coronary angiography as soon as possible after discharge. The patient will have regular outpatient follow-ups and will be prescribed aspirin and atorvastatin for treatment.
Fig. 1.
(A) Echocardiogram revealing pericardial effusion (red arrow). (B) Aortic CTA showing aortic dissection (yellow arrow). (C) Echocardiogram reveals cardiac rupture (yellow arrow). (D) Aortic CTA also demonstrates cardiac rupture (blue arrow). (E) Aortic CTA does not display aortic dissection(red arrow). (F) Cardiac rupture is observed during surgery (black arrow)
Discussion
V-A ECMO is a temporary mechanical circulatory support used for patients with cardiac arrest, cardiogenic shock, or heart failure [3, 4]. This life-saving technology can offer crucial time for patients experiencing early, unexplained cardiogenic shock or cardiac arrest [8]. We report a case of a patient with cardiogenic shock and recurrent pericardial effusion who improved following V-A ECMO and subsequent surgical treatment. Upon emergency admission, considering the patient’s history and auxiliary examinations, our team initially contemplated the possibility of acute myocardial infarction. However, the atypical ECG findings and subsequent aortic CTA examination interfered with our diagnostic considerations. The initial aortic CTA was misinterpreted as an aortic dissection. The primary reason for this misdiagnosis was that V-A ECMO circulation altered the sequence and direction of contrast enhancement, leading to numerous artifacts on the CTA images [7]. When V-A ECMO is in use, it generates two types of blood flow: antegrade cardiac output and retrograde ECMO return. The confluence of these two blood flows creates a watershed area [4, 9]. This watershed area can be located anywhere between the aortic root and the diaphragm, determined by the pressure and flow of left ventricular output in relation to ECMO flow [4]. On first-pass CTA images, the presence of the watershed area can cause flow-related artifacts, where the mixture of contrast-enhanced and non-enhanced blood may lead to non-diagnostic images, easily mistaken for thrombus formation, complete vascular occlusion, or aortic dissection [4, 10]. This was the cause of our initial misdiagnosis of an aortic dissection. Misinterpretation of flow-related CTA artifacts may lead to inappropriate surgical or medical interventions. Therefore, a systematic multiphase approach should be adopted for CTA imaging strategies and interpretation in patients receiving V-A ECMO therapy [7]. The importance of recognizing the watershed area cannot be overstated. Strategies to mitigate the impact on CTA imaging for V-A ECMO patients include temporarily stopping or reducing ECMO flow, directly injecting contrast into the ECMO outflow, and adding a delayed phase [7]. The patient’s second ECMO treatment was necessitated by recurrent pericardial tamponade and obstructive shock caused by pericardial thrombus formation [11], prompting another aortic CTA examination. Learning from the first experience, we lowered the ECMO flow rate to 0.8 L/min during the CTA. Comparing the two aortic CTA examinations (Fig. 1B, E), based on the laboratory findings and bedside echocardiography, the patient was considered to have experienced cardiac rupture secondary to acute myocardial infarction, which was followed by timely surgical repair [12]. The patient experienced recurrent pericardial effusion, and the results of the initial aortic CTA interfered with our accurate diagnosis, leading to a misdiagnosis. For critically ill patients, we should analyze and diagnose their condition from multiple perspectives. Although CTA is the gold standard for diagnosing aortic dissection, bedside echocardiography can be equally important in critically ill ICU patients, allowing dynamic observation and the detection of small details [6]. This patient was considered to have acute myocardial infarction, but further coronary angiography was not performed. For such patients, we recommend that coronary angiography or coronary CTA be conducted. Ideally, the patient should have undergone coronary angiography prior to left ventricular repair to provide a better diagnostic basis for acute myocardial infarction, which could also facilitate the possibility of performing CABG during the left ventricular repair.
Conclusion
The unique hemodynamics of V-A ECMO patients require careful interpretation when performing CTA examinations, taking into account the dual circulation of blood in ECMO patients. There is no universal CTA protocol, and each protocol must be individually tailored to the patient, considering factors such as ECMO cannula placement, site of contrast injection, target area, cardiac output, and ECMO flow rate to identify watershed areas and avoid misdiagnosis. For patients with recurrent pericardial effusion, we should adopt a more comprehensive approach to diagnosis, analyzing the condition from multiple perspectives to avoid misdiagnosis and missed diagnosis. The case can serve as a reference for clinical practitioners encountering similar situations.
Acknowledgements
Not applicable.
Abbreviations
- V-A
Veno-Arterial
- ECMO
Extracorporeal Membrane Oxygenation
- CTA
Computed Tomography Angiography
- ICU
Intensive care unit
- SPO2
Oxygen saturation
- EF
Ejectionfraction
- CABG
Coronary-artery-bypass-grafting
Author contributions
Conception and design: Y.J, review and revision of the manuscript: H.Y., patient treatment and collection of data: M.Z, analysis and interpretation: H.Z, drafting of the manuscript for important intellectual content: W.S, revision of the final manuscript: C.Z. All authors read and approved the manuscript.
Funding
This work was supported by The Science and Technology Project of Taizhou (23ywa47), the Medicines Health Research Fund of Zhejiang, China (2024ky1784), the National Key Research and Development Program of Zhejiang Province (2023C03083).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The patient’s family provided consent for the publication of the images. The patient’s identity has been kept confidential.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White A, Fan E. What is ECMO? Am J Respir Crit Care Med. 2016;193(6):P9–10. [DOI] [PubMed] [Google Scholar]
- 2.Le Gall A, et al. Veno-arterial-ECMO in the intensive care unit: from technical aspects to clinical practice. Anaesth Crit Care Pain Med. 2018;37(3):259–68. [DOI] [PubMed] [Google Scholar]
- 3.Koziol KJ et al. Extracorporeal membrane oxygenation (VA-ECMO) in management of cardiogenic shock. J Clin Med, 2023. 12(17). [DOI] [PMC free article] [PubMed]
- 4.Rao P, et al. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11(9):e004905. [DOI] [PubMed] [Google Scholar]
- 5.Baliga RR, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging. 2014;7(4):406–24. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, et al. Type a aortic dissection-experience over 5 decades: JACC historical breakthroughs in perspective. J Am Coll Cardiol. 2020;76(14):1703–13. [DOI] [PubMed] [Google Scholar]
- 7.Shen J, et al. CT angiography of venoarterial extracorporeal membrane oxygenation. Radiographics. 2022;42(1):23–37. [DOI] [PubMed] [Google Scholar]
- 8.Tsangaris A, et al. Overview of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support for the management of cardiogenic shock. Front Cardiovasc Med. 2021;8:686558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angleitner P, et al. Watershed of veno-arterial extracorporeal life support. Eur J Cardiothorac Surg. 2016;50(4):785. [DOI] [PubMed] [Google Scholar]
- 10.Hoeper MM, et al. Extracorporeal membrane oxygenation watershed. Circulation. 2014;130(10):864–5. [DOI] [PubMed] [Google Scholar]
- 11.Bodson L, Bouferrache K, Vieillard-Baron A. Cardiac tamponade. Curr Opin Crit Care. 2011;17(5):416–24. [DOI] [PubMed] [Google Scholar]
- 12.Matteucci M, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care. 2019;8(4):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.