Abstract
The concept of femoroacetabular impingement syndrome (FAIS) has received much attention over the past 20 years. Currently, it is believed that FAIS can lead to intra-articular pathologies such as labral tears and articular cartilage lesions, resulting in clinical symptoms and subsequent poor clinical outcomes. FAIS-related articular cartilage lesions are common but unique, and their natural course always leads to early osteoarthritis of the hip. However, despite these cartilage lesions having gradually gained considerable attention, limited consensus has been reached on key aspects, such as diagnosis, mechanisms, classification, and management strategies, which limits clinical and research advances. Hence, an intensive comprehensive overview based on the existing evidence is necessary. The purpose of this review was to introduce the general consensus, controversial issues, and recent advances in FAIS-related articular cartilage lesions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05322-6.
Keywords: Femoracetabular impingement, Hip, Cartilage, Review
Publication trends and hot spots
In order to enable readers to understand the research status of femoroacetabular impingement syndrome (FAIS)-related articular cartilage lesions quickly and grasp the potential hot spots, we took the relevant literature data published worldwide and included in the Web of Science Core Collection from 2003 to 2024, utilizing bibliometric content analysis method for integrative analysis. The search criteria were as follows: ((((ALL=(femoroacetabular impingement syndrome)) OR ALL=(femoroacetabular impingement)) OR ALL=(femoro-acetabular impingement)) OR ALL=(femoro acetabular impingement)) AND ALL=(cartilage). In total, 1112 originals articles and reviews were extracted. Three authors reviewed all these studies via titles and abstracts screening, thus we excluded duplicate records, studies in languages other than English, and studies unrelated to the central theme. 788 publications were used to construct the dataset. The dataset was imported into CiteSpace 6.3. R1 (Drexel University, Philadelphia, PA, USA) and the Online Analysis Platform of Literature Metrology (http://bibliometric.com/) for overall analysis and visualization. The top five institutions were ranked by the total citations of manuscripts: University of Bern, University of Utah, University of Zurich, University of Ottawa, and Harvard University. The top five journals were Clinical Orthopaedics and Related Research, The Journal of Bone and Joint Surgery-British Volume, Osteoarthritis and Cartilage, The American Journal of Sports Medicine, and Arthroscopy-The Journal of Arthroscopic & Related Surgery [see Additional file 1]. We identified the top 19 authors who have attracted the most attention over the past two decades in chronological order via a clustering analysis method, including Gan, Leunig, Beck, Philippon, and Tannast et al., who pushed the derivation and growth of this topic, and Schmaranzer, Pascual-Garrido, and Maldonado et al., whose studies reflect the latest academic achievements and cutting-edge dynamics [see Additional file 2]. We also summarize the changes in research hotspots over the past two decades, revealing research trends in this field (Fig. 1). Furthermore, all terms and definitions in this article will be based on the widely accepted literature and consensus we retrieved [1–5].
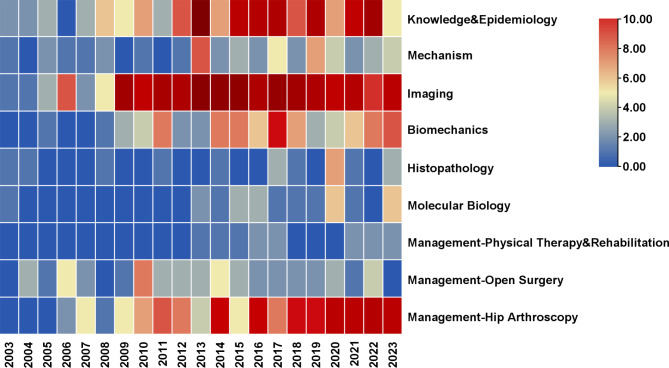
Fig. 1.
Heatmap of research trends of the FAIS-related articular cartilage lesions from 2003 to 2023. Each row represents a research topic, each column represents a year, and the color of each box represents the frequency (the darker the red, the more the related literatures were published; blue indicates that the quantity was close to zero)
All in all, abundant epidemiological evidence has rapidly promoted our understanding of FAIS-related cartilage lesions. In the early stages, scholars described the natural history of FAIS-related cartilage lesions based on clinical observations, and proposed possible mechanisms that dominate disease progression. Further exploration of the mechanisms started with biomechanical research of the hip joint, including computer simulation, motion analysis, and kinetics. While the application of histopathology and molecular biology methods in FAIS cartilage pathology is still in its infancy, the findings have advanced the field and revealed the unique mechanism of the development of hip osteoarthritis in patients with FAIS. Research trends have revealed a gradual shift in the management of FAIS-related cartilage lesions from open surgery to hip arthroscopy. Multiple new technologies, such as tissue engineering, have been introduced for the treatment of FAIS-related cartilage lesions. Physical therapy and rehabilitation have also begun to receive attention; however, there is still a lack of consensus. Interestingly, scholars have always shown great enthusiasm for utilizing imaging methods, especially MRI, to achieve early diagnosis and prognosis prediction.
This review will provide an integrated overview of the current state of FAIS-related articular cartilage lesions and offer a “state-of-the-art” snapshot of this domain.
Epidemiology
More than 80% of patients with FAIS are noted to have acetabular cartilage lesions on surgery, and most of lesions are partial-thickness occurring in the anterosuperior region of the acetabulum accompanying with adjacent labral tears [6–11]. While cartilage lesions on femoral head are rare. FAIS patients with cam morphology have a higher incidence of cartilage lesions and mostly located at chondrolabral junction (CLJ); chondromalacia, debonding, and cleavage are the most common lesion patterns [6, 9, 12–20]. Adolescent patients with FAIS have a lower incidence of cartilage lesions than adults, reaching only 20% [15]. Sex, age, and BMI are predictors of intraoperative cartilage lesions in both adolescent and adult patients with FAIS. For instance, high-grade cartilage lesions are more common in male, older, and higher BMI patients [9, 10, 21–26]. Current evidence suggests that FAIS patients with cartilage lesions tend to have worse clinical outcomes regardless of whether they undergo treatment [8, 21, 27].
Mechanisms
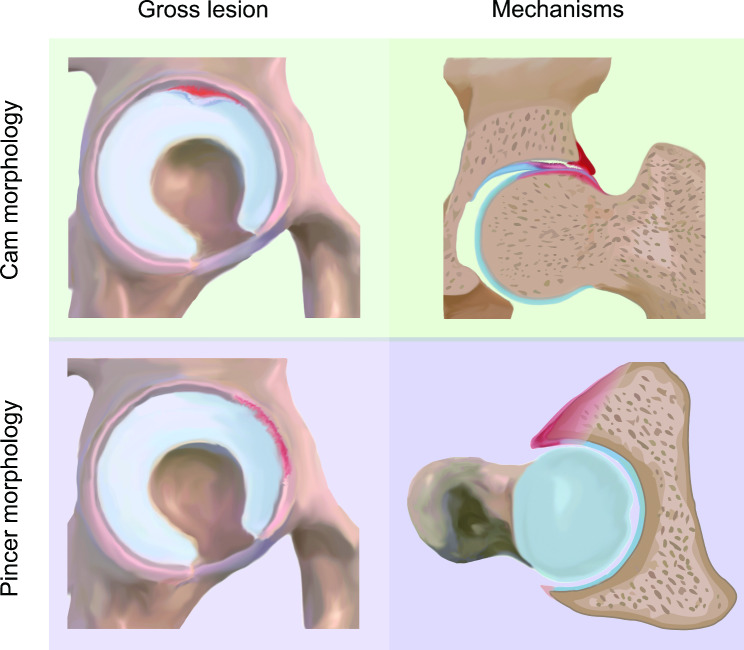
The mechanisms underlying FAIS were first summarized by Ganz et al. [1] in 2003, describing FAIS as an abnormal contact between the femur and rim during the end stage of hip joint motion that leads to intraarticular pathology. It is currently accepted that cartilage lesions in Cam morphology are the result of an outside-in mechanism, while in Pincer morphology, cartilage lesions result from linear contact between the femoral head–neck junction and acetabular rim (Fig. 2) [1, 2, 18, 19, 28, 29]. The radius of the abnormal femoral head-neck junction in the cam morphology gradually increases when sliding into the acetabulum, which develops compression and shear stresses at the CLJ. The cartilage is sheared by the non-spherical femoral head and then stripped from the subchondral bone, causing a cartilage lesion to develop from the outside. In Pincer morphology, in which the femoral neck repeatedly impinges against the abnormal acetabular rim, the labrum is first compressed by deformation, and then force is transmitted to the acetabular cartilage, resulting in a labral tear and strip cartilage lesion. Repeated microtrauma can cause labral ossification, and leverage of the head in the acetabulum causes contrecoup cartilage lesions in the posteroinferior acetabulum. However, hypothesis at the joint level cannot fully explain why not all patients with FAI morphology will exist cartilage lesions. Thus, we summarize the recent research progress and put forward cogent key events as follows: anatomical structures prone to damage, altered biomechanical due to worsening of contact mechanics and sustained abnormal stress, and molecular biological changes represented by chronic inflammation.
Fig. 2.
Diagram of typical gross findings and mechanisms of FAIS-related articular cartilage lesions. During joint motion, cam morphology disrupts the chondrolabral junction and then “peel off” the cartilage, while pincer morphology leads to hip anterosuperior labral tears and cartilage lesions as a result of linear contact between components
Anatomical structures
Cam morphology poses a significant threat to the hip cartilage. It is generally accepted that primary cam morphology develops during skeletal maturation as a normal physiological response to loading, whereas secondary cam morphology is caused by hip disease or acute trauma [4, 30]. Vigorous sporting activity during adolescence leads to trauma or shear stress in the growth plate before epiphyseal closure, increasing the epiphyseal extension along the femoral neck [30]. Thus, Cam morphology is not only more prevalent in males, but also higher and greater than that of females. The CLJ is a sharp and abrupt transition zone between labrum and hyaline cartilage that has to bear different types of stresses, yet it is susceptible to shear stresses due to its vulnerable histological structure (less collagen content and parallel fiber orientation) [31–35].
Biomechanics and contact mechanics
Compared to healthy controls, obvious biomechanical alterations could be seen in hips during walking, squatting, stair climbing, sitting to standing, and jumping to landing in FAIS patients with cam morphology [36–41]. Significantly, hip ROM reduced in all directions except extension in FAIS patients but may not be restricted in asymptomatic FAI morphology [42]. Finite element analysis studies have revealed that cartilage in impingement zone continued to sustain overload contact pressure, tensile strain, and shear stress [43, 44]. The presence of a fluid film within the central compartment of the hip joint influences the pressure distribution pattern at the articular surface. Dwyer et al. [45] demonstrated that cam morphology could reduce the seal of the central compartment during pivoting in cadaveric specimens. Pierannunzii [46] presented a perspective based on contact mechanics in which cam intrusion disrupts the fluid film and increases friction, and shear stresses result in cartilage wear and extracellular matrix (ECM) fragment release, further triggering inflammation pathobiology, which leads to worsening lubrication and enhanced wear through a vicious cycle, maintaining a chronic-recurrent joint inflammation. In summary, multiple factors have a combined effect on cartilage contact mechanics, which may drive the pathological cascade involved in the development of FAIS cartilage lesions.
Molecular biology
Articular cartilage continuously subjected to abnormal mechanical stresses at the impingement zone in FAI hips shows behaviors and composition similar to early osteoarthritis [47–49]. New evidence from molecular biology reveals a molecular link between mechanical impact and cartilage lesions in FAIS (Table 1) [49–56]. However, the pathobiological mechanisms underlying the transition from FAIS to Hip OA are poorly understood. Based on previous research, we found that the loss of cartilage homeostasis in patients with FAIS was more obvious. Chondrocytes exhibit an accelerated OA phenotype with increased expression of pro-inflammatory cytokines. Both anabolic and catabolic activities increase in the early stages and then convert to sustained catabolism as the disease progresses. However, because of ethical issues in obtaining acetabular cartilage samples, almost all studies are based on samples from the anterolateral head-neck junction. Goats and rabbits have been used to develop FAIS models that provide opportunities to explore both the pathogenesis and early events of acetabular cartilage lesions [57, 58]. Identifying possible molecular mechanisms is critical for clinical decision-making, such as whether to perform early intervention for patients with asymptomatic FAI morphology and how we should do so.
Table 1.
Studies on the molecular biology of FAIS-related cartilage lesionsa
| Tissue source | Study group | Methods | Markers | Results | |
|---|---|---|---|---|---|
| Wagner et al. [53] 2003 | anterolateral head-neck junction |
FAIS, n = 22 OA, n = 14 ND, n = 6 |
IHC ISHH |
COMP COL2-3/4 C (long) COL1 COL2 |
Cartilage in patients with FAIS and OA showed similar histological changes. |
| Hashimoto et al. [54] 2013 | anterolateral head-neck junction |
FAIS, n = 25 FAIS-OA, n = 7 DDH, n = 3 |
qRT-PCR |
IL-1β IL-8* CXCL1 CXCL2 CXCL3* CXCL6 CCL3 CCL3L1* MMP-13 ADAMTS-4* COL2A1* ACAN* |
Cartilage in FAIS group was metabolically hyperactive, versus FAIS-OA and DDH group. Cartilage at the cleavage/thinning stage expressed more inflammatory and catabolic mediators. |
| Chinzei et al. [55] 2016 | anterolateral head-neck junction |
FAIS, n = 30 OA, n = 30 |
qRT-PCR |
IL-1β* IL-8* MMP-13* ADAMTS-4* ACAN* COL2A1* |
Cartilage in FAIS group expressed higher inflammatory cytokines and catabolic genes, as well as lower anabolic genes, versus OA group. |
| Haneda et al. [56] 2020 |
anterolateral head-neck junction acetabulum |
FAIS, n = 15 FAIS-OA, n = 15 ND, n = 7 |
IHC |
IL-1β MMP-13 ADAMTS-4 COL2 NITEGE |
Cartilage in patients with FAIS and FAIS-OA showed similar histological changes. |
| Haneda et al. [56] 2020 | anterolateral head-neck junction |
FAIS, n = 15 FAIS-OA, n = 15 DDH OA, n = 15 ND, n = 7 |
IHC |
IL-1β* MMP-13* ADAMTS-4* COL2* NITEGE |
Cartilage in FAIS group was metabolically hyperactive, versus FAIS-OA, DDH OA and ND group. |
| Gao et al. [52] 2021 |
(Bone tissue) anterolateral head-neck junction |
FAIS, n = 12 FNF, n = 6 |
qRT-PCR |
IL-1 IL-6* IL-8 ALP* RANKL* OPG* |
Bone tissue in FAIS group expressed higher inflammatory genes and bone remodeling genes |
| Pascual-Garrido et al. [57] 2022 | anterolateral head-neck junction |
FAIS, n = 9 FAIS-OA, n = 13 |
RNA seq qRT-PCR IF |
AKT1* PPAR-γ* HIF1α* DNMT3B* DNMT1* DNMT3A* |
With disease progression, the expression of PPARγ and DNMT3B were gradually suppressed, while DNMT1/3A was induced. |
| Kamenaga et al. [58] 2023 | anterolateral head-neck junction |
FAIS, n = 12 FAIS-OA, n = 12 ND, n = 5 |
qRT-PCR IF WB MSP |
DNMT3B* ABAT* MMP13 COL10A1 COL2A1 |
Gradual epigenetic dysregulation between during the progression from FAIS to FAIS-OA. |
| Kuhns et al. [59] 2023 | anterolateral head-neck junction |
FAIS, n = 10 FAIS-OA, n = 10 |
RNA seq qRT-PCR IHC |
FGF18* WNT16* MMP13* ADAMTS4* |
FAIS and OA cartilage have distinct genomic expression profiles. Early anabolic signaling is replaced with catabolic signaling in the disease course. |
a FAIS, femoroacetabular impingement syndrome; OA, osteoarthritis; ND, no disease; IHC, immunohistochemistry; ISHH, in situ hybridization histochemistry; DDH, developmental dysplasia of the Hip; qRT-PCR, quantitative real-time polymerase chain reaction; FNF, femoral neck fracture; RNA seq, Ribonucleic acid sequencing; IF, immunofluorescence; WB, western blotting; MSP, methylation specific PCR
* Indicates differential expression in FAIS compared to OA or FAIS-OA
The underlying pathophysiology of the changes in the osteochondral unit cannot be ignored. Acetabular subchondral bone mineral density (BMD) is elevated in patients with Cam-type FAIS [59]. Ng et al. [60, 61] confirmed that the subchondral bone of patients with cam morphology experienced substantially higher peak stresses and shear stresses than those covering the cartilage during squatting. The expression levels of genes associated with inflammation and bone remodeling are higher in the bone tissue of patients with early Cam-type FAIS than in those with normal bone tissue [49].
Diagnosis
The preoperative diagnosis of FAIS-related articular cartilage lesions depends on a detailed interrogation, comprehensive physical examination, and proper imaging. Among patients with motion-related or position-related pain in the hip or groin (sometimes also in the back, buttock, or thigh), other sources of pain must be excluded, such as nonmusculoskeletal (e.g., urinary system disorder, potential nerve entrapment), pathological conditions (e.g., tumors, infections, stress fractures), and competing body regions (e.g., lumbosacral spine), to distinguish between intra-articular and extra-articular sources [3, 62, 63]. Intermittent clicking, buckling, or locking suggests the presence of cartilage lesions but is usually confused with labral lesions [64]. Physical examination should start with gait assessment, including ROM and flexion adduction internal rotation (FADIR) test, meanwhile a comprehensive hip assessment should include tender point, muscle strength, single leg control, flexion abduction external rotation (FABER) test, and straight leg raising against resistance test [3, 62, 64, 65]. While physical diagnostic tests have good sensitivity, they often lack specificity [66]. Therefore, diagnostic imaging provides a more objective approach for preoperatively detecting cartilage lesions [64].
Radiograph
Radiographs provide only an indirect assessment of cartilage, revealing hip morphology, joint space narrowing, and evidence of secondary osteoarthritis (osteophytes, subchondral sclerosis, and subchondral cysts) [67, 68].
An alpha angle above 60°, a femoral offset < 8 mm, and a head-neck offset ratio ≤ 0.15 at the anterior femoral head-neck junction are recommended as the imaging criteria for Cam morphology [4, 67]. A larger alpha angle on radiograph (especially > 65°) is a strong radiographic predictor of severe articular cartilage lesions and labral tears [22, 25, 69]. McClincy et al. [15] found that for each 10° increase in the alpha angle on 45° Dunn radiographs, there was a 1.77-fold increase in the probability of encountering acetabular cartilage lesions during arthroscopy. Similarly, Shapira et al. [24] showed that for every 1° increase in the alpha angle on a 45° Dunn, the odds of severe acetabular cartilage damage increased by 6%.
A joint space of 2 mm or less is considered evidence of high-grade cartilage lesions and is associated with higher hip arthroscopy failure and early conversion to total hip arthroplasty [70, 71]. However, Rosinsky et al. [72] reported that in FAIS patients with Tönnis grade 1 or 0 under the age of 50, joint space narrowing on plain films may not accurately predict cartilage lesions. Relative narrowing of the lateral joint space compared to the medial joint space has been identified as a predictor of cartilage lesions. Mortensen et al. [70] also found that there was no significant correlation between a < 2 mm posterior hip joint narrowing shown on false-profile radiographs and intraoperative high-grade cartilage wear in Cam FAIS patients.
However, Tönnis classification system may underestimate the severity of FAIS-related articular cartilage lesions [21, 70, 73]. Therefore, advanced imaging techniques are required.
MRI
Reports suggest that the standard MRI protocol for FAIS-related articular cartilage lesions should include: (1) unilateral small field-of-view (FOV) sequences, including oblique axial and radial imaging for assessment of cam morphology, and the minimum acceptable number of slices in radial sequences should be 12 slices at 30° intervals around the clock face from 12 o’clock to 11 o’clock positions. (2) femoral torsion assessment; and (3) a fluid-sensitive sequence covering the whole pelvis (in axial or coronal planes, to screen for soft tissue and bone marrow edema beyond the hip) [4, 67, 68, 74].
1.5T direct magnetic resonance arthrography (direct MRA, dMRA) has long been considered the gold standard for diagnosing FAIS-related articular cartilage lesions [75–78]. However, MRA may not easily detect acetabular cartilage delamination, thus the extent of cartilage lesions is often underestimated [79–82]. Axial hip traction has been recommended to improve the sensitivity of MRA [68, 74, 83]. Non-contrast 3.0T MRI is at least equivalent to 1.5 T dMRA in identifying intra-articular hip pathology, but it is a simpler and noninvasive method [67, 68, 78, 84, 85]. Gao et al. [26] retrospectively analyzed preoperative 3.0T MRI data from 233 FAIS patients that were confirmed arthroscopically; the overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 3.0T MRI to identify cartilage lesions were 83.7%, 82%, 74.2%, and 89.1%, respectively, and the intra- and interobserver reliabilities were almost perfect.
The quantitative MRI techniques, employing regional quantitative analysis to detect biochemical changes in cartilage composition, such as delayed gadolinium-enhanced magnetic resonance of cartilage (DGEMRIC), T2 mapping, T2* mapping, and T1ρ mapping, have been seen as the most promising auxiliary diagnostic methods for FAIS-related articular cartilage lesions (Table 2) [71, 82, 84, 86]. These techniques have showed excellent feasibility and reproducibility, as well as strong ability to detect acetabular cartilage delamination [87–90]. Notably, the intrinsic variability of biochemical markers among patients makes it difficult to define a gold-standard threshold for identifying cartilage lesions [91].
Table 2.
The quantitative MRI techniques of FAIS-related cartilage lesionsa
| DGEMRIC | T2 mapping | T2* mapping | T1 rho mapping | |
|---|---|---|---|---|
| Detection | GAG content | Water content and collagen fiber network | Water content and collagen fiber network | Slow-motion interactions between macromolecules (e.g. GAG) and bulk water |
| Normal cartilage |
T1Gd value is positively correlated with GAG content Higher T1Gd value |
T2 value would decrease from superficial zone to calcified zone Lower T2 value |
T2* value would decrease from superficial zone to calcified zone Lower T2* value |
T1ρ value is negatively correlated with GAG content Lower T1ρ value |
| Damaged cartilage | Lower T1Gd value | Higher T2 value | Highter T2* value | Highter T1ρ value |
| Pearls | Clinically validated in hip joint | Without contrast media |
Without contrast media Shorter scan time than T2 mapping Higher resolution than T2 mapping |
Without contrast media More sensitive to earlier changes |
| Pitfalls |
Injection of contrast agent Time consuming |
Magic angle effect Chemical shift artifacts Long scan time Lack of standardized protocol Lack of reference database and abnormal cut-off values |
Magic angle effect Chemical shift artifacts Lack of standardized protocol Lack of reference database and abnormal cut-off values |
Long scan time Poor availability and reproducibility Lack of standardized protocol Lack of reference database and abnormal cut-off values Tissue heating |
aDGEMRIC, delayed gadolinium-enhanced MRI of cartilage; GAG, glycosaminoglycan
Taken together, AP pelvis and Dunn’s 45° view radiographs provide limited but necessary information for treatment decisions and prognosis prediction. A 1.5T dMRA or 3.0T MRI is the first-line modality when a FAIS-related cartilage lesion is suspected. However, the choice depends largely on the institution. Currently, quantitative MRI techniques complement conventional MRI techniques by enabling earlier recognition; however, they have not yet reached clinical maturity.
Classification systems
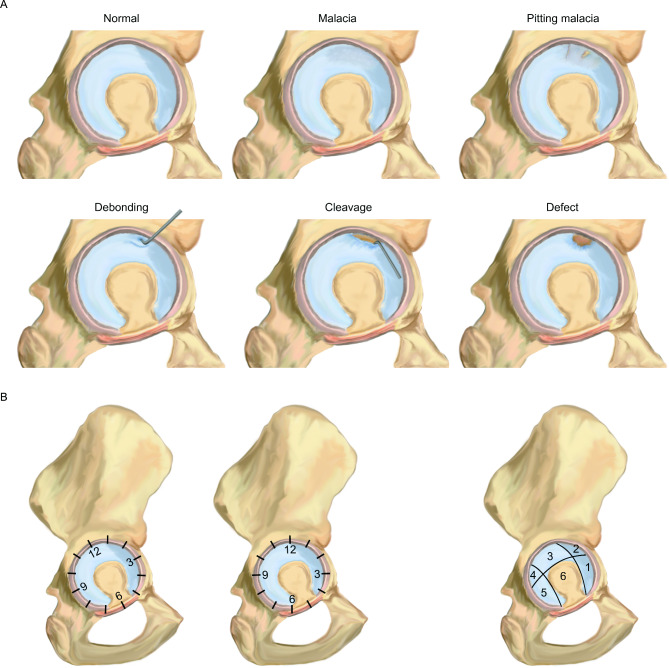
The classification systems currently used for FAIS-related cartilage lesions include Outerbridge classification, Beck classification (Fig. 3A) (various modified Beck classification), acetabular labrum articular disruption (ALAD) classification, Konan/Haddad classification, International Cartilage Repair Society (ICRS) classification, Bern classification, Sampson classification, and Multicenter Arthroscopy of the Hip Outcomes Research Network (MAHORN) classification [64, 92–98]. All these are explained in detail in Table 3. The Outerbridge, Beck, and ALAD classifications are the most commonly used classification systems [99–102]. Meanwhile, the clock-face method and Ilizaliturri’s six acetabular zone method are the two most popular methods employed to map lesion location and describe lesion extent (Fig. 3B) [103]. Current evidence suggests that we can accurately grade and map FAI cartilage lesions using a combination of Beck classification and the clock-face method. Over the years, this simple and reliable combination has been widely used and validated in clinical practice. Future updates of classification systems should focus on characterizing disease progression and prognostic value as well as guide surgical indications.
Fig. 3.
A-B Diagram of the prime classification system of FAlS-related cartilage lesions. Beck classification (A) reflects the different stages of disease progression. Note the differences between the anatomical landmarks of clock-face method and Ilizaliturri’s six acetabular zones method rely on (B)
Table 3.
The classification systems of FAIS-related cartilage lesionsa
| Classification | Grade | Definition | Base | Target area |
|---|---|---|---|---|
| Outerbridge | 1 | Softening and swelling of the cartilage | Gross appearance of cartilage lesions (extent) | Acetabulum and femoral head |
| 2 | Fragmentation and fissuring in an area half an inch or less in diameter | |||
| 3 | Fragmentation and fissuring in an area more than half an inch in diameter. | |||
| 4 | Erosion of cartilage down to bone | |||
| Beck | 0 | Normal-Cartilage macroscopically intact |
Pathological process in disease progression Surgical dislocation findings |
Acetabulum: Chondrolabral junction (transition zone) |
| 1 | Malacia-Fibrillation or roughening of surface | |||
| 2 | Pitting malacia-Roughening, partially thinning and full-thickness defects or deep fissuring to the bone | |||
| 3 | Debonding-Loss of fixation to the subchondral bone, macroscopically sound cartilage; carpet phenomenon | |||
| 4 | Cleavage-Loss of fixation to the Subchondral bone; frayed edges, thinning of the cartilage | |||
| 5 | Defect-Full thickness defect | |||
| ALAD | 0 | Cartilage macroscopically intact |
Pathological process in disease progression Hip arthroscopy findings |
Acetabulum: Chondrolabral junction (transition zone) |
| 1 | Softening of the adjacent cartilage | |||
| 2 | Early peel of the cartilage | |||
| 3 | Large flap of the cartilage | |||
| 4 | Loss of cartilage | |||
| Konan/Haddad | 0 | Normal cartilage |
Pathological process in disease progression Hip arthroscopy findings |
Acetabulum: Chondrolabral junction (transition zone) |
| 1 | Wave sign | |||
| 2 | Cleavage tear | |||
| 3 | Delamination | |||
| 4 | Exposed bone | |||
| Using combined with six acetabular zones method | ||||
| Grades 1, 3 and 4 could be further grouped as A, B and C based on whether the lesion was less than one-third of the distance from the acetabular rim to the cotyloid fossa (A), one-third to two-thirds of this distance (B) or greater than two-thirds of this distance (C). | ||||
| ICRS | 0 | Normal | Gross appearance of cartilage lesions (depth) | Acetabulum and femoral head |
| 1 | Nearly normal-Superficial lesions. Soft indentation (A) and/or superficial fissures and cracks (B) | |||
| 2 | Abnormal-Lesions extending down to < 50% of cartilage depth. | |||
| 3 | Severely Abnormal-Cartilage defects extending down > 50% of cartilage depth (A) as well as down to calcified layer (B) and down to but not through the subchondral bone (C). Blisters are included in this Grade (D). | |||
| 4 | Severely Abnormal-Lesions through the subchondral bone | |||
| Bern | 1 | Normal |
Clinical experience Cover the entire spectrum of early hip cartilage lesions independent of etiology |
Acetabulum: Chondrolabral junction (transition zone) |
| 2 | Discoloration and fibrillation-Macroscopically reddish or yellowish discoloration of the cartilage | |||
| 3 | Softening and thinning-Provocation of a cartilage indentation with the probe in a zone with softening of cartilage | |||
| 4 | Wave sign-Loss of fixation to the subchondral bone without flap formation, carpet phenomenon on palpation by a probe | |||
| 5 | Cleavage tear-Frayed edges in the cartilage, typically near the chondrolabral junction with preserved attachment to the subchondral bone | |||
| 6 | Delamination-Delamination of the cartilage, cartilage flap; loss of fixation to the subchondral bone | |||
| 7 | Exposed bone-Loosening of cartilage with exposed bone, bony palpation with probe | |||
| Sampson | AC0 | No damage |
Treatment strategies Pathological process in disease progression |
Acetabulum: Chondrolabral junction (transition zone) Femoral head |
| AC1 | Softening no wave sign | |||
| AC1w | Softening with wave sign intact labrocartilage junction | |||
| AC1wTj | Softening with wave sign and torn labrocartilage junction | |||
| AC1wD | Softening with wave sign and intact labrocartilage junction with delamination | |||
| AC1wTjD | Softening with wave sign and torn labrocartilage junction with delamination | |||
| AC2 | Fibrillation | |||
| AC2Tj | Fibrillation with torn labrocartilage junction | |||
| AC3 | Exposed bone small area < 1 cm2 | |||
| AC4 | Exposed bone large area > 1 cm2 | |||
| Abbreviations: A, acetabulum; C, cartilage defects; D, with delamination; Tj, Torn labrocartilage junction; w, with wave sign. | ||||
| HC 0 | No damage | |||
| HC 0T | Uniform thinning (T) | |||
| HC 1 | Softening | |||
| HC 2 | Fibrillation | |||
| HC 3 | Exposed bone | |||
| HC 4 | Any delamination | |||
| HTD | traumatic defect (size in mm) | |||
| HDZ | demarcation zone from FAI | |||
| Abbreviations: HC, femoral head cartilage; T, thinning; TD, traumatic defect; DZ, demarcation zone from FAI. | ||||
| MAHORN | 0 | Normal-Macroscopically sound cartilage |
Pathological process in disease progression Hip arthroscopy findings |
Acetabulum: Chondrolabral junction (transition zone) |
| 1 | Focal defect or extensive softening | |||
| 2 | Bubble-Detached cartilage from bone with intact periphery | |||
| 3 | Pocket-Detached cartilage from bone with one free edge | |||
| 4 | Flap-Detached cartilage from bone with more than one free edge | |||
| 5 | Exposed bone |
aMAHORN, Multicenter Arthroscopy of the Hip Outcomes Research Network
Treatment
Controversial issues
Non-surgical treatment versus surgical-treatment
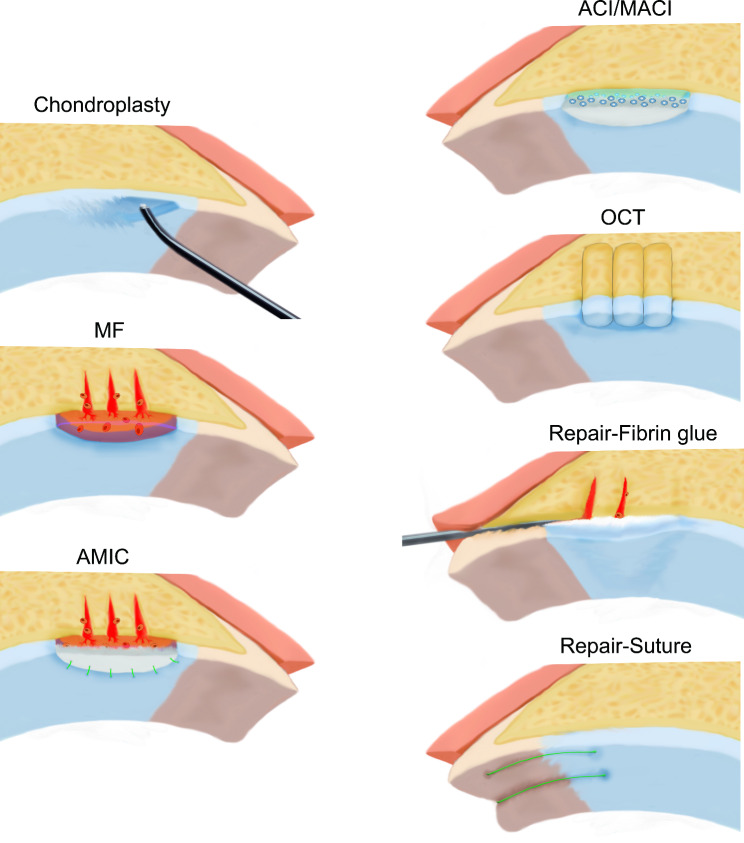
There is no high-level evidence to identify optimal treatment strategies for FAIS-related articular cartilage lesions. Nonsurgical treatment is still the first choice. The initial treatment strategy consists of patient education, rest, lifestyle and activity modification, nonsteroidal anti-inflammatory drugs (NSAIDs), and physiotherapy. If no improvement is observed after 4–6 weeks of treatment, an advanced review including an MRI for identifying cartilage status, or a diagnostic intra-articular injection for both pain relief and pain source distinguishing should be conducted [65]. It is now generally accepted that patients diagnosed with FAIS-related cartilage lesions should undergo surgical intervention within six months of symptom onset and conservative treatment failure [11, 104]. There are limited studies on the surgical treatment of cartilage lesions in adolescent patients owing to concerns about potential iatrogenic epiphyseal injury. It is difficult to determine a rigid upper age limit for surgical treatment because patient age is not completely associated with cartilage status. At present, chondroplasty, microfracture (MF), enhanced MF, autologous chondrocyte implantation (ACI), autologous matrix-induced chondrogenesis (AMIC), osteochondral transplantation (OCT), platelet-rich plasma, and stem cell therapy have been successfully applied for the treatment of FAIS-related cartilage lesions (Fig. 4). We summarized these surgical strategies in Table 4, that intend to assist clinicians in making decisions. And we found that surgical treatment of FAIS-related cartilage lesions changed from simple morphological repair to histological repair, emphasizing the importance of the microenvironment of chondrocytes and the cellular matrix.
Fig. 4.
Diagram of common surgical-treatment methods of FAlS-related cartilage lesions. The figure shows radiofrequency-based chondroplasty, MF, AMIC fixed with suturing, OCT, cartilage repairing with fibrin glue and MF, as well as cartilage repairing with suturing. MF, microfracture; AMIC, autologous matrix-induced chondrogenesis; ACI, autologous chondrocyte implantation; OCT, osteochondral transplantation
Table 4.
The surgical management strategies of FAIS-related cartilage lesionsa
| Non-full-thickness cartilage lesions | Cartilage delamination or cartilage flap | Full-thickness cartilage lesions | ||
|---|---|---|---|---|
| chondroplasty | < 2 cm2 |
Repair First: Fibrin glue Second: Suture or other adhesive techniques |
< 2cm2 | Debridement or MF |
| > 2 cm2 | Seen as full-thickness lesions after debridement | 2-4cm2 |
First: MF Second: OCT, enhanced MF, ACI/MACI |
|
| 4–6 cm2 |
First: enhanced MF, ACI/MACI Second: OCT |
|||
| >6 cm2 |
First: ACI/MACI Second: OCT, THA |
|||
aMF, microfracture; OCT, osteochondral transplantation; ACI, autologous chondrocyte implantation; MACI, matrix-induced autologous chondrocyte implantation; THA, total hip arthroplasty
Open surgery versus hip arthroscopy
Open surgery for FAIS-related cartilage lesions can be performed using surgical hip dislocation (SHD) and the anterior mini-open approach (AMO) [105, 106]. SHD, namely anterior dislocation with trochanteric flip osteotomy, can achieve complete exposure of the acetabulum and femoral head and allow easy repair of the joint capsule [107]. However, it carries the risks of avascular necrosis, heterotopic ossification (HO), and trochanteric nonunion. The AMO utilizes the internervous plane between the superior gluteal nerve (tensor fasciae latae) and the femoral nerve (sartorius) to minimize tissue damage around the hip. However, this approach carries the risk of iatrogenic injury to the lateral femoral cutaneous nerve, femoral nerve, and the ascending branch of the lateral femoral circumflex artery. With advances in surgical techniques, hip arthroscopy (HA) has become increasingly popular for diagnosing and treating FAIS-related articular cartilage lesions. HA offers shorter recovery times and fewer complications than open surgery, with adequate visualization and satisfactory short- and mid-term outcomes [108–111]. Maffulli et al. confirmed that HA permitted the significant reduction of revisions-rate and significant increase in ROM for FAIS patients [112]. According to a systematic review and meta-analysis, AMO had a significantly higher rate of complications (mainly lateral femoral cutaneous nerve injury) than HA and SHD, while SHD had the highest rate of conversion to THA [113]. HA is not without shortcomings: the limited joint space available requires continuous traction, and instrumentation may cause iatrogenic cartilage damage. In addition, attention should be paid to capsular repair in HA because inappropriate capsule management may lead to postoperative joint instability. The arthroscopic capsular suture-lifting technique for treating patients could achieve better anterior stability of the hip joint and is more reliable than previous suturing techniques [114].
In short, hip arthroscopy has become the first-line treatment, while open surgery still plays an irreplaceable role in patients with significant dysplasia or malformation, excessive cartilage lesions, or osteochondral transplantation.
Remove versus repair
There is significant controversy over whether the delamination and chondral flaps should be retained in patients with FAIS. The traditional strategy is debridement followed by microfracture, which completely removes the delaminated cartilage and promotes fibrocartilage formation in defect areas. However, the delaminated cartilage may appear normal grossly and still have a large number of viable articular chondrocytes. Therefore, some scholars claim to conserve the delaminated cartilage and then perform a repair operation to bond the cartilage to the underlying subchondral bone.
Wright et al. [115] assessed the viability of chondral flaps using live/dead staining immediately after biopsy, and the percentage of live cells was 87% ± 10%. Hariri et al. [116] determined the DNA, hydroxyproline, glycosaminoglycan, and cellular viability of the flaps. The results showed that the biochemical characteristics of these flaps were abnormal, and cellular viability was only 39%. Rodriguez-Fontan et al. [117] compared cellular viability and tissue quality between chondral flaps and non-weight-bearing cartilage around the fossa. They confirmed the loss of viability (54.6%±25.6%) and tissue degeneration of chondral flaps. Levinson et al. [118] believed that despite the presence of viable chondrocytes (50 ± 19%), these cells residing in pathological ECM may have limited migration ability, and it is difficult to produce sufficient ECM for stable re-attachment.
Despite histological evidence not supporting the retention of delaminated cartilage and chondral flaps, clinical reports of repair surgery have indicated optimistic results, which are discussed in another section below.
Chondroplasty
Chondroplasty, also known as debridement, aims to reduce unstable flaps, prevent the development of loose bodies, and eliminate potential mechanical blocks. It has been considered the first-line treatment for small lesions sized < 2 cm2 and is effective for relieving pain as well as mechanical symptoms. Arthroscopic chondroplasty for FAIS-related articular cartilage lesions is associated with encouraging short- and medium-term postoperative functional outcomes. Radiofrequency devices can provide better mechanical stability and less release of inflammatory mediators compared with mechanical shavers [119]. However, inappropriate intraoperative radiofrequency or iatrogenic injury may lead to chondrolysis after hip arthroscopy [120]. Scraping calcified cartilage during debridement may induce fibrocartilage formation [121]. According to data from the Danish hip arthroscopy registry (DHAR), chondroplasty has become the most common treatment strategy for FAIS-related cartilage lesions, accounting for 81.6% [8]. Similarly, data from a North American cohort showed that chondroplasty was performed in more than 40% of patients with FAIS with acetabular cartilage lesions [7].
Microfracture and enhanced microfracture
Microfracture
Arthroscopic microfracture is a frequently used strategy for FAIS-related cartilage lesions, with reported success rates ranging from 82.4% to-96.7%, and is suitable for focal full-thickness cartilage lesions (Outerbridge grade IV) on the acetabulum and femoral head with a size 1 to 4 cm2 and Tönnis grade ≤ 1 [65, 122–129].However, the violation of the subchondral bone carried by MF increases the risk of subchondral fracture, intralesional osteophyte formation, and subchondral cyst formation, and iatrogenic injury of the subchondral plate may counteract revision surgery [121]. Overall, arthroscopic microfractures were associated with significant improvements in short-term PRO scores [21, 121, 129–132].
Patients with lesions greater than 400 mm2 or age > 50 years may could also benefit from MF [124]. Chaharbakhshi et al. [133] reported the effect of lesion size on clinical outcomes after arthroscopic microfracture was performed with concomitant treatment for labral tears and FAIS. Lesion size did not affect clinical improvements at a minimum 2-year follow-up, but patients with larger cartilage lesions (≥ 300 mm2) had a higher rate of conversion to THA. Carreira et al. reviewed 347 patients who underwent hip arthroscopy and found that surgeons would not perform MF in Beck grade 1 and 2 lesions, while whether to perform MF in Beck grade 3 and 4 lesions depended on age, and lesion size in patients aged ≥ 50 years and small size were more prone to MF treatment [21]. Two studies have provided second-look histological evidence that the final mean filling rate was over 90% and the repaired tissues were primarily fibrocartilage [134, 135].
Enhanced microfracture
Enhanced microfractures are considered superior to standard microfractures [136]. Enhanced microfracture is achieved by improving cell proliferation and differentiation as well as by increasing the stability of fibrin clots after standard MF. Biologics such as bone marrow mesenchymal stem cells (BM-MSCs), bone marrow aspirate concentrate (BMAC), and platelet-rich plasma (PRP) can be injected into the MF site or into the joint to facilitate the proliferation and differentiation of stem cells into cartilage [137]. Biomaterials, mainly scaffolds, can be fixed to MF sites to protect clots and cells from excessive shear and compressive stress. Multiple natural and synthetic biomaterials have been used in a dry powdered, gel, or membrane form.
Autologous matrix-induced chondrogenesis
AMIC is an enhanced microfracture technique recommended for the treatment of medium-to-large full-thickness FAIS-related cartilage lesions (> 2 cm2) [138, 139]. Collagen and chitosan scaffolds have been extensively used in AMIC procedures, and have demonstrated clinically validated efficacy in promoting cartilage regeneration.
Chondrogide®(Geistlich Pharma AG, Wolhusen, Switzerland), a bilayer collagen I/III membrane made from porcine collagen. Thorey et al. [139] reported positive outcomes of arthroscopic AMIC using Chondrogide® for mid-sized cartilage lesions in the acetabulum of amateur athletes. Fontana et al. [140] provided evidence for the stability and efficacy of AMIC with Chondrogide® for FAIS-related cartilage lesions. Patients with arthroscopic microfracture had visibly deteriorated at 36 months after surgery, but AMIC showed durable results. Similar favorable long-term outcomes were obtained from 5 to 8 years of follow-up [141–143].
BST-CarGel® (Smith and Nephew Inc., Andover, MA) is an injectable chitosan-based biopolymer which was delivered in a dropwise manner to fill the MF site during operation without additional fixation [144–146]. Large lesions (> 6 cm2) could also benefit from BST-CarGel® [147]. Tey et al. [144, 148] provided a detailed account of the hip arthroscopic AMIC technique with BST-CarGel® for FAIS cartilage delamination, and in which the clinical improvements could be maintained for more than two years. T2 mapping showed that BST-CarGel® produced homogenous repair tissue similar to the native cartilage after AMIC [149]. A randomized controlled trial reported that AMIC with BST-CarGel® led to greater lesion filling and superior repair tissue quality compared to isolated MF at 12 months after surgery [150]. Similarly, BST-CarGel® promoted a significant decrease in progressive loss of joint space and conversion to THA [145].
Recently, an absorbable gel implant consisting of collagen Type I from rats, known as ChondroFiller® (Meidrix Biomedicals, Esslingen, Germany), was reported, which allowed chondrocytes and stem cells to migrate into the collagen matrix without MF [151, 152]. Mazek et al. [151] reported encouraging long-term results with ChondroFiller® in patients with FAIS-related cartilage lesions. MRI showed significant healing of the defect after five years of follow-up. A case series demonstrated that in the 1-year MRI evaluation, the thickness of regenerative tissue approached normal cartilage [152].
Autologous chondrocyte implantation and matrix-induced autologous chondrocyte implantation
ACI and matrix-induced autologous chondrocyte implantation (MACI) have been established as good treatment options to deal with focal large full-thickness cartilage lesions(> 4cm2) in FAIS patients [65, 153]. Both techniques require a 2-stage surgical procedure: cartilage biopsy for chondrocyte culture and implantation of cultured chondrocytes with a scaffold at the defect site after debridement [153]. Currently, most scholars choose to harvest chondrocytes from the non-weight-bearing area of the femoral head or acetabular fossa as seed cells; however, additional harvesting procedures may induce secondary lesions in donor areas. Two studies have validated the feasibility of harvesting donor chondrocytes from the covered cartilage of cam morphology, although hyaline cartilage in this area has been demonstrated to exhibit clear signs of degeneration and inflammation [154, 155].
BioSeed-C® (BioTissue Technologies GmbH, Freiburg, Germany) is a bioresorbable polyglycolic acid/polylactic acid (PGA/PLA) polymer scaffold that embeds chondrocytes in a gel-like porous three-dimensional textile structure when used in MACI. Fontana et al. [156] compared the efficacy of arthroscopic debridement with arthroscopic BioSeed-C® MACI for post-traumatic hip cartilage lesions. After a mean follow-up of 74 months, the MACI group showed more significant postoperative improvements in the mHHS score. Another study showed that BioSeed-C® MACI and AMIC provided the same beneficial effects and long-term outcomes in repairing mid-sized (2-4cm2) FAIS-related cartilage lesions [157].
Chondrosphere®(co.don® AG, Berlin, Germany) is composed of injectable 3-dimensional autologous chondral spheroids with excellent self-adhesive ability [158, 159]. Körsmeier et al. [160] reported good short-term outcomes for Chondrosphere® in FAIS patients. The promising results of Chondrosphere® for treating larger cartilage lesions ranging from 2 cm2 to 6 cm2 have also been shown in several clinical studies [159, 161, 162].
NOVOCART® Inject (TETEC Tissue Engineering Technologies AG, Reutlingen, Germany) is an in situ cross-linkable albumin-hyaluronan-based hydrogel that includes two components: a hydrogel suspension containing autologous cells and a cross-linker. It can be injected into the prepared site of the defect via a dual-chamber syringe. Thier et al. [163] reported the positive short-term outcomes of arthroscopic NOVOCART® Inject MACI for small cartilage lesions in the hip. And they compared the clinical outcomes of NOVOCART® Inject with Chondrosphere® in treating FAIS-related cartilage lesions [164]. There were no significant differences in the outcomes between the two products. Another study also showed that patients treated with arthroscopic NOVOCART® Inject MACI combined with FAIS surgery presented the complete integration of the transplant [165].
Platelet-rich plasma and stem-cell therapy
Platelet-rich plasma and stem cell therapies are often used as adjunctive strategies in the treatment of FAIS-related cartilage lesions. These injectable biologics are injected into the joint cavity directly or at the defect site after MF to promote the regeneration of articular cartilage. However, the protocols for the delivery and preparation of current clinical products vary widely. The use of PRP and stem cell preparations in combination with tissue-engineered scaffolds seems intriguing its best potential.
The theoretical benefits of PRP in intra-articular hip disorders include promoting healing and reducing postoperative inflammation [166]. It is generally accepted that PRP can relieve hip pain associated with early osteoarthritis but has limited effects on cartilage repair [166–168]. Schallmo et al. [137] introduced an arthroscopic microfracture procedure enhanced with BioCartilage Extracellular Matrix®(Arthrex, Naples, FL) and PRP for the treatment of symptomatic full-thickness chondral defects of the hip. BioCartilage® is a biologically active scaffold containing dehydrated and micronized allograft cartilage and primary articular cartilage extracellular matrix [137, 169]. They mixed leukocyte-reduced PRP with BioCartilage® and input it into the defect areas of MF, that exhibited excellent survivorship and significant improvement after at least 1 year of follow-up [169].
Several stem-cell treatment strategies, including intra-articular injections of expanded MSCs, BMAC, and micro-fragmented adipose tissue transplantation (MATT), have been successfully applied to patients with FAIS, and result in more significant pain reduction and clinical improvement [170–172]. The optimal dose for intra-articular injection of expanded MSCs requires further investigation, while current evidence shows a positive correlation between dose and curative effect [173].Murata et al. [174] showed that MSCs from the cotyloid fossa synovium of patients with FAIS had higher proliferation and differentiation potential than those from the paralabral synovium, which should be considered a better source for stem cell therapy. Remarkably, some studies have suggested that intra-articular injection of MSCs or BMAC is inefficient [175–177]. The injected cells were distributed throughout the joint cavity and adhered preferentially to the synovium. Thus some scholars have used BMAC to infiltrate the AMIC matrix, and obtaining satisfactory results [178]. In microfragmented adipose tissue transplantation, autologous subcutaneous fat tissue is refined to cluster as a natural 3-dimensional biological scaffold that contains MSCs and a supportive vascular stromal niche that preserves cells in their native environment. Ivone et al. [172] treated cartilage delamination with a size of 1–2 cm2 by transplanting microfragmented autologous adipose tissue into the delamination gap, and confirmed that MATT led to better clinical outcomes compared with MF.
Osteochondral transplantation (OCT)
OCT is often used to deal with medium-to-large full-thickness cartilage or osteochondral lesions in weight-bearing areas. Depending on the source of transplantation, it can be divided into osteochondral autograft transplantation (OAT) and osteochondral allograft transplantation (OCA). Hip OCT usually exposes the articular surface through surgical dislocation or anterior approach (Smith-Petersen approach) rather than hip arthroscopy [179, 180]. Short- and medium-term evidence suggested that OAT combined with SHD was a reliable treatment strategy for large femoral head cartilage defects of young patients [181–187]. And it is also suitable for the treatment of “apple-bite” defects at the femoral head and neck junction due to excessive cam morphology [188].
Garcia-Mansilla et al. [189] introduced their experience about OCA combined with osteoplasty of the head/neck junction for Cam FAIS and concomitant chondral lesion in femoral head. The other two studies reported the good clinical and radiological outcomes of acetabular osteochondral defects treated with fresh OCA [190, 191].Field et al. [192] contributed the only report on the treatment of FAIS acetabular cartilage defects with arthroscopic OCT. They used a positioning device to create a bone tunnel from the region of the iliac crest to the acetabular articular surface and inserted a synthetic osteochondral plug (TruFit plug) from the external joint in a retrograde manner and positioned flush with arthroscopy. TruFit Plug (Smith & Nephew, San Antonio, TX, USA) is a synthetic resorbable acellular biphasic scaffold composed of polylactide-coglycolide copolymer (PLGA), calcium sulfate, polyglycolide fibers, and surfactant [192, 193].
Particulated cartilage transplantation (PCT) is a new technique. Autologous or allogeneic particulated cartilage tissue granules with sizes ranging from 1 to 2 mm2 were used as implant units, including chondrocytes and natural chondrocyte matrix. According to knee experience, PCT is mainly appropriate for ages from 18 to 55 years and full-thickness cartilage lesions with a size from 2 to 5 cm2, while combined subchondral bone damage or huge defects (> 5 cm2) are contraindications for PCT treatment [194]. Pascual-Garrido et al. [195] reported arthroscopic implantation of particulate juvenile allograft cartilage (DeNovo® Natural Tissue, DeNovo NT) (Zimmer Biomet®, Warsaw, Indiana, USA) to treat hip cartilage lesions. Similarly, Craig et al. [196] used an arthroscopic planer attached with a suction tube device (GraftNet; Arthrex, Naples, FL) to collect fragmented articular cartilage at the femoral head-neck junction and mixed them with chondral extracellular matrix, growth factors, and autologous peripheral blood to prepare grafts and achieve transplantation via a single operation.
Cartilage repair techniques
Currently, there are two main repair strategies for cartilage delamination and chondral flaps, including adhesive agents, like fibrin glue, and mechanical fixation, like suture anchors; however, it is unknown whether these strategies have satisfactory long-term outcomes [106, 197, 198]. Scholars believe that cartilage delamination less than 2 cm2 in size should be repaired [199].
Fibrin glue is usually used in conjunction with microfractures to bond delaminated cartilage or flaps to the underlying subchondral bone, acting as a glue as well as a scaffold for cells [106]. When the CLJ is intact, we need to determine the location of the delamination according to the carpet and wave signs. A small incision was made close to the acetabular rim on the external articular side of the adjacent labrum to form a pocket connecting the spaces between the delaminated cartilage and subchondral bone where the MF was performed. Once the pocket was filled with fibrin glue, the delaminated cartilage was pressed back in place until solidification. At one year after fibrin glue reparing, patients showed favorable functional outcomes and macroscopically healthy repair tissue could be noted on the second examination of the repair area [200, 201]. Kucharik et al. [202] proposed that using BMAC to stick chondral flap.
Two suture techniques have been reported [203–206]. In the first technique, suture anchors are placed on top of the acetabular rim near the area of delamination or flaps, then passing the sutures around the cartilage and labrum as a unit. In the second technique, all-suture anchors are inserted in the medial acetabulum, which are then placed through the cartilage and the mid substance of the labrum toward the rim in a mattress configuration, and these sutures are fixed with outer row anchors. The limited cases reported to date have showed positive short- to midterm-term clinical and second-look arthroscopy outcomes [203, 206]. However, it is noteworthy that sutures may cut the cartilage and lead to femoral head cartilage wear [198]. Recently, Dong et al. [207] introduced the technique of biochondral nail fixation for acetabular cartilage delamination. The detached cartilage was refixed by inserting an absorbable chondral nail perpendicular to the delamination surface, which maintained the articular surface smooth and flat. The nail surface has grooves that allow cell migration, which can be regarded as a combination of cartilage fixation and microfracture surgery.
A cadaveric study compared the biomechanical stability of chondral flap repair techniques under physiological gait cycles [204]. The results showed that the fibrin glue and cyanoacrylate repairs always failed midway through the test, while the repairs of both suture and hydrogel scaffolds were sufficiently stable. We believe that these results do not represent the real repair process in vivo but could be served as a reference for initial rehabilitation activities.
Rehabilitation
Current rehabilitation protocols of FAIS-related articular cartilage lesions are mainly based on personal experience or expert recommendations. The limited research has only covered patients who underwent chondroplasty and microfracture surgery. In short, postoperative rehabilitation for FAIS-related articular cartilage lesions should include patient education, adjuvant therapy (e.g., cryotherapy and cold compression therapy), use of braces and crutches, neuromuscular electrical stimulation (NMES), continuous passive motion (CPM), weight-bearing and ROM limiting, manual therapy and soft tissue mobilization, strength training, proprioceptive training, functional assessment, gait assessment, and preparation for returning to sports.
The patient’s weight-bearing restriction and motion progression depended on the surgical procedure performed. In general, a restricted weight-bearing protocol is recommended, except for isolated chondroplasty and injection therapy, to promise more extensive biological healing [63, 208–210]. There is increasing clinical data to support weight-bearing as tolerated (WBAT) after chondroplasty. A WBAT protocol allows immediate weight bearing in a progressive, controlled manner, as tolerated by each patient, providing a more comfortable rehabilitation process [211]. Weight-bearing is typically restricted for 3 to 8 weeks after microfracture of FAIS-related articular cartilage lesions, with an average of 4.97 ± 2.35 weeks in 68 studies and a median of 6 weeks in 31 protocols published online [208, 212].
The postoperative rehabilitation protocol should consist of multiple phases, and physiotherapist will play an important role in individualized rehabilitation. Domb et al. [213] described a four-phase structured rehabilitation protocol, and patient-reported hip outcomes showed that patients with arthroscopic chondroplasty and MF could resume satisfactory ADL under this protocol. Some studies have also described similar four-phase rehabilitation protocols and the aims of each phase are relatively consistent [209]. Thus, we summarized these protocols and proposed a four-phase framework to help surgeons establish rehabilitation protocols conveniently (Table 5).
Table 5.
Four-phase framework for rehabilitation protocols of FAIS-related cartilage lesionsa
| Phase | Goal | Modality | Duration |
|---|---|---|---|
|
Phase I Protection |
Relieve pain Protect repaired tissue Early restoration of ROM Avoid muscle weakness and hip contracture |
Adjuvant therapy Restricted motion and weight-bearing Isometric exercises Manual mobilization Moderate quadriceps and gluteus activation |
0–4 weeks post-op, up to 6 weeks |
|
Phase II Restoration Stabilization Strengthening |
Protect repaired tissue Restoration of full pain-free weight-bearing, ROM, and gait patterns Core stabilization Restoration of muscle strength (4-) |
Strengthening and stabilization exercises of lower limb, pelvic, lumbar, and core musculature Closed kinetic chain exercises Resistance training Manual therapy Gait assessment |
4–8 weeks post-op, up to 12 weeks |
|
Phase III Strengthening |
Restoration of muscle strength (5) Improve balance, proprioception, and cardiovascular endurance |
Motor control Strength training Advanced closed kinetic chain exercises Proprioceptive retraining Dynamic stabilization exercises |
8–12 weeks post-op, up to 20 weeks |
|
Phase IV Return to activity |
Return to daily activities and sports Athletes: return to play |
Sport-specific training |
>12 weeks post-op Return to play: 6–9 months post-op |
aROM, range of motion; post-op, post-operation
Prognosis
A systematic review reported that hip preservation procedures for cartilage lesions demonstrated a high success rate, ranging from 85.6–99.7% [214]. In general, the symptoms of cartilage lesions can be relieved by treatment, and most FAIS patients can return to sports (RTS) [215]. However, in addition to providing short-term pain relief from debridement, surgery for FAIS patient who already has extensive cartilage lesions (Tönnis ≥ grade-3) leads to poor therapeutic effects [6, 73]. The stage of chondral lesion, time elapsed from the onset of symptoms and preoperative functional status predict the functional outcomes following surgery [8, 21, 46, 104, 216, 217]. Confirmed subchondral cysts and chondral damage exceeding 2 h on the acetabular clock-face and central acetabular osteophytes indicates poor prognosis [68]. Lighter weight and younger age at baseline may positively associated with post-operative sport activity level, while patients with labral debridement, pathologic acetabular index, and higher BMI are more at risk for a subsequent THA after surgical treatment [218, 219]. Despite microfracture allows athletes with FAIS-related cartilage lesions to return to play at the professional level, including hockey, soccer, football, baseball, tennis, and golf, long-term clinical evidence of prognosis is still lacking [220–222]. And it cannot be ignored that the rate of athletes who cannot RTS after arthroscopic treatment for FAIS was approximately 11–12%, which will worse within the presence of cartilage lesions [63, 219, 223–225].
Conclusion
Now a deeper understanding of FAIS-related aceabular cartilage lesions has been achieved and some consensus has been reached on the mechanism, diagnosis, classification, treatment and rehabilitation. The latest clinical attention of FAIS-related aceabular cartilage lesions has focused on the exploration of molecular biological mechanisms and the application of arthroscopic tissue engineering technology in order to provide better treatment. With increased clinical data and technological advancement, the evidence-based management of FAIS-related aceabular cartilage lesions doesn’t seem far away anymore.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Ahamed Fazloon Fathima Farha, Xinyu Qu, Jie Li, and Zeng Lin for advice on writing.
Abbreviations
- FAIS
Femoroacetabular impingement syndrome
- OA
Osteoarthritis
- CLJ
Chondrolabral junction
- ROM
Range of motion
- ECM
Extracellular matrix
- FADIR
Flexion adduction internal rotation
- FABER
Flexion abduction external rotation
- AP
Anteroposterior
- MRI
Magnetic resonance imaging
- CT
Computed tomography
- FOV
Field-of-view
- dMRA
Direct magnetic resonance arthrography
- PPV
Positive predictive value
- NPV
Negative predictive value
- DGEMRIC
Delayed gadolinium-enhanced magnetic resonance of cartilage
- ALAD
Acetabular labrum articular disruption
- ICRS
International Cartilage Repair Society
- MAHORN
Multicenter Arthroscopy of the Hip Outcomes Research Network
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- MF
Microfracture
- ACI
Autologous chondrocyte implantation
- AMIC
Autologous matrix-induced chondrogenesis
- OCT
Osteochondral transplantation
- SHD
Surgical hip dislocation
- AMO
Anterior mini-open approach
- HA
Hip arthroscopy
- HE
Hematoxylin and eosin
- mHHS
Modified Harris Hip Score
- BM
MSCs-Bone marrow mesenchymal stem cells
- BMAC
Bone marrow aspirate concentrate
- PRP
Platelet-rich plasma
- MACI
Matrix-induced autologous chondrocyte implantation
- PGA/PLA
Polyglycolic acid/polylactic acid
- MATT
Micro-fragmented adipose tissue transplantation
- OAT
Osteochondral autograft transplantation
- OCA
Osteochondral allograft transplantation
- PLGA
Polylactide-coglycolide copolymer
- PCT
Particulated cartilage transplantation
- ADL
Activities of daily living
- NMES
Neuromuscular electrical stimulation
- CPM
Continuous passive motion
- WBAT
Weight-bearing as tolerated
- RTS
Return to sports
Author contributions
All the authors contributed to the drafting of the manuscript, revised it critically for intellectual content, and approved the final review article. ZL: Conceptualization, Methodology, Formal analysis, Investigation, Writing-Original Draft, Visualization; JWY: Investigation, Writing-Original Draft, Writing-Review & Editing; PTA: Investigation, Writing-Original Draft, Writing-Review & Editing; WGZ: Conceptualization, Methodology, Writing-Review & Editing; KT: Conceptualization, Methodology, Writing-Review & Editing, Supervision, Funding Acquisition.
Funding
This work was supported by the National Natural Science Foundation of China under Grant No.81601901, Natural Science Foundation of Liaoning, China under Grant No.2019-MS-079; Peak Climbing Program, Dalian under Grant No.2022DF012, and Dalian Science and Technology Innovation Fund under Grant No.2023JJ13SN051.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhi Li, Jiangwei Yu and Peitong An contributed equally to this paper and share the first authorship.
Contributor Information
Weiguo Zhang, Email: dlmedu@outlook.com.
Kang Tian, Email: dmu-tiankang@outlook.com.
References
- 1.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for Osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–20. 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 2.Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of Osteoarthritis of the hip: an Integrated Mechanical Concept. Clin Orthop Relat Res. 2008;466(2):264–72. 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin DR, Dickenson EJ, O’Donnell J, Agricola R, Awan T, Beck M, et al. The Warwick Agreement on femoroacetabular impingement syndrome (FAI syndrome): an international consensus statement. Br J Sports Med. 2016;50(19):1169–76. 10.1136/bjsports-2016-096743. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra HP, Mc Auliffe S, Ardern CL, Kemp JL, Mosler AB, Price A, et al. Oxford consensus on primary cam morphology and femoroacetabular impingement syndrome: part 1—definitions, terminology, taxonomy and imaging outcomes. Br J Sports Med. 2023;57(6):325–41. 10.1136/bjsports-2022-106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Klij P, Heerey J, Waarsing JH, Agricola R. The prevalence of Cam and Pincer Morphology and its Association with Development of Hip Osteoarthritis. J Orthop Sports Phys Therapy. 2018;48(4):230–8. 10.2519/jospt.2018.7816. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer MK, Tumpowsky C, Boone A, Lee J, McCarthy JC. What is the Association between Articular Cartilage Damage and subsequent THA 20 years after hip arthroscopy for Labral tears? Clin Orthop Relat Res. 2019;477(5):1211–20. 10.1097/CORR.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clohisy JC, Baca G, Beaulé PE, Kim YJ, Larson CM, Millis MB, et al. Descriptive epidemiology of Femoroacetabular Impingement: a north American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348–56. 10.1177/0363546513488861. [DOI] [PubMed] [Google Scholar]
- 8.Lund B, Nielsen TG, Lind M. Cartilage status in FAI patients – results from the Danish hip Arthroscopy Registry (DHAR). SICOT-J. 2017;3:44. 10.1051/sicotj/2017023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual-Garrido C, Li DJ, Grammatopoulos G, Yanik EL, Group ANCHOR, Clohisy JC. The pattern of Acetabular Cartilage wear is hip morphology-dependent and patient demographic-dependent. Clin Orthop Relat Res. 2019;477(5):1021–33. 10.1097/CORR.0000000000000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez-Ahedo C, Gui C, Rabe SM, Chandrasekaran S, Lodhia P, Domb BG. Acetabular Chondral lesions in Hip Arthroscopy: relationships between Grade, Topography, and demographics. Am J Sports Med. 2017;45(11):2501–6. 10.1177/0363546517708192. [DOI] [PubMed] [Google Scholar]
- 11.Claßen T, Körsmeier K, Kamminga M, Beck S, Rekowski J, Jäger M, et al. Is early treatment of cam-type femoroacetabular impingement the key to avoiding associated full thickness isolated chondral defects? Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2332–7. 10.1007/s00167-014-3332-7. [DOI] [PubMed] [Google Scholar]
- 12.Saberi Hosnijeh F, Zuiderwijk ME, Versteeg M, Smeele HTW, Hofman A, Uitterlinden AG, et al. Cam Deformity and Acetabular Dysplasia as Risk factors for hip osteoarthritis. Arthritis Rheumatol. 2017;69(1):86–93. 10.1002/art.39929. [DOI] [PubMed] [Google Scholar]
- 13.Wyles CC, Heidenreich MJ, Jeng J, Larson DR, Trousdale RT, Sierra RJ. The John Charnley Award: redefining the natural history of Osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Relat Res. 2017;475(2):336–50. 10.1007/s11999-016-4815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoch A, Schenk P, Jentzsch T, Rahm S, Zingg PO. FAI morphology increases the risk for osteoarthritis in young people with a minimum follow-up of 25 years. Arch Orthop Trauma Surg. 2021;141(7):1175–81. 10.1007/s00402-020-03522-3. [DOI] [PubMed] [Google Scholar]
- 15.McClincy MP, Lebrun DG, Tepolt FA, Kim YJ, Yen YM, Kocher MS. Clinical and Radiographic Predictors of Acetabular Cartilage Lesions in adolescents undergoing hip arthroscopy. Am J Sports Med. 2018;46(13):3082–9. 10.1177/0363546518801848. [DOI] [PubMed] [Google Scholar]
- 16.Cho YJ, Rhyu KH, Chun YS, Kim MS. Patterns of labral tears and cartilage injury are different in femoroacetabular impingement and dysplasia. J Hip Preservation Surg. 2022;9(3):151–7. 10.1093/jhps/hnac026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapron AL, Aoki SK, Weiss JA, Krych AJ, Maak TG. Isolated focal cartilage and labral defects in patients with femoroacetabular impingement syndrome may represent new, unique injury patterns. Knee Surg Sports Traumatol Arthrosc. 2019;27(10):3057–65. 10.1007/s00167-018-4861-2. [DOI] [PubMed] [Google Scholar]
- 18.Kraeutler MJ, Goodrich JA, Fioravanti MJ, Garabekyan T, Mei-Dan O. The Outside-In lesion of hip impingement and the Inside-Out Lesion of Hip Dysplasia: two distinct patterns of Acetabular Chondral Injury. Am J Sports Med. 2019;47(12):2978–84. 10.1177/0363546519871065. [DOI] [PubMed] [Google Scholar]
- 19.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012–8. 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 20.Tamura S, Nishii T, Takao M, Sakai T, Yoshikawa H, Sugano N. Differences in the locations and modes of labral tearing between dysplastic hips and those with femoroacetabular impingement. Bone Joint J. 2013;95–B(10):1320–5. 10.1302/0301-620X.95B10.31647. [DOI] [PubMed] [Google Scholar]
- 21.Carreira DS, Shaw DB, Ueland TE, Wolff AB, Christoforetti JJ, Salvo JP, et al. Acetabular Cartilage lesions Predict Inferior Mid-term outcomes for Arthroscopic Labral Repair and Treatment of Femoroacetabular Impingement Syndrome. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(12):3152–8. 10.1016/j.arthro.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Beaulé PE, Hynes K, Parker G, Kemp KA. Can the Alpha Angle Assessment of Cam Impingement Predict Acetabular Cartilage Delamination? Clin Orthop Relat Res. 2012;470(12):3361–7. 10.1007/s11999-012-2601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagen MS, Hannay WM, Saluan Q, Lynch TS, Westermann RW, Rosneck J. Magnetic Resonance Imaging Predictors of Chondral Lesions in patients with Femoroacetabular Impingement: an analysis of 545 cases. Arthroscopy: J Arthroscopic Relat Surg. 2021;37(8):2497–501. 10.1016/j.arthro.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 24.Shapira J, Owens JS, Jimenez AE, Maldonado DR, Rosinsky PJ, Ankem HK, et al. Dunn View Alpha Angle more useful than femoral Head-Neck Offset to Predict Acetabular cartilage damage in patients with Femoroacetabular Impingement Syndrome undergoing hip arthroscopy. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(4):1193–200. 10.1016/j.arthro.2021.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in Arthroscopy. Am J Sports Med. 2011;39(2):296–303. 10.1177/0363546510384787. [DOI] [PubMed] [Google Scholar]
- 26.Gao G, Dong H, Wang J, Ao Y, Xu Y. Accuracy of Magnetic Resonance Imaging in the diagnosis of Acetabular Chondral Delamination in Femoroacetabular Impingement. Orthop J Sports Med. 2022;10(8):23259671221119225. 10.1177/23259671221119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mygind-Klavsen B, Lund B, Nielsen TG, Maagaard N, Kraemer O, Hölmich P, et al. Danish hip Arthroscopy Registry: predictors of outcome in patients with femoroacetabular impingement (FAI). Knee Surg Sports Traumatol Arthrosc. 2019;27(10):3110–20. 10.1007/s00167-018-4941-3. [DOI] [PubMed] [Google Scholar]
- 28.Eijer H, Hogervorst T. Femoroacetabular impingement causes osteoarthritis of the hip by migration and micro-instability of the femoral head. Med Hypotheses. 2017;104:93–6. 10.1016/j.mehy.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Kohl S, Hosalkar HS, Mainil-Varlet P, Krueger A, Buechler L, Siebenrock K. Histology of damaged Acetabular Cartilage in Symptomatic Femoroacetabular Impingement: an observational analysis. HIP Int. 2011;21(2):154–62. 10.5301/hip.2011.6515. [DOI] [PubMed] [Google Scholar]
- 30.Morris WZ, Li RT, Liu RW, Salata MJ, Voos JE. Origin of Cam morphology in Femoroacetabular Impingement. Am J Sports Med. 2018;46(2):478–86. 10.1177/0363546517697689. [DOI] [PubMed] [Google Scholar]
- 31.Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of Femoroacetabular Impingement. Clin Orthop Relat Res. 2008;466(2):273–80. 10.1007/s11999-007-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jannelli E, Parafioriti A, Acerbi A, Ivone A, Fioruzzi A, Fontana A. Acetabular Delamination: Epidemiology, histological features, and treatment. CARTILAGE. 2019;10(3):314–20. 10.1177/1947603518768096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulé PE, OʼNeill M, Rakhra K. Acetabular Labral tears. J Bone Joint Surgery-American Volume. 2009;91(3):701–10. 10.2106/JBJS.H.00802. [DOI] [PubMed] [Google Scholar]
- 34.Türker M, Kılıçoğlu Ö, Göksan B, Bilgiç B. Vascularity and histology of fetal labrum and chondrolabral junction: its relevance to chondrolabral detachment tears. Knee Surg Sports Traumatol Arthrosc. 2012;20(2):381–6. 10.1007/s00167-011-1566-1. [DOI] [PubMed] [Google Scholar]
- 35.Cashin M, Uhthoff H, O’Neill M, Beaulé PE. Embryology of the acetabular labral- chondral complex. J Bone Joint Surg. 2008;90:8. [DOI] [PubMed] [Google Scholar]
- 36.Yarwood W, Sunil Kumar KH, Ng KCG, Khanduja V. Biomechanics of Cam Femoroacetabular Impingement: a systematic review. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(1):174–89. 10.1016/j.arthro.2021.05.066. [DOI] [PubMed] [Google Scholar]
- 37.Diamond LE, Bennell KL, Wrigley TV, Hinman RS, Hall M, O’Donnell J, et al. Trunk, pelvis and hip biomechanics in individuals with femoroacetabular impingement syndrome: strategies for step ascent. Gait Posture. 2018;61:176–82. 10.1016/j.gaitpost.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 38.King MG, Lawrenson PR, Semciw AI, Middleton KJ, Crossley KM. Lower limb biomechanics in femoroacetabular impingement syndrome: a systematic review and meta-analysis. Br J Sports Med. 2018;52(9):566–80. 10.1136/bjsports-2017-097839. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Wang W, Thoreson AR, Zhao C, Zhu W, Dou P. Finite element prediction of contact pressures in cam-type femoroacetabular impingement with varied alpha angles. Comput Methods Biomech BioMed Eng. 2017;20(3):294–301. 10.1080/10255842.2016.1224861. [DOI] [PubMed] [Google Scholar]
- 40.Diamond LE, Bennell KL, Wrigley TV, Hinman RS, O’Donnell J, Hodges PW. Squatting biomechanics in individuals with symptomatic femoroacetabular impingement. Med Sci Sports Exerc. 2017;49(8):1520–9. 10.1249/MSS.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 41.Naili JE, Stålman A, Valentin A, Skorpil M, Weidenhielm L. Hip joint range of motion is restricted by pain rather than mechanical impingement in individuals with femoroacetabular impingement syndrome. Arch Orthop Trauma Surg. 2022;142(8):1985–94. 10.1007/s00402-021-04185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albertoni DB, Gianola S, Bargeri S, Hoxhaj I, Munari A, Maffulli N, et al. Does femoroacetabular impingement syndrome affect range of motion? A systematic review with meta-analysis. Br Med Bull. 2023;145(1):45–59. 10.1093/bmb/ldac027. [DOI] [PubMed] [Google Scholar]
- 43.Ng KCG, Lamontagne M, Labrosse MR, Beaulé PE. Hip Joint Stresses Due to Cam-Type Femoroacetabular Impingement: A Systematic Review of Finite Element Simulations. Lammi M, ed. PLoS ONE. 2016;11(1):e0147813. 10.1371/journal.pone.0147813 [DOI] [PMC free article] [PubMed]
- 44.Todd JN, Maak TG, Anderson AE, Ateshian GA, Weiss JA. How does Chondrolabral damage and Labral Repair Influence the mechanics of the hip in the setting of Cam morphology? A finite-element modeling study. Clin Orthop Relat Res. 2022;480(3):602–15. 10.1097/CORR.0000000000002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwyer MK, Jones HL, Field RE, McCarthy JC, Noble PC. Femoroacetabular impingement negates the Acetabular Labral Seal during pivoting maneuvers but not Gait. Clin Orthop Relat Res. 2015;473(2):602–7. 10.1007/s11999-014-3760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierannunzii L. Femoroacetabular impingement: question-driven review of hip joint pathophysiology from asymptomatic skeletal deformity to end-stage osteoarthritis. J Orthop Traumatol. 2019;20(1):32. 10.1186/s10195-019-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grantham WJ, Philippon MJ. Etiology and Pathomechanics of Femoroacetabular Impingement. Curr Rev Musculoskelet Med. 2019;12(3):253–9. 10.1007/s12178-019-09559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Speirs AD, Beaulé PE, Huang A, Frei H. Properties of the cartilage layer from the cam-type hip impingement deformity. J Biomech. 2017;55:78–84. 10.1016/j.jbiomech.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Gao G, Wu R, Liu R, Wang J, Ao Y, Xu Y. Genes associated with inflammation and bone remodeling are highly expressed in the bone of patients with the early-stage cam-type femoroacetabular impingement. J Orthop Surg Res. 2021;16(1):348. 10.1186/s13018-021-02499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner S, Hofstetter W, Chiquet M, Mainil-Varlet P, Stauffer E, Ganz R, et al. Early osteoarthritic changes of human femoral head cartilage subsequent to femoro-acetabular impingement. Osteoarthr Cartil. 2003;11(7):508–18. 10.1016/S1063-4584(03)00075-X. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto S, Rai MF, Gill CS, Zhang Z, Sandell LJ, Clohisy JC. Molecular characterization of articular cartilage from young adults with femoroacetabular impingement. J Bone Joint Surg. 2013;95(16):1457–64. 10.2106/JBJS.L.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chinzei N, Hashimoto S, Fujishiro T, Hayashi S, Kanzaki N, Uchida S, et al. Inflammation and degeneration in cartilage samples from patients with femoroacetabular impingement. J Bone Joint Surg. 2016;98(2):135–41. 10.2106/JBJS.O.00443. [DOI] [PubMed] [Google Scholar]
- 53.Haneda M, Rai MF, O’Keefe RJ, Brophy RH, Clohisy JC, Pascual-Garrido C. Inflammatory response of articular cartilage to Femoroacetabular Impingement in the hip. Am J Sports Med. 2020;48(7):1647–56. 10.1177/0363546520918804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascual-Garrido C, Kamenaga T, Brophy RH, Shen J, O’Keefe RJ, Clohisy JC. Otto Aufranc Award: identification of Key Molecular players in the progression of Hip Osteoarthritis through transcriptomes and epigenetics. J Arthroplast. 2022;37(7):S391–9. 10.1016/j.arth.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamenaga T, Shen J, Wu M, Brophy RH, Clohisy JC, O’Keefe RJ, et al. Epigenetic dysregulation of articular cartilage during progression of hip femoroacetabular impingement disease. J Orthop Res. 2023;41(8):1678–86. 10.1002/jor.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhns BD, Reuter JM, Hansen VL, Soles GL, Jonason JH, Ackert-Bicknell CL, et al. Whole‐genome RNA sequencing identifies distinct transcriptomic profiles in impingement cartilage between patients with femoroacetabular impingement and hip osteoarthritis. J Orthop Res. 2023;41(7):1517–30. 10.1002/jor.25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zurmühle CA, Schmaranzer F, Nuss K, Wolfer N, Ryan MK, Zheng G, et al. Proof of concept: hip joint damage occurs at the zone of femoroacetabular impingement (FAI) in an experimental FAI sheep model. Osteoarthr Cartil. 2019;27(7):1075–83. 10.1016/j.joca.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Kamenaga T, Haneda M, Brophy RH, O’Keefe RJ, Clohisy JC, Pascual-Garrido C. A novel model of hip femoroacetabular impingement in immature rabbits reproduces the distinctive Head-Neck Cam Deformity. Am J Sports Med. 2022;50(7):1919–27. 10.1177/03635465221090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speirs AD, Beaulé PE, Rakhra KS, Schweitzer ME, Frei H. Increased acetabular subchondral bone density is associated with cam-type femoroacetabular impingement. Osteoarthr Cartil. 2013;21(4):551–8. 10.1016/j.joca.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 60.Ng KCG, Mantovani G, Lamontagne M, Labrosse MR, Beaulé PE. Cam FAI and smaller Neck angles increase subchondral bone stresses during squatting: a finite element analysis. Clin Orthop Relat Res. 2019;477(5):1053–63. 10.1097/CORR.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng KCG, Rouhi G, Lamontagne M, Beaulé PE. Finite element analysis examining the effects of Cam FAI on hip joint mechanical loading using subject-specific geometries during Standing and Maximum Squat. HSS Journal®: Musculoskelet J Hosp Special Surg. 2012;8(3):206–12. 10.1007/s11420-012-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiman MP, Agricola R, Kemp JL, Heerey JJ, Weir A, Van Klij P, et al. Consensus recommendations on the classification, definition and diagnostic criteria of hip-related pain in young and middle-aged active adults from the International hip-related Pain Research Network, Zurich 2018. Br J Sports Med. 2020;54(11):631–41. 10.1136/bjsports-2019-101453. [DOI] [PubMed] [Google Scholar]
- 63.Takla A, O’Donnell J, Voight M, Byrd T, Dienst M, Martin RR, et al. The 2019 International Society of Hip Preservation (ISHA) physiotherapy agreement on assessment and treatment of femoroacetabular impingement syndrome (FAIS): an international consensus statement. J Hip Preservation Surg. 2021;7(4):631–42. 10.1093/jhps/hnaa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampson TG. Arthroscopic treatment for Chondral lesions of the hip. Clin Sports Med. 2011;30(2):331–48. 10.1016/j.csm.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 65.Makhni EC, Stone AV, Ukwuani GC, Zuke W, Garabekyan T, Mei-Dan O, et al. A critical review: management and Surgical options for articular defects in the hip. Clin Sports Med. 2017;36(3):573–86. 10.1016/j.csm.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Jones AC, Stewart TD, Maher N, Holton C. Can a computational model predict the Effect of Lesion Location on Cam-type hip impingement? Clin Orthop Relat Res. 2023;481(7):1432–43. 10.1097/CORR.0000000000002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mascarenhas VV, Castro MO, Rego PA, Sutter R, Sconfienza LM, Kassarjian A, et al. The Lisbon Agreement on Femoroacetabular Impingement Imaging—part 1: overview. Eur Radiol. 2020;30(10):5281–97. 10.1007/s00330-020-06822-9. [DOI] [PubMed] [Google Scholar]
- 68.Castro MO, Mascarenhas VV, Afonso PD, Rego P, Schmaranzer F, Sutter R, et al. The Lisbon Agreement on Femoroacetabular Impingement Imaging—part 3: imaging techniques. Eur Radiol. 2021;31(7):4652–68. 10.1007/s00330-020-07501-5. [DOI] [PubMed] [Google Scholar]
- 69.Rogers MJ, Sato EH, LaBelle MW, Ou Z, Presson AP, Maak TG. Association of Cam Deformity on Anteroposterior pelvic radiographs and more severe Chondral damage in Femoroacetabular Impingement Syndrome. Am J Sports Med. 2022;50(11):2980–8. 10.1177/03635465221111565. [DOI] [PubMed] [Google Scholar]
- 70.Mortensen AJ, Philippi MT, Karns MR, Kahn TL, Adeyemi TF, Maak TG, et al. A narrow posterior Joint Space on a false Profile Radiograph does not correlate with posterior joint cartilage degeneration in hip preservation patients. Arthroscopy: J Arthroscopic Relat Surg. 2020;36(12):2984–91. 10.1016/j.arthro.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 71.Pun S, Kumar D, Lane NE. Femoroacetabular impingement. Arthritis Rheumatol. 2015;67(1):17–27. 10.1002/art.38887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosinsky PJ, Chen JW, Lall AC, Wojnowski NM, Shapira J, Maldonado DR, et al. Can Radiographic Joint Space accurately predict chondral damage during hip arthroscopy? A cross-sectional analysis. Arthroscopy: J Arthroscopic Relat Surg. 2020;36(6):1565–e15721. 10.1016/j.arthro.2020.01.034. [DOI] [PubMed] [Google Scholar]
- 73.Horisberger M, Brunner A, Herzog RF. Arthroscopic treatment of femoral Acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy: J Arthroscopic Relat Surg. 2010;26(5):623–9. 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Schmaranzer F, Kheterpal AB, Bredella MA. Best practices: hip femoroacetabular impingement. Am J Roentgenol. 2021;216(3):585–98. 10.2214/AJR.20.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saied AM, Redant C, El-Batouty M, El-Lakkany MR, El-Adl WA, Anthonissen J, et al. Accuracy of magnetic resonance studies in the detection of chondral and labral lesions in femoroacetabular impingement: systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18(1):83. 10.1186/s12891-017-1443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sutter R, Zubler V, Hoffmann A, Mamisch-Saupe N, Dora C, Kalberer F, et al. Hip MRI: how useful is Intraarticular contrast material for evaluating surgically proven lesions of the Labrum and articular cartilage? Am J Roentgenol. 2014;202(1):160–9. 10.2214/AJR.12.10266. [DOI] [PubMed] [Google Scholar]
- 77.Hanke MS, Steppacher SD, Anwander H, Werlen S, Siebenrock KA, Tannast M. What MRI findings predict failure 10 years after surgery for Femoroacetabular Impingement? Clin Orthop Relat Res. 2017;475(4):1192–207. 10.1007/s11999-016-5040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang P, Li C, Wang W, Zhang B, Miao W, Liu Y. 3.0 T MRI is more recommended to detect acetabular labral tears than MR Arthrography: an updated meta-analysis of diagnostic accuracy. J Orthop Surg Res. 2022;17(1):126. 10.1186/s13018-022-02981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaragoza E, Lattanzio PJ, Beaule PE. Magnetic Resonance Imaging with Gadolinium Arthrography to Assess Acetabular Cartilage Delamination. [DOI] [PubMed]
- 80.Konstantinidis G, Mitchell M, Boyd G, Coady C, Ghosh S, Wong I. Poor sensitivity of magnetic resonance arthrography to detect hip chondral delamination: a Retrospective Follow-Up of 227 FAI-Operated patients. CARTILAGE. 2021;12(2):162–8. 10.1177/1947603518816453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfirrmann CWA, Duc SR, Zanetti M, Dora C, Hodler J. MR Arthrography of Acetabular Cartilage Delamination in Femoroacetabular Cam Impingement 1. Radiology. 2008;249(1):236–41. 10.1148/radiol.2491080093. [DOI] [PubMed] [Google Scholar]
- 82.Bittersohl B, Hosalkar HS, Hesper T, Tiderius CJ, Zilkens C, Krauspe R. Advanced Imaging in Femoroacetabular Impingement: current state and future prospects. Front Surg. 2015;2. 10.3389/fsurg.2015.00034. [DOI] [PMC free article] [PubMed]
- 83.Schmaranzer F, Klauser A, Kogler M, Henninger B, Forstner T, Reichkendler M, et al. Diagnostic performance of direct traction MR Arthrography of the hip: detection of chondral and labral lesions with arthroscopic comparison. Eur Radiol. 2015;25(6):1721–30. 10.1007/s00330-014-3534-x. [DOI] [PubMed] [Google Scholar]
- 84.Mascarenhas VV, Caetano A, Dantas P, Rego P. Advances in FAI Imaging: a focused review. Curr Rev Musculoskelet Med. 2020;13(5):622–40. 10.1007/s12178-020-09663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linda DD, Naraghi A, Murnaghan L, Whelan D, White LM. Accuracy of non-arthrographic 3T MR imaging in evaluation of intra-articular pathology of the hip in femoroacetabular impingement. Skeletal Radiol. 2017;46(3):299–308. 10.1007/s00256-016-2551-z. [DOI] [PubMed] [Google Scholar]
- 86.Ellermann J, Ziegler C, Nissi MJ, Goebel R, Hughes J, Benson M, et al. Acetabular Cartilage Assessment in patients with femoroacetabular impingement by using T2* mapping with Arthroscopic Verification. Radiology. 2014;271(2):512–23. 10.1148/radiol.13131837. [DOI] [PubMed] [Google Scholar]
- 87.Hesper T, Neugroda C, Schleich C, Antoch G, Hosalkar H, Krauspe R, et al. T2*-Mapping of Acetabular cartilage in patients with Femoroacetabular Impingement at 3 Tesla: comparative analysis with arthroscopic findings. CARTILAGE. 2018;9(2):118–26. 10.1177/1947603517741168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samaan MA, Zhang AL, Gallo MC, Schwaiger BJ, Link TM, Souza RB, et al. Quantitative magnetic resonance arthrography in patients with femoroacetabular impingement: quantitative MRA in FAI patients. J Magn Reson Imaging. 2016;44(6):1539–45. 10.1002/jmri.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Samaan MA, Pedoia V, Zhang AL, Gallo MC, Link TM, Souza RB, et al. A novel mr-based method for detection of cartilage delamination in femoroacetabular impingement patients. J Orthop Res. 2018;36(3):971–8. 10.1002/jor.23667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siebenrock KA, Kienle KP, Steppacher SD, Tannast M, Mamisch TC, Von Rechenberg B. Biochemical MRI predicts hip osteoarthritis in an experimental ovine femoroacetabular impingement model. Clin Orthop Relat Res. 2015;473(4):1318–24. 10.1007/s11999-014-3849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Eliezer N, Raya JG, Babb JS, Youm T, Sodickson DK, Lattanzi R. A New Method for cartilage evaluation in Femoroacetabular Impingement using quantitative T2 magnetic resonance imaging: preliminary validation against arthroscopic findings. CARTILAGE. 2021;13(1suppl):S1315–23. 10.1177/1947603519870852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Outerbridge RE, THE ETIOLOGY OF CHONDROMALACIA PATELLAE. J Bone Joint Surg. 1961;43(4). [DOI] [PubMed]
- 93.Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;418:67–73. [PubMed] [Google Scholar]
- 94.Kelly BT, Philippon MJ et al. Arthroscopic Hip Anatomy. In: Callaghan JJ, Rosenberg AG, Rubash HE, editors. The Adult Hip, 2nd Edition. Lippincott Williams & Wilkins, 2007.
- 95.Konan S, Rayan F, Meermans G, Witt J, Haddad FS. Validation of the classification system for acetabular chondral lesions identified at arthroscopy in patients with femoroacetabular impingement. J Bone Joint Surg Br Volume. 2011;93–B(3):332–6. 10.1302/0301-620X.93B3.25322. [DOI] [PubMed] [Google Scholar]
- 96.Brittberg M, Winalski CS, EVALUATION OF CARTILAGE, INJURIES AND REPAIR. J Bone Joint Surgery-American Volume. 2003;85:58–69. 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 97.Safran MR, Hariri S. Hip Arthroscopy Assessment Tools and outcomes. Oper Tech Orthop. 2010;20(4):264–77. 10.1053/j.oto.2010.09.014. [Google Scholar]
- 98.Yamamoto T, Zurmühle CA, Stetzelberger VM, Schwab JM, Steppacher SD, Tannast M. The New Bern Chondrolabral classification is Reliable and reproducible. Clin Orthop Relat Res. 2021;479(5):1002–13. 10.1097/CORR.0000000000001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Logan ZS, Redmond JM, Spelsberg SC, Jackson TJ, Domb BG. Chondral Lesions of the Hip. Clin Sports Med. 2016;35(3):361–72. 10.1016/j.csm.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Mayer SW, Fauser TR, Marx RG, Ranawat AS, Kelly BT, Lyman S, et al. Reliability of the classification of cartilage and labral injuries during hip arthroscopy. J Hip Preservation Surg. 2021;7(3):448–57. 10.1093/jhps/hnaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amenabar T, Piriz J, Mella C, Hetaimish BM, O’Donnell J. Reliability of 3 different arthroscopic classifications for Chondral damage of the Acetabulum. Arthroscopy: J Arthroscopic Relat Surg. 2015;31(8):1492–6. 10.1016/j.arthro.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 102.Nepple JJ, Larson CM, Smith MV, Kim YJ, Zaltz I, Sierra RJ, et al. The reliability of arthroscopic classification of Acetabular Rim Labrochondral Disease. Am J Sports Med. 2012;40(10):2224–9. 10.1177/0363546512457157. [DOI] [PubMed] [Google Scholar]
- 103.Ilizaliturri VM, Byrd JWT, Sampson TG, Guanche CA, Philippon MJ, Kelly BT, et al. A Geographic Zone Method to describe Intra-articular Pathology in Hip Arthroscopy: cadaveric study and preliminary Report. Arthroscopy: J Arthroscopic Relat Surg. 2008;24(5):534–9. 10.1016/j.arthro.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 104.Aprato A, Jayasekera N, Villar R. Timing in hip arthroscopy: does surgical timing change clinical results? Int Orthop (SICOT). 2012;36(11):2231–4. 10.1007/s00264-012-1655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119–24. 10.1302/0301-620x.83b8.11964. [DOI] [PubMed] [Google Scholar]
- 106.Bagheri K, Sierra F, Jamali AA. Acetabular cartilage repair: state of the art in surgical treatment. J Hip Preservation Surg. 2020;7(2):205–24. 10.1093/jhps/hnaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sankar WN, Matheney TH, Zaltz I. Femoroacetabular impingement: current concepts and controversies. Orthop Clin North Am. 2013;44(4):575–89. 10.1016/j.ocl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 108.Byrd JWT, Jones KS. Arthroscopic management of Femoroacetabular Impingement: Minimum 2-Year follow-up. Arthroscopy: J Arthroscopic Relat Surg. 2011;27(10):1379–88. 10.1016/j.arthro.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 109.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91(1):16–23. 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 110.Jackson M, Poskttt EME. The effects of high-energy feeding on energy balance and growth in infants with congenital heart disease and failure to thrive. Br J Nutr. 1991;65(2):131–43. 10.1079/BJN19910075. [DOI] [PubMed] [Google Scholar]
- 111.Migliorini F, Liu Y, Catalano G, Trivellas A, Eschweiler J, Tingart M, et al. Medium-term results of arthroscopic treatment for femoroacetabular impingement. Br Med Bull. 2021;138(1):68–84. 10.1093/bmb/ldaa038. [DOI] [PubMed] [Google Scholar]
- 112.Migliorini F, Liu Y, Eschweiler J, Baroncini A, Tingart M, Maffulli N. Increased range of motion but otherwise similar clinical outcome of arthroscopy over open osteoplasty for femoroacetabular impingement at midterm follow-up: a systematic review. Surgeon. 2022;20(3):194–208. 10.1016/j.surge.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 113.Addai D, Zarkos J, Pettit M, Sunil Kumar KH, Khanduja V. Outcomes following surgical management of femoroacetabular impingement: a systematic review and meta-analysis of different surgical techniques. Bone Joint Res. 2021;10(9):574–90. 10.1302/2046-3758.109.BJR-2020-0443.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tian K, Gao G, Dong H, Zhang S, Zhang W, Wang J, et al. Arthroscopic capsular suture-lifting technique for treating femoroacetabular impingement patients with a high risk of postoperative anterior instability. Arthrosc Techniques. 2023;12(2):e307–12. 10.1016/j.eats.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wright VJ, McCrum CL, Li H, Tranovich MJ, Huard J. Significant chondrocyte viability is Present in Acetabular Chondral flaps Associated with Femoroacetabular Impingement. Am J Sports Med. 2018;46(1):149–52. 10.1177/0363546517732751. [DOI] [PubMed] [Google Scholar]
- 116.Hariri S, Truntzer J, Smith RL, Safran MR. Biochemical and Cellular Assessment of Acetabular Chondral flaps Identified during Hip Arthroscopy. Arthroscopy: J Arthroscopic Relat Surg. 2015;31(6):1077–83. 10.1016/j.arthro.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez-Fontan F, Payne KA, Chahla J, Mei-Dan O, Richards A, Uchida S, et al. Viability and tissue quality of cartilage flaps from patients with femoroacetabular hip impingement: a matched-control comparison. Orthop J Sports Med. 2017;5(8):2325967117723608. 10.1177/2325967117723608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levinson C, Naal FD, Salzmann GM, Zenobi-Wong M, Leunig M. Is there a scientific rationale for the refixation of Delaminated Chondral flaps in Femoroacetabular Impingement? A Laboratory Study. Clin Orthop Relat Res. 2020;478(4):854–67. 10.1097/CORR.0000000000001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rocco P, Lorenzo DB, Guglielmo T, Michele P, Nicola M, Vincenzo D. Radiofrequency energy in the arthroscopic treatment of knee chondral lesions: a systematic review. Br Med Bull. 2016;117(1):149–56. 10.1093/bmb/ldw004. [DOI] [PubMed] [Google Scholar]
- 120.Más Martínez J, Sanz Reig J, Morales Santias M, Bustamante Suarez De Puga D. Chondrolysis after Hip Arthroscopy. Arthroscopy: J Arthroscopic Relat Surg. 2015;31(1):167–72. 10.1016/j.arthro.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 121.Hevesi M, Bernard C, Hartigan DE, Levy BA, Domb BG, Krych AJ. Is microfracture necessary? Acetabular Chondrolabral Debridement/Abrasion Demonstrates Similar Outcomes and survival to microfracture in hip arthroscopy: a Multicenter Analysis. Am J Sports Med. 2019;47(7):1670–8. 10.1177/0363546519845346. [DOI] [PubMed] [Google Scholar]
- 122.Nakano N, Gohal C, Duong A, Ayeni OR, Khanduja V. Outcomes of cartilage repair techniques for chondral injury in the hip—a systematic review. Int Orthop (SICOT). 2018;42(10):2309–22. 10.1007/s00264-018-3862-6. [DOI] [PubMed] [Google Scholar]
- 123.MacDonald AE, Bedi A, Horner NS, De Sa D, Simunovic N, Philippon MJ, et al. Indications and outcomes for microfracture as an Adjunct to hip arthroscopy for treatment of Chondral defects in patients with femoroacetabular impingement: a systematic review. Arthroscopy: J Arthroscopic Relat Surg. 2016;32(1):190–e2002. 10.1016/j.arthro.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 124.Trask DJ, Keene JS. Analysis of the current indications for microfracture of Chondral Lesions in the Hip Joint. Am J Sports Med. 2016;44(12):3070–6. 10.1177/0363546516655141. [DOI] [PubMed] [Google Scholar]
- 125.Marquez-Lara A, Mannava S, Howse EA, Stone AV, Stubbs AJ. Arthroscopic management of hip Chondral defects: a systematic review of the literature. Arthroscopy: J Arthroscopic Relat Surg. 2016;32(7):1435–43. 10.1016/j.arthro.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 126.Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and Chondroplasty. Sports Med Arthrosc Rev. 2010;18(2):83–9. 10.1097/JSA.0b013e3181de1189. [DOI] [PubMed] [Google Scholar]
- 127.O’Connor M, Minkara AA, Westermann RW, Rosneck J, Lynch TS. Outcomes of Joint Preservation Procedures for Cartilage Injuries in the hip: a systematic review and Meta-analysis. Orthop J Sports Med. 2018;6(6):2325967118776944. 10.1177/2325967118776944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg. 2010;92(5). [DOI] [PubMed]
- 129.Riedl M, Banke IJ, Goronzy J, Sobau C, Steimer O, Thier S, et al. Patients with small Acetabular cartilage defects caused by Femoroacetabular Impingement do Not Benefit from Microfracture. JCM. 2022;11(21):6283. 10.3390/jcm11216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Domb BG, Gupta A, Dunne KF, Gui C, Chandrasekaran S, Lodhia P. Microfracture in the hip: results of a matched-cohort controlled Study with 2-Year follow-up. Am J Sports Med. 2015;43(8):1865–74. 10.1177/0363546515588174. [DOI] [PubMed] [Google Scholar]
- 131.Domb BG, Redmond JM, Dunne KF, Stake CE, Gupta A. A matched-pair controlled study of microfracture of the hip with average 2-Year Follow-up: do full-thickness Chondral defects portend an Inferior Prognosis in Hip Arthroscopy? Arthroscopy: J Arthroscopic Relat Surg. 2015;31(4):628–34. 10.1016/j.arthro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 132.Lodhia P, Gui C, Chandrasekaran S, Suarez-Ahedo C, Vemula SP, Domb BG. Microfracture in the hip: a matched-control study with average 3-year follow-up. J Hip Preserv Surg. 2015;hnv073. 10.1093/jhps/hnv073. Published online December 10. [DOI] [PMC free article] [PubMed]
- 133.Chaharbakhshi EO, Hartigan DE, Spencer JD, Perets I, Lall AC, Domb BG. Do larger Acetabular Chondral defects portend inferior outcomes in patients undergoing arthroscopic Acetabular microfracture? A matched-controlled study. Arthroscopy: J Arthroscopic Relat Surg. 2019;35(7):2037–47. 10.1016/j.arthro.2019.01.047. [DOI] [PubMed] [Google Scholar]
- 134.Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in Acetabular Chondral defects? Arthroscopy: J Arthroscopic Relat Surg. 2008;24(1):46–50. 10.1016/j.arthro.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 135.Karthikeyan S, Roberts S, Griffin D. Microfracture for Acetabular Chondral defects in patients with Femoroacetabular Impingement: results at second-look arthroscopic surgery. Am J Sports Med. 2012;40(12):2725–30. 10.1177/0363546512465400. [DOI] [PubMed] [Google Scholar]
- 136.Rayes J, Sparavalo S, Wong I. Biological augments for Acetabular Chondral defects in hip Arthroscopy—A scoping review of the current clinical evidence. Curr Rev Musculoskelet Med. 2021;14(6):328–39. 10.1007/s12178-021-09721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schallmo MS, Marquez-Lara A, Luo TD, Rosas S, Stubbs AJ. Arthroscopic treatment of hip chondral defect with microfracture and platelet-rich plasma–infused micronized cartilage allograft augmentation. Arthrosc Techniques. 2018;7(4):e361–5. 10.1016/j.eats.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leunig M, Tibor LM, Naal FD, Ganz R, Steinwachs MR. Surgical technique: second-generation bone marrow stimulation via Surgical dislocation to treat hip cartilage lesions. Clin Orthop Relat Res. 2012;470(12):3421–31. 10.1007/s11999-012-2466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thorey F, Malahias MA, Giotis D. Sustained benefit of autologous matrix-induced chondrogenesis for hip cartilage repair in a recreational athletic population. Knee Surg Sports Traumatol Arthrosc. 2020;28(7):2309–15. 10.1007/s00167-019-05801-y. [DOI] [PubMed] [Google Scholar]
- 140.Fontana A. Microfracture or AMIC for Arthroscopic Repair of Acetabular cartilage defects in Femoroacetabular Impingement. Arthroscopy: J Arthroscopic Relat Surg. 2013;29(10):e50. 10.1016/j.arthro.2013.07.032. [Google Scholar]
- 141.Fontana A, De Girolamo L. Sustained five-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. Bone Joint J. 2015;97–B(5):628–35. 10.1302/0301-620X.97B5.35076. [DOI] [PubMed] [Google Scholar]
- 142.De Girolamo L, Jannelli E, Fioruzzi A, Fontana A. Acetabular Chondral lesions Associated with Femoroacetabular Impingement treated by Autologous Matrix-Induced Chondrogenesis or microfracture: a comparative study at 8-Year Follow-Up. Arthroscopy: J Arthroscopic Relat Surg. 2018;34(11):3012–23. 10.1016/j.arthro.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 143.Fontana A. Autologous membrane Induced Chondrogenesis (AMIC) for the treatment of acetabular chondral defect. Muscles Ligaments Tendons J. 2016;6(3):367–71. 10.11138/mltj/2016.6.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tey M, Mas J, Pelfort X, Monllau JC. Arthroscopic treatment of hip Chondral defects with bone marrow stimulation and BST-CarGel. Arthrosc Techniques. 2015;4(1):e29–33. 10.1016/j.eats.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.John R, Ma J, Wong I. Better clinicoradiological results of BST-CarGel treatment in cartilage repair compared with microfracture in Acetabular Chondral defects at 2 years. Am J Sports Med. 2020;48(8):1961–6. 10.1177/0363546520924841. [DOI] [PubMed] [Google Scholar]
- 146.Tahoun MF, Tey M, Mas J, Abd-Elsattar Eid T, Monllau JC. Arthroscopic repair of Acetabular Cartilage lesions by Chitosan-based Scaffold: clinical evaluation at Minimum 2 years follow-up. Arthroscopy: J Arthroscopic Relat Surg. 2018;34(10):2821–8. 10.1016/j.arthro.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 147.Rhee C, Amar E, Glazebrook M, Coday C, Wong IH. Safety Profile and short-term outcomes of BST-CarGel as an Adjunct to microfracture for the Treatment of Chondral Lesions of the hip. Orthop J Sports Med. 2018;6(8):2325967118789871. 10.1177/2325967118789871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tahoun M, Shehata TA, Ormazabal I, Mas J, Sanz J, Tey Pons M. Results of arthroscopic treatment of chondral delamination in femoroacetabular impingement with bone marrow stimulation and BST-CarGel ®. SICOT-J. 2017;3:51. 10.1051/sicotj/2017031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tahoun MF, Tey M, Ormazabal I, Elsayed AS, Said HG, Monllau JC. Promising radiological outcome after repair of acetabular chondral defects by microfracture augmented with Chitosan-based scaffold: mid-term T2 mapping evaluation. Knee Surg Sports Traumatol Arthrosc. 2021;29(1):324–8. 10.1007/s00167-020-06068-4. [DOI] [PubMed] [Google Scholar]
- 150.Stanish WD, McCormack R, Forriol F, Mohtadi N, Pelet S, Desnoyers J, et al. Novel Scaffold-based BST-CarGel treatment results in Superior Cartilage Repair compared with microfracture in a Randomized Controlled Trial. J Bone Joint Surg. 2013;95(18):1640–50. 10.2106/JBJS.L.01345. [DOI] [PubMed] [Google Scholar]
- 151.Mazek J, Gnatowski M, Salas AP, O’Donnell JM, Domżalski M, Radzimowski J. Arthroscopic utilization of ChondroFiller gel for the treatment of hip articular cartilage defects: a cohort study with 12- to 60-month follow-up. J Hip Preservation Surg. 2021;8(1):22–7. 10.1093/jhps/hnab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Lucas Villarrubia JC, Méndez Alonso MÁ, Sanz Pérez MI, Trell Lesmes F, Panadero Tapia A. Acellular Matrix-Induced chondrogenesis technique improves the results of Chondral lesions Associated with Femoroacetabular Impingement. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(4):1166–78. 10.1016/j.arthro.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 153.Jannelli E, Fontana A. Arthroscopic treatment of chondral defects in the hip: AMIC, MACI, microfragmented adipose tissue transplantation (MATT) and other options. SICOT-J. 2017;3:43. 10.1051/sicotj/2017029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rogers MJ, Kondo M, Kim K, Okano T, Maak TG. Femoral Head Chondrocyte viability at the Cam deformity in patients with Femoroacetabular Impingement Syndrome. Am J Sports Med. 2020;48(14):3586–93. 10.1177/0363546520962788. [DOI] [PubMed] [Google Scholar]
- 155.Wilken F, Slotta-Huspenina J, Laux F, Blanke F, Schauwecker J, Vogt S, et al. Autologous chondrocyte transplantation in Femoroacetabular Impingement Syndrome: growth and redifferentiation potential of chondrocytes harvested from the Femur in Cam-Type Deformities. CARTILAGE. 2021;12(3):377–86. 10.1177/1947603519833138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fontana A, Bistolfi A, Crova M, Rosso F, Massazza G. Arthroscopic treatment of hip Chondral defects: autologous chondrocyte transplantation Versus simple Debridement—A pilot study. Arthroscopy: J Arthroscopic Relat Surg. 2012;28(3):322–9. 10.1016/j.arthro.2011.08.304. [DOI] [PubMed] [Google Scholar]
- 157.Mancini D, Fontana A. Five-year results of arthroscopic techniques for the treatment of acetabular chondral lesions in femoroacetabular impingement. Int Orthop (SICOT). 2014;38(10):2057–64. 10.1007/s00264-014-2403-1. [DOI] [PubMed] [Google Scholar]
- 158.Fickert S, Schattenberg T, Niks M, Weiss C, Thier S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: a case series. Arch Orthop Trauma Surg. 2014;134(7):971–8. 10.1007/s00402-014-1997-5. [DOI] [PubMed] [Google Scholar]
- 159.David RK, Daniel K, Maximilian B, Markus G, Michael S, Carsten P, et al. Is a minimal invasive autologous chondrocyte implantation (ACI) in the hip possible? A feasibility and safety study of arthroscopic treatment of full thickness Acetabular Cartilage defects with an Injectable ACI. J Orthop Surg Tech. 2017;1(1). 10.36959/453/512.
- 160.Körsmeier K, Claßen T, Kamminga M, Rekowski J, Jäger M, Landgraeber S. Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2032–7. 10.1007/s00167-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 161.Krueger DR, Gesslein M, Schuetz M, Perka C, Schroeder JH. Injectable autologous chondrocyte implantation (ACI) in acetabular cartilage defects-three-year results. J Hip Preserv Surg. 2018;5(4):386–92. 10.1093/jhps/hny043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schroeder JH, Hufeland M, Schütz M, Haas NP, Perka C, Krueger DR. Injectable autologous chondrocyte transplantation for full thickness acetabular cartilage defects: early clinical results. Arch Orthop Trauma Surg. 2016;136(10):1445–51. 10.1007/s00402-016-2510-0. [DOI] [PubMed] [Google Scholar]
- 163.Thier S, Baumann F, Weiss C, Fickert S. Feasibility of arthroscopic autologous chondrocyte implantation in the hip using an injectable hydrogel. HIP Int. 2018;28(4):442–9. 10.5301/hipint.5000580. [DOI] [PubMed] [Google Scholar]
- 164.Thier S, Weiss C, Fickert S. Arthroscopic autologous chondrocyte implantation in the hip for the treatment of full-thickness cartilage defects: a case series of 29 patients and review of the literature. SICOT-J. 2017;3:72. 10.1051/sicotj/2017037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bretschneider H, Trattnig S, Landgraeber S, Hartmann A, Günther KP, Dienst M, et al. Arthroscopic matrix-associated, injectable autologous chondrocyte transplantation of the hip: significant improvement in patient-related outcome and good transplant quality in MRI assessment. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1317–24. 10.1007/s00167-019-05466-7. [DOI] [PubMed] [Google Scholar]
- 166.Garcia FL, Williams BT, Polce EM, Heller DB, Aman ZS, Nwachukwu BU, et al. Preparation methods and clinical outcomes of platelet-rich plasma for intra-articular hip disorders: a systematic review and Meta-analysis of Randomized clinical trials. Orthop J Sports Med. 2020;8(10):2325967120960414. 10.1177/2325967120960414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rafols C, Monckeberg JE, Numair J, Botello J, Rosales J. Platelet-Rich plasma augmentation of arthroscopic hip surgery for Femoroacetabular Impingement: a prospective study with 24-Month follow-up. Arthroscopy: J Arthroscopic Relat Surg. 2015;31(10):1886–92. 10.1016/j.arthro.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 168.Foo GL, Knudsen JS, Bacon CJ, Mei-Dan O, McConkey MO, Brick MJ. Peri-operative platelet-rich plasma in arthroscopic femoroacetabular impingement surgery: a randomized controlled trial. J Hip Preservation Surg. 2021;8(1):14–21. 10.1093/jhps/hnab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luo TD, Beck EC, Trammell AP, Koulopoulos MW, Edge CC, Marquez-Lara A, et al. Hip arthroscopic microfracture augmented with platelet-rich plasma-infused micronized cartilage allograft significantly improves functional outcomes. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(10):2819–e28261. 10.1016/j.arthro.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 170.Mardones R, Via AG, Jofré C, Minguell J, Rodriguez C, Tomic A, et al. Cell therapy for cartilage defects of the hip. Muscles Ligaments Tendons J. 2016;6(3):361–6. 10.11138/mltj/2016.6.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rivera E, Seijas R, Rubio M, García-Balletbó M, Vilar JM, Boada PL, et al. Outcomes at 2-Years Follow-Up after hip arthroscopy combining bone marrow Concentrate. J Invest Surg. 2020;33(7):655–63. 10.1080/08941939.2018.1535010. [DOI] [PubMed] [Google Scholar]
- 172.Ivone A, Fioruzzi A, Jannelli E, Castelli A, Ghiara M, Ferranti Calderoni E, et al. Micro-fragmented adipose tissue transplantation (MATT) for the treatment of acetabular delamination. A two years follow up comparison study with microfractures. Acta Bio Med Atenei Parmensis. 2019;90(12–S):69–75. 10.23750/abm.v90i12-S.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14(1):246. 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Murata Y, Uchida S, Utsunomiya H, Hatakeyama A, Nakashima H, Chang A, et al. Synovial mesenchymal stem cells derived from the Cotyloid Fossa Synovium have higher self-renewal and differentiation potential than those from the Paralabral Synovium in the Hip Joint. Am J Sports Med. 2018;46(12):2942–53. 10.1177/0363546518794664. [DOI] [PubMed] [Google Scholar]
- 175.Gelse K, Schneider H. Ex vivo gene therapy approaches to cartilage repair☆. Adv Drug Deliv Rev. 2006;58(2):259–84. 10.1016/j.addr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 176.Hori J, Deie M, Kobayashi T, Yasunaga Y, Kawamata S, Ochi M. Articular cartilage repair using an intra-articular magnet and synovium‐derived cells. J Orthop Res. 2011;29(4):531–8. 10.1002/jor.21267. [DOI] [PubMed] [Google Scholar]
- 177.Hernigou J, Vertongen P, Rasschaert J, Hernigou P. Role of Scaffolds, Subchondral, Intra-articular injections of Fresh Autologous Bone Marrow Concentrate Regenerative cells in treating human knee cartilage lesions: different approaches and different results. IJMS. 2021;22(8):3844. 10.3390/ijms22083844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sobti AS, Baryeh KW, Woolf R, Chana R. Autologous matrix-induced chondrogenesis and bone marrow aspirate concentrate compared with microfracture for arthroscopic treatment of femoroacetabular impingement and chondral lesions of the hip: bridging the osteoarthritis gap and facilitating enhanced recovery. J Hip Preservation Surg. 2021;7(3):503–10. 10.1093/jhps/hnaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Emre TY, Uzun M. Mosaicplasty for the Treatment of the Osteochondral Lesion in the Femoral Head. [PubMed]
- 180.Viamont-Guerra MR, Bonin N, May O, Le Viguelloux A, Saffarini M, Laude F. Promising outcomes of hip mosaicplasty by minimally invasive anterior approach using osteochondral autografts from the ipsilateral femoral head. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):767–76. 10.1007/s00167-019-05442-1. [DOI] [PubMed] [Google Scholar]
- 181.Nam D, Shindle MK, Buly RL, Kelly BT, Lorich DG. Traumatic Osteochondral Injury of the femoral Head treated by Mosaicplasty: a report of two cases. HSS Journal®: Musculoskelet J Hosp Special Surg. 2010;6(2):228–34. 10.1007/s11420-010-9159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hart R, Janec̆ek M, Vis̆n̆a P, Buc̆ek P, Koc̆is̆ J. Mosaicplasty for the treatment of femoral head defect after incorrect resorbable screw insertion. Arthroscopy: J Arthroscopic Relat Surg. 2003;19(10):e137–41. 10.1016/j.arthro.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 183.Zelken JA. First-Person Long-Term follow-up using Autologous Mosaicplasty for Osteochondral Lesion accompanying femoral Head fracture. J Orthop Trauma. 2016;30(2):e70–4. 10.1097/BOT.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 184.Anthonissen J, Rommens PM, Hofmann A. Mosaicplasty for the treatment of a large traumatic osteochondral femoral head lesion: a case report with 2 year follow-up and review of the literature. Arch Orthop Trauma Surg. 2016;136(1):41–6. 10.1007/s00402-015-2352-1. [DOI] [PubMed] [Google Scholar]
- 185.Evans KN, Providence BC. Case Report: fresh-stored osteochondral allograft for treatment of Osteochondritis dissecans the femoral head. Clin Orthop Relat Res. 2010;468(2):613–8. 10.1007/s11999-009-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Girard J, Roumazeille T, Sakr M, Migaud H. Osteochondral Mosaicplasty of the femoral head. HIP Int. 2011;21(5):542–8. 10.5301/HIP.2011.8659. [DOI] [PubMed] [Google Scholar]
- 187.Kılıçoğlu Öİ, Polat G, Erşen A, Birişik F. Long-term result of Mosaicplasty for femoral Head Osteochondral Lesion: a Case Report with 8 years follow-up. HIP Int. 2015;25(6):589–92. 10.5301/hipint.5000244. [DOI] [PubMed] [Google Scholar]
- 188.El Bitar YF, Lindner D, Jackson TJ, Domb BG. Joint-preserving Surgical options for Management of Chondral injuries of the hip. J Am Acad Orthop Surg. 2014;22(1):46–56. 10.5435/JAAOS-22-01-46. [DOI] [PubMed] [Google Scholar]
- 189.Garcia-Mansilla I, Jones KJ, Sassoon AA. Surgical hip dislocation and Fresh Osteochondral Allograft Transplantation for Femoroacetabular Impingement and Concomitant Chondral Lesion. Arthrosc Techniques. 2020;9(12):e1857–63. 10.1016/j.eats.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oladeji LO, Cook JL, Stannard JP, Crist BD. Large fresh osteochondral allografts for the hip: growing the evidence. HIP Int. 2018;28(3):284–90. 10.5301/hipint.5000568. [DOI] [PubMed] [Google Scholar]
- 191.Krych AJ, Lorich DG, Kelly BT. Treatment of focal Osteochondral defects of the Acetabulum with Osteochondral Allograft Transplantation. Orthopedics. 2011;34(7). 10.3928/01477447-20110526-24. [DOI] [PubMed]
- 192.Field RE, Rajakulendran K, Strambi F. Arthroscopic grafting of Chondral defects and subchondral cysts of the Acetabulum. HIP Int. 2011;21(4):479–86. 10.5301/HIP.2011.8542. [DOI] [PubMed] [Google Scholar]
- 193.Verhaegen J, Clockaerts S, Van Osch GJVM, Somville J, Verdonk P, Mertens P. TruFit Plug for Repair of Osteochondral defects—where is the evidence? Systematic review of literature. CARTILAGE. 2015;6(1):12–9. 10.1177/1947603514548890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Farr J, Cole B, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25(01):023–30. 10.1055/s-0031-1299652. [DOI] [PubMed] [Google Scholar]
- 195.Pascual-Garrido C, Hao J, Schrock J, Mei-Dan O, Chahla J. Arthroscopic juvenile allograft cartilage implantation for cartilage lesions of the hip. Arthrosc Techniques. 2016;5(4):e929–33. 10.1016/j.eats.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Craig MJ, Maak TG. Single-stage arthroscopic autologous matrix–enhanced Chondral Transplantation (AMECT) in the hip. Arthrosc Techniques. 2020;9(3):e399–403. 10.1016/j.eats.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ross JR, Larson CM, Bedi A. Indications for Hip Arthroscopy. Published online 2017. [DOI] [PMC free article] [PubMed]
- 198.Hernigou J, Verdonk P, Homma Y, Verdonk R, Goodman SB, Hernigou P. Nonoperative and operative bone and cartilage regeneration and Orthopaedic Biologics of the hip: an Orthoregeneration Network (ON) Foundation Hip Review. Arthroscopy: J Arthroscopic Relat Surg. 2022;38(2):643–56. 10.1016/j.arthro.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 199.Hevesi M, Jacob G, Shimomura K, Ando W, Nakamura N, Krych AJ. Current hip cartilage regeneration/repair modalities: a scoping review of biologics and surgery. Int Orthop (SICOT). 2021;45(2):319–33. 10.1007/s00264-020-04789-2. [DOI] [PubMed] [Google Scholar]
- 200.Tzaveas AP, Villar RN. Arthroscopic repair of Acetabular Chondral Delamination with Fibrin Adhesive. HIP Int. 2010;20(1):115–9. 10.1177/112070001002000117. [DOI] [PubMed] [Google Scholar]
- 201.Stafford GH, Bunn JR, Villar RN. Arthroscopic repair of Delaminated Acetabular articular cartilage using Fibrin Adhesive. Results at one to three years. HIP Int. 2011;21(6):744–50. 10.5301/HIP.2011.8843. [DOI] [PubMed] [Google Scholar]
- 202.Kucharik MP, Abraham PF, Nazal MR, Varady NH, Eberlin CT, Meek WM, et al. Treatment of full-thickness Acetabular Chondral flaps during Hip Arthroscopy: bone marrow aspirate Concentrate Versus microfracture. Orthop J Sports Med. 2021;9(12):23259671211059170. 10.1177/23259671211059170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sekiya JK, Martin RL, Lesniak BP. Arthroscopic repair of Delaminated Acetabular articular cartilage in Femoroacetabular Impingement. Orthopedics. 2009;32(9):692–6. 10.3928/01477447-20090728-44. [DOI] [PubMed] [Google Scholar]
- 204.Cassar-Gheiti AJ, Byrne DP, Kavanagh E, Mulhall KJ. Comparison of four chondral repair techniques in the hip joint: a biomechanical study using a physiological human cadaveric model. Osteoarthr Cartil. 2015;23(6):1018–25. 10.1016/j.joca.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 205.Kaya M, Hirose T, Yamashita T. Bridging suture repair for Acetabular Chondral Carpet Delamination. Arthrosc Techniques. 2015;4(4):e345–8. 10.1016/j.eats.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Arriaza CR, Sampson TG, Olivos Meza A, Mendez-Vides AC. Findings on repaired full-thickness acetabular articular cartilage defects during revision hip arthroscopy allowing a second look. J Hip Preservation Surg. 2020;7(1):122–9. 10.1093/jhps/hnz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dong H, Tian K, Gao G, Liu R, Zhang S, Liu Z, et al. Arthroscopic repair of Acetabular Cartilage Delamination using chondral nail fixation in patients with Femoroacetabular Impingement. Arthrosc Techniques. 2024;13(5):102950. 10.1016/j.eats.2024.102950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cvetanovich GL, Lizzio V, Meta F, Chan D, Zaltz I, Nho SJ et al. Variability and comprehensiveness of north American online available physical therapy protocols following hip arthroscopy for Femoroacetabular Impingement and Labral Repair. Arthroscopy: J Arthroscopic Relat Surg. Published online September 2017:S0749806317306862. 10.1016/j.arthro.2017.06.045 [DOI] [PubMed]
- 209.Grzybowski JS, Malloy P, Stegemann C, Bush-Joseph C, Harris JD, Nho SJ. Rehabilitation following hip arthroscopy †a systematic review. Front Surg. 2015;2. 10.3389/fsurg.2015.00021. [DOI] [PMC free article] [PubMed]
- 210.Migliorini F, Maffulli N, Bell A, Cuozzo F, Hildebrand F, Weber CD. Midterm results after arthroscopic femoral neck osteoplasty combined with labral debridement for cam type femoroacetabular impingement in active adults. J Orthop Surg Res. 2023;18(1):67. 10.1186/s13018-023-03543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Avnieli IB, Vidra M, Factor S, Atzmon R, Persitz J, Safran N, et al. Postoperative Weightbearing protocols after arthroscopic surgery for Femoroacetabular Impingement does not affect patient outcome: a comparative study with Minimum 2-Year follow-up. Arthroscopy: J Arthroscopic Relat Surg. 2020;36(1):159–64. 10.1016/j.arthro.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 212.Reiman MP, Boyd J, Ingel N, Reichert A, Westhoven M, Peters S. There is Limited and Inconsistent Reporting of Postoperative Rehabilitation for Femoroacetabular Impingement Syndrome: a scoping review of 169 studies. J Orthop Sports Phys Ther. 2020;50(5):252–8. 10.2519/jospt.2020.9189. [DOI] [PubMed] [Google Scholar]
- 213.Domb BG, Sgroi TA, VanDevender JC. Physical therapy protocol after hip arthroscopy: clinical guidelines supported by 2-Year outcomes. Sports Health: Multidisciplinary Approach. 2016;8(4):347–54. 10.1177/1941738116647920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meiron R, Brenner J, Gluckman A, Avraham R, Trainin Z. Humoral and cellular responses in calves experimentally infected with bovine leukemia virus (BLV). Vet Immunol Immunopathol. 1985;9(2):105–14. 10.1016/0165-2427(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 215.Lucenti L, Maffulli N, Bardazzi T, Saggini R, Memminger M, Simeone F, et al. Return to Sport following arthroscopic management of Femoroacetabular Impingement: a systematic review. J Clin Med. 2024;13(17):5219. 10.3390/jcm13175219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Seijas R, Barastegui D, López-de-Celis C, Montaña F, Cuscó X, Alentorn-Geli E, et al. Preoperative risk factors in hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1502–9. 10.1007/s00167-021-06484-0. [DOI] [PubMed] [Google Scholar]
- 217.Domb BG, Chen SL, Go CC, Shapira J, Rosinsky PJ, Meghpara MB, et al. Predictors of clinical outcomes after hip arthroscopy: 5-Year follow-up analysis of 1038 patients. Am J Sports Med. 2021;49(1):112–20. 10.1177/0363546520968896. [DOI] [PubMed] [Google Scholar]
- 218.Migliorini F, Maffulli N, Baroncini A, Eschweiler J, Tingart M, Betsch M. Revision surgery and progression to total hip Arthroplasty after Surgical correction of Femoroacetabular Impingement: a systematic review. Am J Sports Med. 2022;50(4):1146–56. 10.1177/03635465211011744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Migliorini F, Baroncini A, Eschweiler J, Knobe M, Tingart M, Maffulli N. Return to sport after arthroscopic surgery for femoroacetabular impingement. Surgeon. 2023;21(1):21–30. 10.1016/j.surge.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 220.McDonald JE, Herzog MM, Philippon MJ. Return to play after hip arthroscopy with microfracture in Elite athletes. Arthroscopy: J Arthroscopic Relat Surg. 2013;29(2):330–5. 10.1016/j.arthro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 221.McDonald JE, Herzog MM, Philippon MJ. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):915–9. 10.1007/s00167-013-2691-9. [DOI] [PubMed] [Google Scholar]
- 222.Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for Femoroacetabular Impingement in Professional Soccer players. Am J Sports Med. 2018;46(2):273–9. 10.1177/0363546517738741. [DOI] [PubMed] [Google Scholar]
- 223.Weber AE, Bolia IK, Mayfield CK, Ihn H, Kang HP, Bedi A, et al. Can we identify why athletes fail to return to Sport after Hip Arthroscopy for Femoroacetabular Impingement Syndrome? A systematic review and Meta-analysis. Am J Sports Med. 2021;49(6):1651–8. 10.1177/0363546520956292. [DOI] [PubMed] [Google Scholar]
- 224.Bolia IK, Ihn H, Kang HP, Mayfield CK, Briggs KK, Bedi A, et al. Cutting, impingement, contact, endurance, flexibility, and Asymmetric/Overhead sports: is there a difference in Return-to-Sport rate after arthroscopic femoroacetabular impingement surgery? A systematic review and Meta-analysis. Am J Sports Med. 2021;49(5):1363–71. 10.1177/0363546520950441. [DOI] [PubMed] [Google Scholar]
- 225.Nawabi DH, Bedi A, Tibor LM, Magennis E, Kelly BT. The demographic characteristics of high-level and recreational athletes undergoing hip arthroscopy for femoroacetabular impingement: a sports-specific analysis. Arthroscopy: J Arthroscopic Relat Surg. 2014;30(3):398–405. 10.1016/j.arthro.2013.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.