Abstract
Background
This study investigates the effectiveness and safety of aripiprazole oral solution in Chinese patients with schizophrenia.
Methods
This was a multicenter, single-arm phase IV study involving 134 patients in China in the acute stage of schizophrenia from May 2021 to July 2022. The patients received aripiprazole oral solution 10 − 30 mg/d for 12 weeks. The effectiveness endpoints included the Positive and Negative Symptom Scale (PANSS) and the Clinical Global Impression (CGI) scale score. The safety endpoints included adverse events, laboratory inspection indicators (including the serum prolactin level [PRL]), and waist circumferences (WC).
Results
Ultimately, 86 patients (64.18%) completed the trial, and 21 patients (15.67%) dropped out due to poor effectiveness. From baseline to week eight, 43.28% of patients had a PANSS reduction of ≥ 50%, 82.84% of patients improved in the CGI-Improvement (CGI-I scale score of 1 − 3), and the percentage of patients with abnormal PRL and waist circumferences decreased significantly. In total, 45 patients (33.58%) experienced mild adverse drug reactions predominately manifested as extrapyramidal symptoms (EPSs; 9.70%), constipation (8.96%), and palpitations (7.46%). Upon further subgroup analysis, aripiprazole oral solution demonstrated significantly improved effectiveness in first-episode schizophrenia patients and those with symptoms of agitation.
Conclusions
Aripiprazole oral solution displayed positive clinical effectiveness and favorable tolerability in Chinese patients in the acute stage of schizophrenia.
Clinical trial registration
Clinical trial registration number: ChiCTR2100044653. Name of trial registration: A real-world study of Aripiprazole Oral Solution in the treatment of schizophrenia (Registration date: 25/03/2021). The full trial protocol can be accessed at the Chinese Clinical Trial Registry ( http://www.chictr.org.cn/).
Keywords: Aripiprazole oral solution, Chinese, Schizophrenia
Background
Schizophrenia is a cerebral disorder that is characterized by mental dysfunction and behavioral disturbances. Clinical symptoms of schizophrenia, including positive and negative symptoms and cognitive impairment, are often seen among people 15 − 45 years of age [1]. Schizophrenia often requires long-term treatment with antipsychotic drugs as the cornerstone, with psychotherapy and rehabilitation as adjuvant therapies [2]. Patients with schizophrenia are a special population who have poor medication adherence; the rate of non-adherence has been reported to be as high as 60% during the initial treatment stages. Non-adherence or partial adherence to antipsychotic medication may lead to relapse or steady deterioration, ultimately affecting the prognosis of the disease [3]. Therefore, improving the medication adherence of patients with schizophrenia is a feasible approach to innovate the dosage forms of antipsychotic drugs.
Aripiprazole is the first dopamine system stabilizer (DSS) approved by the US Food and Drug Administration (FDA). It acts as a partial dopamine D2 and 5-HT1A receptor agonist and a partial 5-HT2A receptor antagonist. By functioning as a partial D2 dopaminergic agonist and 5-HT2A antagonist, aripiprazole exerts its antipsychotic effects by reducing dopaminergic-mediated neurotransmission in areas of the brain with dopaminergic hyperactivity, such as the mesolimbic system. In addition, aripiprazole reduces the risk of extrapyramidal symptoms (EPS) and improves the negative symptoms and cognitive impairments in schizophrenia by increasing dopaminergic-mediated neurotransmission in areas of the brain with dopaminergic hypoactivity, such as the nigrostriatal and mesocortical systems [4–6]. As a partial 5-HT1A receptor agonist, aripiprazole alleviates anxiety [7]. Aripiprazole can effectively reduce the plasma prolactin level due to its partial inhibitory effect on the pituitary D2 receptor [8]. Aripiprazole’s unique mechanism of action provides it with many clinical advantages; therefore, many experts recognize it as a "third-generation antipsychotic drug."
Aripiprazole oral solution is formulated with solubilization and taste-masking techniques to overcome the low water solubility of aripiprazole and ensure its convenience in administration. Compared with conventional tablets, the oral solution offers numerous advantages, such as ease of ingestion, convenient medication management, precise dose adjustment, and improved medication adherence [9]. In China, the schizophrenia patient population is complex and diverse, and treatment often requires continuous adjustments based on individual patient conditions. The oral solution offers this flexibility, enabling better optimization of treatment plans, reducing side effects, and improving overall therapeutic outcomes. The oral solution was approved for marketing by the US FDA in late 2004 and by the NMPA(National Medical Products Administration) of China in 2020. However, despite its widespread use internationally, there is currently a lack of clinical trial data and related literature specifically for Chinese patients with schizophrenia. Therefore, this clinical study was conducted to observe and evaluate the effectiveness and safety of the oral solution in patients with schizophrenia in China in real-world settings. The results may serve as a foundation for clinical rational drug use.
Methods
Study design
The study was a 12-week (eight-week treatment and four-week extended observation) prospective, multicenter, single-arm phase IV clinical trial. It was conducted in nine psychiatric hospitals in China from May 2021 to July 2022. According to inclusion and exclusion criteria, physicians initially perform a subjective screening of all hospitalized patients with acute schizophrenia. For those who meet the criteria, physicians will communicate the informed consent, and only those who agree and sign will proceed to the formal screening phase. Following a one-week screening period, eligible patients received treatment with aripiprazole oral solution (Chengdu Kanghong Pharmaceutical Group Co., Ltd.), and clinical evaluations were conducted at baseline and weeks 1, 2, 4, 8, and 12.
Guided by the principles of the Declaration of Helsinki, the study was conducted after obtaining the approval from the Ethics Committee of Beijing Anding Hospital (approval No. 202119FS-2) and being registered at ClinicalTrials.gov (ChiCTR2100044653). All of the patients and their legal guardians were fully informed about and understood the clinical trial details and voluntarily signed the informed consent forms.
Patients
The study enrolled patients aged 13 − 65 years who met the diagnostic criteria for schizophrenia by the International Classification of Diseases (ICD)−10 and who were in the acute phase (with a total Positive and Negative Symptom Scale [PANSS] score of ≥ 70) [10]. The exclusion criteria were: (1) patients diagnosed with refractory schizophrenia; (2) patients with a poor response to aripiprazole tablets or a history of allergy to aripiprazole; (3) patients with aspartate aminotransferase (AST), alanine aminotransferase (ALT), or blood urea nitrogen (BUN) > 2 × upper limit of normal (ULN); (4) patients with a prolonged QTc interval (QTc ≥ 450 ms in males and QTc ≥ 470 ms in females); (5) patients who received modified electroconvulsive therapy (MECT) within two month prior to enrollment; (6) patients participating in other drug clinical trials within one month prior to enrollment; (7) patients with severe suicidal ideations or serious physical illnesses; and (8) patients with a history of alcohol or drug abuse or dependence in the past 12 months.
Inclusion criteria for the agitation group were: patients with a total PANSS-Excited Component score (P4 Excitement, P7 Hostility, G4 Tension, G8 Uncooperativeness, and G14 Poor Impulse Control) ≥ 16. Other patients were classified as the no-agitation group. The definition for first-episode schizophrenia patients was: patients who met the diagnostic criteria for schizophrenia for the first time, with a disease duration of less than five years [11]. Other patients were classified as the chronic schizophrenia group.
Treatment
This was a real-world study. The usages and dosages were determined by the research doctors within the dosage range specified in the medication package insert. The following recommendations were presented during the research protocol: (1) for first-episode acute schizophrenia patients: aripiprazole 10 mg/d initially, increasing to 15 mg/d on day 3, to 20 mg/d on day 5, and to the target dose of 30 mg/d on day 7 [12]; (2) for acute schizophrenia patients in the agitation group: aripiprazole 15 mg/d initially, increasing to the target dose of 30 mg/d on days 2 − 5 [13]; and (3) for acute-phase patients with chronic schizophrenia: aripiprazole 15 mg/d initially, increasing to the target dose of 30 mg/d within 48 h [14]. In this study, research doctors were authorized to adjust the drug dosage and titration speed based on the patient's condition and tolerance.
For patients who were on other antipsychotic drugs prior to enrollment, a cross-titration strategy was implemented to prevent a withdrawal response, which should be done within two weeks of treatment initiation. During the study, concomitant use of other antipsychotic, antidepressant, or mood-stabilizing drugs was prohibited. However, it was permissible to concurrently use certain drugs to alleviate patient symptoms. For example, the use of benzodiazepines or β-adrenergic receptor blockers to alleviate anxiety and akathisia, the use of anticholinergic drugs to relieve EPSs such as dystonia and tremor, and the use of sedative-hypnotic drugs in severe insomnia patients for no more than seven consecutive days. Concomitant use of psychological or physical therapy was also not allowed in the study.
Clinical assessment
The primary effectiveness endpoint was the change in the PANSS total score from baseline to the end of eight weeks. The secondary effectiveness endpoints were changes in the scores of PANSS subscales and the clinical global impression (CGI). A clinical response was defined as a reduction of ≥ 50% in the PANSS score compared to baseline after eight weeks of treatment [15]. Potential adverse drug reactions were assessed using the UKU Side Effect Rating Scale (UKU-SERS), the Simpson's Rating Scale for Extrapyramidal Side Effects (RSESE), and the Barnes Akathisia Rating Scale (BARS). Parameters, such as body mass index (BMI), waist circumference, prolactin level, and liver function, were monitored at each visit from baseline to the end of the study.
Dropouts
Follow-up was conducted when patients returned for a hospital assessment or via home visits, phone calls, and other methods. If the researchers failed to contact the patients after three or more attempts, the patients were considered unable to undergo follow-up. Patients with poor effectiveness, poor drug adherence, or who for other reasons were unwilling to continue participating in the study could freely withdraw from the study.
Statistical analysis
All of the statistical analyses were completed using SAS 9.4 software (SAS Institute, Cary, North Carolina, USA). The demographic data, treatment exposure, effectiveness, and safety were summarized using descriptive statistical methods. Paired t-tests were used to compare each visit time point with baseline. The inter-group comparisons were analyzed using the mixed-effects model repeated measures (MMRM). All of the statistical analyses and effectiveness data were tested at a two-tailed significance level of 0.05. Data were analyzed with the last observation carried forward (LOCF) method.
Results
Demographic and treatment exposure
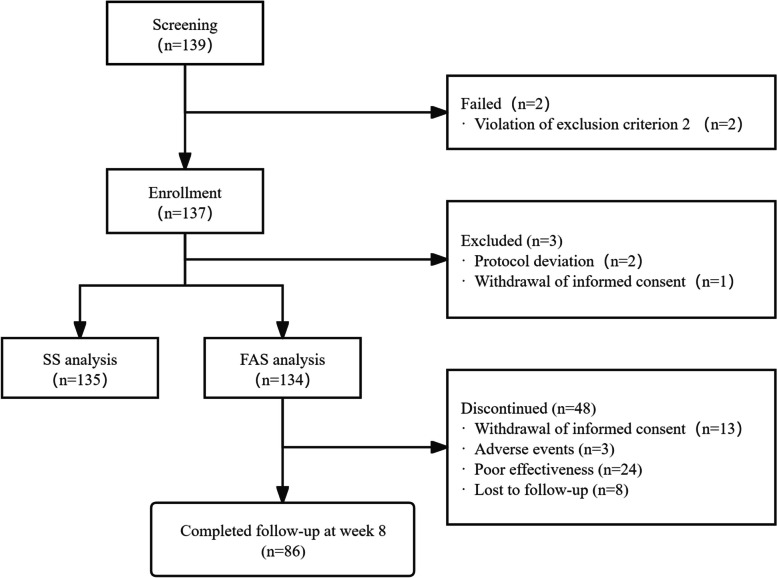
This research screened 139 participants and enrolled 137 patients who met the inclusion criteria. FAS included 134 subjects, while SS contained 135 subjects (Table 1; Fig. 1). Ultimately, 125, 113, 86, and 61 patients completed the 2-, 4-, 8-, and 12-week follow-ups, respectively. Due to the fact that acute schizophrenia treatment typically requires four to eight weeks, patients who completed the eight-week follow-up were considered to have completed the study. A total of 48 patients (35.82%) dropped out of the trial due to various reasons that included poor effectiveness (n = 24; 17.91%), adverse events (n = 3; 2.24%), withdrawal of informed consent (n = 13; 9.70%), and loss to follow-up (n = 8; 5.97%). This study included 49 patients (36.57%) with first-episode schizophrenia and 60 patients (44.78%) fulfilled the predetermined criteria for the agitation group. Among the enrolled patients, the majority of the participants (68.66%) were female, and half (50.75%) had a history of psychiatric medication treatment.
Table 1.
Summary of study results
| Variable | ||
|---|---|---|
| Baseline characteristics (FAS, n = 134) | Male | 42 (31.34) |
| Age, years | 32.31 ± 12.34 | |
| Family history | 33 (24.63) | |
| Body mass index, kg/m2 | 23.16 ± 4.33 | |
| Disease duration, months | 71.43 ± 81.44 | |
| Previous treatment for schizophrenia | 68 (50.75%) | |
| Treatment exposure | Mean daily dose aripiprazole during 12-week study duration, ml | 19.65 ± 4.04 |
| Target dose, ml | 21.27 ± 4.63 | |
| Average time to reach target dose, days | 11.76 ± 10.39 | |
| Use of concomitant medicationa | 126 (94.03) | |
| Effectiveness | ≥ 50% decrease in PANSS total score, week 8 | 58 (43.28) |
| CGI-I improvement, week 8 | 111 (82.84) | |
| Safety | Adverse event | 75 (55.97) |
| Serious adverse event | 1 (0.75) | |
| Adverse drug reactions | 45 (33.58) | |
| Extrapyramidal syndrome | 13 (9.70) | |
| Gastrointestinal disorders: nausea and constipation | 13 (9.70) | |
| Palpitation | 10 (7.46%) | |
| Dyslipidemia | 3 (2.24) | |
| Dysfunction of liver | 2 (1.49) | |
| Agitation | 2 (1.49) |
Data are reported as n (%) or mean ± SD. SD, standard deviation. FAS, full analysis set. PANSS, Positive and Negative Syndrome Scale
CGI-I, Clinical Global Impression-Improvement. CGI-I improvement is defined as a score of 1–3
aConcomitant medications were used to implement a cross-titration strategy in the first two weeks, improve insomnia symptoms, manage adverse reactions, treat other comorbidities in patients
Fig. 1.
Flow chart
The mean daily dose of aripiprazole oral solution was 19.65 ± 4.04 ml. A total of 41 patients (30.60%) underwent a cross-titration strategy, involving the use of eight antipsychotics administered a total of 51 times. The frequency and percentage of each antipsychotic used in the cross-titration were as follows: aripiprazole orally disintegrating tablets or tablets (n = 21, 41.18%), amisulpride (n = 2, 3.92%), olanzapine (n = 14, 27.45%), quetiapine (n = 4, 7.84%), risperidone (n = 4, 7.84%), paliperidone (n = 4, 7.84%), sulpiride (n = 1, 1.96%), and ziprasidone (n = 1, 1.96%). Additionally, a haloperidol injection was administered to 15 patients (11.19%) on 16 occasions within the first two weeks. Most patients received concomitant medications that were permitted by the study protocol: benzodiazepines (72.39%), anticholinergic drugs (47.01%), β-adrenergic receptor blockers (23.13%), purgative (5.97%), and a hepatoprotective (1.49%). These concomitant medications were used to improve insomnia symptoms, manage adverse reactions, and treat other comorbidities in patients.
Effectiveness
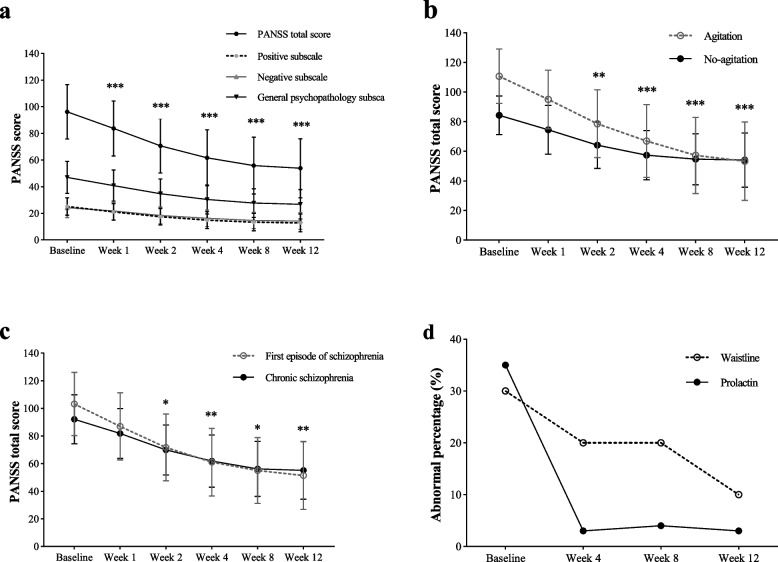
Statistically, there was a significant difference in the PANSS total score from the baseline after one week of treatment, and the difference sharpened with the increase in the treatment time until the end of eight weeks (Table 2). After eight weeks of treatment, the PANSS total score (D-value: –40.37 ± 25.45) and each sub-scale score of the patients with schizophrenia had been significantly reduced (Fig. 2a). A total of 43.28% of patients achieved a clinical response. In addition, the CGI-Severity (CGI-S) score (D-value: –1.95 ± 1.36) was significantly reduced compared to baseline (t = 16.49, P < 0.001). A total of 82.84% of patients improved their CGI-Improvement scores (CGI-I scale score of 1–3).
Table 2.
Differences in primary effectiveness indicators between each visit time point and baseline
| Baseline (n = 134) |
1 week (n = 133) |
2 weeks (n = 125) |
4 weeks (n = 113) |
8 weeks (n = 86) |
12 weeks (n = 61) |
Baseline VS 1 week | Baseline VS 2 weeks | Baseline VS 4 weeks | Baseline VS 8 weeks | Baseline VS 12 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All participants | T | P | T | P | T | P | T | P | T | P | ||||||
| PANSS total score | 96.16 (20.44) | 83.69 (20.67) | 70.56 (20.48) | 61.61 (21.05) | 55.78 (21.37) | 53.77 (22.25) | –12.70 | < 0.001 | –17.35 | < 0.001 | –18.49 | < 0.001 | –18.36 | < 0.001 | –17.22 | < 0.001 |
| CGI-S score | 5.24 (0.72) | 4.64 (0.88) | 4.08 (1.13) | 3.65 (1.21) | 3.28 (1.36) | 3.16 (1.42) | –9.03 | < 0.001 | –12.30 | < 0.001 | –15.32 | < 0.001 | –16.50 | < 0.001 | –16.22 | < 0.001 |
Data are reported as mean (SD). SD standard deviation, PANSS Positive and Negative Syndrome Scale, CGI-S Clinical Global Impression-Severity. Data were analyzed with the last observation carried forward (LOCF) method
Fig. 2.
a: Change of PANSS score in patients with schizophrenic who were treated with aripiprazole oral solution for 12 weeks. b: Comparison of the decrease of PANSS scores between agitated and no-agitated schizophrenia patients treated with aripiprazole oral solution for 12 weeks. c: Comparison of the decrease of PANSS scores between first-episode and chronic schizophrenia patients treated with aripiprazole oral solution for 12 weeks. d: Change of abnormal percentage of prolactin and waistline in patients with schizophrenic who were treated with aripiprazole oral solution for 12 weeks. *There is a significant difference in the PANSS score reduction from baseline (Fig. 2a). *There is a significant difference in the PANSS score reduction between the two groups (Fig. 2bcd). *P < 0.05, **P < 0.01, ***P < 0.001
The subgroup analysis showed a significant difference in the reduction of the PANSS total score between the agitation group and the no-agitation group (t = 2.64, P = 0.009; Fig. 2b, Table 3) after two weeks of treatment, and the difference persisted until the end of the eighth week. There was a significantly greater reduction in the PANSS total score in the first-episode schizophrenia patients than in the chronic patients (t = 2.58, P = 0.01; Fig. 2c, Table 3) that was detected two weeks after treatment, and the difference persisted until the end of the eighth week. Furthermore, the PANSS total score of the first-episode and chronic schizophrenia patients decreased significantly after 12 weeks of treatment (Table 3). A significant difference in PANSS score reduction between the adolescent (13–17 years old) and adult (≥ 18 years old) groups was observed only at the 4-week follow-up (t = 2.02, P = 0.045). No significant differences were found at the other time points (weeks 1, 2, 8, and 12). The oral solution of aripiprazole did not show significant gender differences in patients with schizophrenia (Table 3).
Table 3.
Changes in the primary effectiveness indicators of each subgroup compared to baseline
| The change of PANSS total score | 1 week (n = 133) |
2 weeks (n = 125) |
4 weeks (n = 113) |
8 weeks (n = 86) |
12 weeks (n = 61) |
|---|---|---|---|---|---|
| Agitation group | –15.72 (9.40) | –32.18 (17.75) | –43.85 (22.60) | –53.53 (26.87) | –57.42 (30.80) |
| No-agitation group | –9.84 (12.181) | –20.26 (14.56) | –27.00 (17.63) | –29.70 (18.42) | –30.20 (19.40) |
| Agitation VS No-agitation group |
T = 0.89 P = 0.37 |
T = 2.64 P = 0.009 |
T = 3.57 P < 0.001 |
T = 4.93 P < 0.001 |
T = 5.26 P < 0.001 |
| First-episode group | –16.16 (9.63) | –31.47 (15.87) | –42.06 (19.78) | –48.18 (23.87) | –51.76 (28.74) |
| Chronic group | –10.34 (11.79) | –22.21 (16.91) | –30.21 (21.57) | –35.87 (25.38) | –36.99 (27.07) |
| First-episode VS Chronic group |
T = 1.91 P = 0.06 |
T = 2.58 P = 0.01 |
T = 2.80 P = 0.006 |
T = 2.50 P = 0.01 |
T = 2.77 P = 0.006 |
| Male group | −14.18 (1.78) | −23.23 (2.46) | −30.82(3.07) | −35.61 (3.59) | −36.58 (4.00) |
| Female group | −12.57 (1.18) | −27.56 (1.65) | −37.13 (2.06) | −43.43 (2.41) | −45.92 (2.69) |
| Male VS Female group |
T = 0.75 P = 0.45 |
T = −1.47 P = 0.15 |
T = −1.71 P = 0.09 |
T = −1.81 P = 0.07 |
T = −1.94 P = 0.06 |
| Youth group, | −14.37 (3.09) | −30.45 (4.37) | −45.22 (5.43) | −48.30 (6.39) | −50.61 (7.18) |
| Adult group | −12.51 (1.02) | −25.32 (1.43) | −33.65 (1.78) | −39.77 (2.10) | −41.75 (2.36) |
| Youth VS Adult group |
T = 0.57 P = 0.57 |
T = 1.12 P = 0.27 |
T = 2.02 P = 0.045 |
T = 1.27 P = 0.21 |
T = 1.17 P = 0.24 |
Data are reported as mean (SD). SD, standard deviation. PANSS, Positive and Negative Syndrome Scale. Data were analyzed with the last observation carried forward (LOCF) method
Safety
An abnormal serum prolactin level was defined as a PRL > 25 ng/mL (530 mIU/L), and an abnormal waist circumference was defined as a waist circumference ≥ 90 cm for males and ≥ 85 cm for females. After 12 weeks of treatment with aripiprazole oral solution, the percentage of patients with abnormal prolactin levels decreased from 35.07% to 2.99%, and the percentage of patients with abnormal waist circumferences decreased from 32.84% to 11.94% (Fig. 2d). There was no significant change in BMI values during the treatment period.
Throughout the study, 55.97% of patients experienced adverse events, and 33.58% of patients experienced adverse drug reactions. Most of the adverse reactions the patients experienced were mild. EPSs (9.70%), including akathisia and dystonia, gastrointestinal disorders (9.70%) including nausea and constipation, and palpitations (7.46%) were the most common adverse reactions (Table 1). One of the patients committed suicide by jumping from a building at home for unknown reasons, and this was judged as a serious adverse event by the investigator as unrelated to the investigational drug.
Discussion
This real-world clinical study showed that aripiprazole oral solution treatment for 12 weeks effectively improved clinical symptoms and corrected abnormal serum prolactin levels and waist circumferences in patients with schizophrenia in China. A subgroup analysis revealed that aripiprazole oral solution showed significantly enhanced effectiveness in first-episode schizophrenia patients and those with agitation symptoms. The incidence of adverse reactions of aripiprazole oral solution was relatively low, and the degree was mild.
There have been no clinical studies that have reported on aripiprazole oral liquid until now. Instead, there have been many studies on the treatment of acute exacerbation of schizophrenia with aripiprazole tablets. A series of four- to six-week placebo-controlled studies reported within a dose range of 10 − 30 mg/d showed that both positive and negative symptoms significantly improved [16]. L. Citrome summarized data from four short-term (4 − 6 weeks) studies on oral aripiprazole (10 − 30 mg/d) in adults with schizophrenia, finding that the clinical response rate for a PANSS total score reduction of over 30% was 38% [17]. In another study of the long-acting injectable aripiprazole lauroxil (AL) over 12 weeks, using the same response rate definition, the clinical response rate was 52% for AL 441 mg/month and 56% for AL 882 mg/month [18]. In research related to the schizophrenic population in China, the clinical response rate (a reduction in PANSS score of ≥ 50%) was 26.6% after eight weeks of treatment with aripiprazole tablets (10 − 30 mg/d) [19]. In two other studies that used aripiprazole tablets for four weeks (15 mg/d) and six weeks (10 − 30 mg/d), the response rates (a reduction in PANSS score of > 30%) were 51% and 71%, respectively [20, 21]. In this study, the clinical response rate was 43.28% after eight weeks of treatment (dose range: 15 − 30 mg/d). Although it is difficult to directly compare clinical reactions due to the different criteria for effectiveness assessment in each study, the above results suggest that aripiprazole oral solution is roughly equivalent to the tablets in terms of improving clinical symptoms. In addition, there are only two 8-week clinical studies on other antipsychotic medications targeting the Chinese population [19, 22]. Due to the heterogeneity of the study designs and the lack of direct comparative studies, it is currently not possible to directly compare the efficacy of aripiprazole oral solution with other antipsychotic medications. However, existing research suggests that aripiprazole has fewer side effects, which may help improve patient adherence and achieve better long-term treatment outcomes [19, 22, 23].
Psychomotor agitation symptoms may be related to dopaminergic hyperactivity in the mesolimbic pathway. Aripiprazole, a partial dopamine D2 receptor agonist, competes with dopamine for D2 receptors and reduces dopamine activity. Serotonergic neurotransmitters are also believed to play a role in controlling agitation symptoms [24]. Several studies have demonstrated that aripiprazole injection rapidly controls agitated and restless behaviors in adults [24, 25]. Aripiprazole tablets (15 − 30 mg) for five days can also rapidly control agitation in patients with acute schizophrenia [13]. This study adopted the same dose adjustment scheme for patients with agitation symptoms and found aripiprazole oral solution had significant effectiveness in patients who have schizophrenia with agitation symptoms in China. An improved experimental design is required in the future to rule out the impact of concomitant medication on drug effectiveness. In addition, this study also revealed that aripiprazole oral solution was more effective in first-episode patients, possibly because these patients were more sensitive to antipsychotic pharmacotherapy, thus leading to a better overall response than multiple-episode patients [26].
Hyperprolactinemia (HPRL), a common adverse effect of antipsychotic drugs, is related to dopamine D2 receptor blockade in the tuberoinfundibular pathway and occurs in approximately 70% of patients who take antipsychotic drugs [27]. Two recent meta-analyses compared the effectiveness of adjuvant treatment with aripiprazole, herbal medicine, and metformin in reducing HPRL, and the results showed that low-dose aripiprazole (< 5 mg/d) was the most effective [8, 28]. Although the aripiprazole oral solution dose taken by the patients in this study was relatively large, the percentage of patients with abnormal serum prolactin levels decreased significantly after eight weeks of treatment, indicating similar effectiveness. Moreover, a meta-analysis discovered that < 10% of patients on aripiprazole experienced weight gain related to its antagonistic activity against histamine H1 receptors [29, 30]. In this study, BMI did not change significantly, and the percentage of patients with abnormal waist circumferences gradually decreased, demonstrating that aripiprazole oral solution had a negligible effect on body weight.
Consistent with the results of previous clinical trials of aripiprazole tablets and long-acting injections, EPSs were identified as the most frequently occurring adverse effects in this study, although the incidence was relatively low when compared with other antipsychotic drugs [29, 30]. The therapeutic window for an antipsychotic effect of dopamine receptor antagonists in relation to striatal dopamine 2 to 3 (D2/D3) receptors occupancy in the striatum was 60% − 80%. In addition, when the occupancy exceeded 80%, the incidence of EPSs increased [31]. Positron emission tomography (PET) scans showed that, at the clinically used doses, the striatal D2/D3 receptor occupancy was greater than 80% [32], but the aripiprazole-induced EPSs were mild in severity, which was related to the particular mechanism of action where aripiprazole exerts its effects as an agonist and antagonist in areas of the brain with dopaminergic hypoactivity and hyperactivity, respectively [32, 33]. Similarly, a small number of patients in this study experienced arrhythmias, nausea, and other uncomfortable symptoms after 12 weeks of treatment with aripiprazole oral solution [29, 30]. Individual differences in the sensitivity to aripiprazole's effects on presynaptic dopamine receptors may explain the occasional adverse effects of aripiprazole, such as agitation [32]. In this study, only a few patients dropped out due to adverse reactions, indicating a high level of safety.
Nevertheless, this study still had some limitations. First, this study was a real-world, one-arm study with no control group, a small sample size, and a short study period. Second, concomitant use of antipsychotic medications was present during the first two weeks, which may affect the observation of early efficacy (1 − 2 weeks). Although the proportion of concomitant medication use in the agitation and non-agitation groups is approximately the same, resulting in minimal impact on subgroup analysis, this concomitant use may still introduce potential confounding factors, which should be considered when interpreting early efficacy (1 − 2 weeks) results. Third, this study used a physician’s clinical decision-making approach for preliminary patient screening, which may introduce selection bias. Although subgroup analysis showed no gender differences in efficacy, the effect of enrollment bias cannot be ruled out, as more male patients may have been excluded. Lastly, the sample size of the adolescent group in this study is relatively small, which limits the extrapolation of its statistical analysis results. The above influencing factors may lead to overestimation or underestimation of certain clinical effects. Therefore, when applying the study results to patient populations in different contexts, these factors should be carefully considered.
Conclusions
This study showed that aripiprazole oral solution was a safe and effective antipsychotic drug for patients with schizophrenia in China, and it was particularly effective in first-episode schizophrenia patients or those with agitation symptoms. In general, aripiprazole oral solution has similar clinical effectiveness and safety as aripiprazole tablets in the Chinese population.
Acknowledgements
We are very grateful to all of the patients and researchers for their time and effort in this research. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Authors’ contributions
Data acquisition and collation: Fang Dong, Junhong Zhu, Xiangdong Du, Gang Wu, Huaili Deng, Xueqin Yu, Jintong Liu, Shiping Xie, Xiaowei Tang. Feng Li managed the literature searches. Anning Li undertook the statistical analysis. Zhen Mao wrote the complete first draft. Final review and editing by Fang Dong and Gang Wang. All authors have read and approved the manuscript.
Author’s information
All researchers in the study were trained regarding the protocol and Good Clinical Practice guidelines.
Funding
This study was supported by Chengdu Kanghong Pharmaceutical Group Co., Ltd. (China). No financial or other incentives were given to study participants.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All procedures of the present study were performed in accordance with the Declaration of Helsinki. The study protocols were approved by the clinical research ethics committees of Beijing Anding Hospital, Capital Medical University. All the individuals were aware of the purpose of the study and signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhen Mao and Fang Dong are co-first author.
References
- 1.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28(2):325–34. [DOI] [PubMed] [Google Scholar]
- 2.Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335(7610):91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curto M, Fazio F, Ulivieri M, Navari S, Lionetto L, Baldessarini RJ. Improving adherence to pharmacological treatment for schizophrenia: a systematic assessment. Expert Opin Pharmacother. 2021;22(9):1143–55. [DOI] [PubMed] [Google Scholar]
- 4.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J Clin Psychiatry. 2001;62(11):841–2. [DOI] [PubMed] [Google Scholar]
- 5.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 2001;62(12):923–4. [DOI] [PubMed] [Google Scholar]
- 6.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26(5):649–66. [DOI] [PubMed] [Google Scholar]
- 7.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83–244. [DOI] [PubMed] [Google Scholar]
- 8.Zhang. L, Qi. H, Xie. Y-Y, Zheng. W, Liu. X-H, Cai. D-B, Ng. CH, Ungvari. GS, Xiang. Y-T: Efficacy and Safety of Adjunctive Aripiprazole, Metformin, and Paeoniae-Glycyrrhiza Decoction for Antipsychotic-Induced Hyperprolactinemia: A Network Meta-Analysis of Randomized Controlled Trials. Front Psychiatry. 2021;12:728204. [DOI] [PMC free article] [PubMed]
- 9.Parab PV, Chou JTI. Aripiprazole oral solution. EP1381367 B1; 2005.
- 10.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: diagnostic criteria for research. Geneva: Clinical Descriptions & Diagnostic Guidelines; 1993. [Google Scholar]
- 11.Lieberman JA, Phillips M, Gu H, Stroup S, Zhang P, Kong L, Ji Z, Koch G, Hamer RM. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28(5):995–1003. [DOI] [PubMed] [Google Scholar]
- 12.Findling RL, Robb A, Nyilas M, Forbes RA, Jin N, Ivanova S, Marcus R, McQuade RD, Iwamoto T, Carson WH. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432–41. [DOI] [PubMed] [Google Scholar]
- 13.Kinon BJ, Stauffer VL, Kollack-Walker S, Chen L, Sniadecki J. Olanzapine versus aripiprazole for the treatment of agitation in acutely ill patients with schizophrenia. J Clin Psychopharmacol. 2008;28(6):601–7. [DOI] [PubMed] [Google Scholar]
- 14.Fagiolini A, Brugnoli R, Sciascio GD, Filippis SD, Maina G. Switching antipsychotic medication to aripiprazole: position paper by a panel of Italian psychiatrists. Expert Opin Pharmacother. 2015;16(5):727–37. [DOI] [PubMed] [Google Scholar]
- 15.Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J Clin Psychiatry. 2014;75(Suppl 1):8–14. [DOI] [PubMed] [Google Scholar]
- 16.Kinghorn WA, McEvoy JP. Aripiprazole: pharmacology, efficacy, safety and tolerability. Expert Rev Neurother. 2005;5(3):297–307. [DOI] [PubMed] [Google Scholar]
- 17.Citrome L. The ABC’s of dopamine receptor partial agonists - aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract. 2015;69(11):1211–20. [DOI] [PubMed] [Google Scholar]
- 18.Citrome L, Risinger R, Cutler AJ, Du Y, Zummo J, Nasrallah HA, Silverman BL. Effect of aripiprazole lauroxil in patients with acute schizophrenia as assessed by the Positive and Negative Syndrome Scale-supportive analyses from a Phase 3 study. CNS Spectr. 2018;23(4):284–90. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Z, Yuan Y, Han X, Yang L, Cai S, Yang F, Lu Z, Wang C, Deng H, Zhao J, et al. An open-label randomised comparison of aripiprazole, olanzapine and risperidone for the acute treatment of first-episode schizophrenia: Eight-week outcomes. J Psychopharmacol. 2019;33(10):1227–36. [DOI] [PubMed] [Google Scholar]
- 20.Chan H-Y, Lin W-W, Lin S-K, Hwang T-J. Su T-PT, Chiang S-C, Hwu H-G: Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial. J Clin Psychiatry. 2007;68(1):29–36. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Luo J, Wang C, Xie S, Xu X, Wang X, Yu W, Gu N, Kane JM. Efficacy and safety of aripiprazole in Chinese Han schizophrenia subjects: a randomized, double-blind, active parallel-controlled, multicenter clinical trial. Schizophr Res. 2014;157(1–3):112–9. [DOI] [PubMed] [Google Scholar]
- 22.Liang Y, Cao C, Zhu C, Wang C, Zhang C, Dong F, Yang F, Deng H, Yu J, Tang J, et al. The effectiveness and safety of amisulpride in Chinese patients with schizophrenia: An 8-week, prospective, open-label, multicenter, single-arm study. Asia-Pacific psychiatry : official journal of the Pacific Rim College of Psychiatrists. 2016;8(3):241–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna P, Suo T, Komossa K, Ma H, Rummel-Kluge C, El-Sayeh HG, Leucht S, Xia J. Aripiprazole versus other atypical antipsychotics for schizophrenia. The Cochrane database of systematic reviews. 2014;1:CD006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanford M, Scott LJ. Intramuscular aripiprazole : a review of its use in the management of agitation in schizophrenia and bipolar I disorder. CNS Drugs. 2008;22(4):335–52. [DOI] [PubMed] [Google Scholar]
- 25.Ostinelli EG, Jajawi S, Spyridi S, Sayal K, Jayaram MB. Aripiprazole (intramuscular) for psychosis-induced aggression or agitation (rapid tranquillisation). The Cochrane database of systematic reviews. 2018;1:CD008074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correll CU, Martin A, Patel C, Benson C, Goulding R, Kern-Sliwa J, Joshi K, Schiller E, Kim E. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia. 2022;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, Josiassen RC. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004;65(11):1491–8. [DOI] [PubMed] [Google Scholar]
- 28.Lu Z, Sun Y, Zhang Y, Chen Y, Guo L, Liao Y, Kang Z, Feng X, Yue W. Pharmacological treatment strategies for antipsychotic-induced hyperprolactinemia: a systematic review and network meta-analysis. Transl Psychiatry. 2022;12(1):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belgamwar RB, El-Sayeh HGG. Aripiprazole versus placebo for schizophrenia. The Cochrane database of systematic reviews. 2011;8:CD006622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preda A, Shapiro BB. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin Drug Saf. 2020;19(12):1529–38. [DOI] [PubMed] [Google Scholar]
- 31.Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538–44. [DOI] [PubMed] [Google Scholar]
- 32.Gründer G, Fellows C, Janouschek H, Veselinovic T, Boy C, Brcheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C. Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry. 2008;165(8):988–95. [DOI] [PubMed] [Google Scholar]
- 33.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;20(6):612–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.