Abstract
Background
Ischemic stroke is a major contributor to global morbidity and mortality, particularly in critically ill patients in intensive care units (ICUs). While advances in stroke management have improved outcomes, predicting mortality remains challenging due to the involvement of complex metabolic and cardiovascular factors. The triglyceride-glucose (TyG) index, a marker for insulin resistance, has gained attention for its potential to predict adverse outcomes in stroke patients. Furthermore, the TyG-BMI index, which combines TyG with body mass index (BMI), may offer a more comprehensive measure by accounting for obesity-related metabolic burden. However, the comparative impact of these indices on short- and long-term mortality among critically ill ischemic stroke patients remains unclear.
Methods
This retrospective cohort study analyzed data from the Medical Information Mart for Intensive Care IV (MIMIC-IV 3.0) database, including 1,334 critically ill ischemic stroke patients. The patients were divided into four groups based on TyG and TyG-BMI quartiles, respectively. Cox proportional hazards models were employed to assess the association of these indices with 30-day, 90-day, 180-day, and 1-year all-cause mortality (ACM). Kaplan-Meier survival analysis was used to compare survival rates across different index levels. We utilized restricted cubic splines (RCS) to examine the association between the TyG, TyG-BMI index and the specified outcomes. Furthermore, TyG and TyG-BMI index were utilized to establish logistic regression models for mortality across different time periods, and corresponding Receiver Operating Characteristic (ROC) curves were generated.
Results
Kaplan-Meier survival analysis show that Higher TyG levels were associated with significantly increased mortality risk at all time points, with patients in the highest TyG quartile exhibiting the greatest risk. Conversely, patients having a lower TyG-BMI level faced a heightened risk of long-term ACM. The RCS analysis results demonstrated that the TyG index did not exhibit a statistically significant nonlinear relationship with mortality across all time points. However, a significant nonlinear relationship was observed between the TyG index and long-term mortality. From the ROC curve, it can be observed that TyG performs better in predicting short-term mortality. Conversely, TyG-BMI demonstrates superior performance in predicting long-term mortality. The analysis revealed that while the TyG index alone is a strong predictor of mortality, the TyG-BMI index enhances the ability to predict long-term outcomes.
Conclusion
This finding suggests both the TyG and TyG-BMI indices serve as valuable predictors of mortality in critically ill ischemic stroke patients. However, significant differences were observed across the various follow-up periods. Based on the distinct characteristics of these two indicators, future research should focus on the selective integration of TyG and TyG-BMI indices into clinical risk assessment models, tailored to the metabolic profiles of ischemic stroke patients in the ICU. This approach could enhance the precision of mortality risk stratification and optimize patient management strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04450-5.
Keywords: Ischemic stroke, MIMIC-IV 3.0 database, TyG, TyG-BMI, All-cause mortality, Differences
Introduction
Ischemic stroke remains a leading cause of morbidity and mortality worldwide [1, 2], particularly in critically ill patients. Despite advances in acute stroke management, predicting mortality in this patient population remains a significant challenge due to the involvement of complex metabolic, cardiovascular, and inflammatory factors [3]. Stroke patients in ICUs are especially vulnerable to adverse outcomes, including both short-term and long-term mortality, as critical illness often exacerbates underlying metabolic dysregulation [4, 5]. Identifying reliable prognostic markers in this context is essential to improving clinical decision-making, optimizing patient management strategies, and reducing the overall burden of stroke [6].
One such marker that has gained increasing attention in recent years is the TyG index [7]. The TyG index is a readily calculated and cost-effective surrogate marker of insulin resistance, which has been closely linked to cardiovascular diseases, including stroke [7–9]. Insulin resistance plays a central role in the development of atherosclerosis, hypertension, and dyslipidemia, all of which are established risk factors for ischemic stroke [10]. Furthermore, studies have suggested that insulin resistance may exacerbate stroke severity and contribute to worse outcomes by increasing the likelihood of post-stroke complications such as recurrent strokes and cardiovascular events [11, 12].
While the TyG index is recognized as a robust predictor of cardiovascular outcomes, its role in predicting mortality specifically in critically ill ischemic stroke patients remains understudied [13]. Recent evidence has suggested that the TyG index may be a valuable tool for predicting short-term mortality in these patients, as elevated TyG levels are often indicative of acute metabolic disturbances, such as hyperglycemia and hypertriglyceridemia, that can worsen stroke prognosis [14]. However, less is known about the index’s ability to predict long-term outcomes in this population.
In addition to the TyG index, the combination of the TyG index with BMI, known as the TyG-BMI index, may offer a more comprehensive assessment of metabolic risk [15]. BMI, a commonly used measure of obesity, has been associated with both increased stroke risk and post-stroke mortality [16]. Obesity is a significant contributor to chronic conditions such as type 2 diabetes and hypertension, both of which exacerbate the metabolic burden in stroke patients [17]. By combining the TyG index with BMI, the TyG-BMI index captures not only insulin resistance but also the obesity-related metabolic dysfunction that may influence long-term outcomes in critically ill ischemic stroke patients.
Despite the growing interest in both the TyG and TyG-BMI indices, the comparative impact of these indices on short- and long-term mortality in critically ill ischemic stroke patients has yet to be fully explored. Understanding how these indices influence mortality at different time points could inform clinical risk stratification models and improve patient management in the ICU setting. Therefore, this study aims to assess the differential impact of TyG and TyG-BMI indices on short-term and long-term mortality among critically ill ischemic stroke patients using data from the MIMIC-IV 3.0 database [18].
Methods
Source of data
This retrospective study was conducted using health-related data obtained from the MIMIC-IV, version 3.0 database, an extensive resource of high quality developed and maintained by the Massachusetts Institute of Technology Computational Physiology Laboratory [18]. The database contains detailed medical records of patients hospitalized in the intensive care units at the Beth Israel Deaconess Medical Center. Patients diagnosed with ischemic stroke were identified through the International Classification of Diseases, 9th and 10th Revisions. The exclusion criteria included: (1) patients younger than 18 years at the time of their first admission; (2) patients with multiple ICU admissions for ischemic stroke, for whom only data from the initial admission were used; (3) patients with an ICU stay of less than 3 h; and (4) patients lacking sufficient data for triglycerides (TG) and fasting blood glucose (FBG) on the first day of admission. Ultimately, 1,334 patients were enrolled in the study and stratified into four groups based on the TYG index and the TYG-BMI index.
Variable extraction
Information extraction was performed using PostgreSQL (version 13.7.2) and Navicat Premium (version 16), both utilizing Structured Query Language (SQL). Possible variables were segmented into four primary categories: (1) demographic factors such as age, race, gender, weight, height, and BMI; (2) comorbid health conditions, including heart failure, diabetes, kidney disease, and paraplegia; (3) laboratory markers encompassing red blood cells (RBC), white blood cells (WBC), hemoglobin, platelets, serum sodium, serum creatinine, high-density lipoprotein (HDL), FBG, and TG; and (4) severity scores upon admission, including the Acute Physiology Score III (APSIII), the Simplified Acute Physiology Score II (SAPS-II), the Oxford Acute Severity of Illness Score (OASIS), and the Sepsis-related Organ Failure Assessment (SOFA) score [19]. The follow-up period commenced on the admission date and concluded on the date of death or at the end of the study period. The outcomes assessed included all-cause mortality (ACM) at 30 days, 90 days, 180 days, and 1 year. All blood metrics were documented during the initial measurements following admission to the ICU. There were no variables in the dataset with more than 20% missing values, and all missing data were imputed using multiple imputation techniques [20].
Definition of related concepts
The TyG index is used as a proxy for insulin resistance and is calculated based on FBG and TG levels. Initially, participants had their FBG and TG levels measured. for TyG and TyG-BMI were computed using the following formulas: TyG index = Ln [fasting TG (mg/dL) × FBG (mg/dL) / 2]; TyG-BMI index = TyG index × BMI [21]. This study aimed to assess the outcomes related to ACM in patients with ischemic stroke, using data from the MIMIC-IV database. The primary endpoints were based on ACM and were evaluated at 30 days, 90 days, 180 days, and 1 year following admission.
Statistical analysis
This study used R 4.3.0 statistical analysis software. Normally distributed continuous data were expressed as Mean ± SD, and the two groups were compared using the t-test of two independent samples. Categorical data were expressed as n (%) and the chi-square test or Fisher’s exact probability method was used. The differences between the groups were considered statistically significant when P < 0.05. The Kaplan-Meier method for survival analysis was utilized to evaluate the frequency of endpoints across various groups according to the TyG, TyG-BMI index levels, with their differences determined using log-rank tests. To determine the hazard ratio (HR) and 95% confidence interval (CI) among the TyG, TyG-BMI index and endpoints, Cox proportional hazards models were employed, with adjustments made for certain models. The multivariate model incorporated variables pertinent to clinical conditions and prognoses: model 1: unadjusted; model 2: adjusted for age, gender, and ethnicity; model 3: adjusted for Gender, Age, Race, BMI, Respiratory Failure, Diabetes, Congestive Heart Failure, Paraplegia, and renal disease. Furthermore, TyG, TyG-BMI was analyzed continuously using RCS to elucidate dose–effect relationships between dosage and effect in relation to the risk of outcomes. When dealing with nonlinear correlations, the inflection points among TyG, TyG-BMI, and ACM were identified by a recursive algorithm. Furthermore, TyG and TyG-BMI index were utilized to establish logistic regression models for mortality across different time periods, and corresponding ROC curves were generated. This study conducted stratified analyzes on the basis of Age (< 60 or ≥ 60 years old), Gender, Race, and Diabetes.
Results
Baseline characteristics of individuals
A total of 1334 people were included in this study, of which 923 people (69.19%) had no death with 1 year, and 411 people (30.81%) had death with 1 year. The average age significantly differed between the two groups, with mean ages of 68.57 years for the non-death group and 78.31 years for the death group (t = -12.30, p < 0.001). The death group had a significantly lower average RBC count (3.92) compared to the non-death group (4.20) (t = 6.57, p < 0.001). WBC counts were found to be higher in the death group (11.47) compared to the non-death group (10.12) (t = -4.44, p < 0.001). There was a statistically significant difference in hemoglobin levels, with the death group averaging 11.60 compared to 12.54 in the non-death group (t = 7.26, p < 0.001). The death group also exhibited higher mean creatinine levels (1.27) compared to the non-death group (1.04) (t = -3.85, p < 0.001). A significant association was found, with 35.52% of the death group experiencing respiratory failure compared to 11.48% in the non-death group (χ² = 107.25, p < 0.001). Rates of congestive heart failure were also significantly different between groups, showing higher prevalence in the death group (35.04%) relative to the non-death group (19.61%) (χ² = 36.72, p < 0.001). The document presents a slightly higher percentage of females in the death group (56.45%) compared to the non-death group (48.86%) (χ² = 6.55, p = 0.010). Data on race indicates varied distributions, with a higher proportion of white individuals in the non-death group compared to the death group (57.42% vs. 49.39%, χ² = 9.85, p = 0.043) (Table 1). Characteristics and outcomes of participants categorized by TyG index (illustrated in Additional file 1, Table S1) and TyG-BMI index (illustrated in Additional file 2, Table S2).
Table 1.
Baseline characteristics of study subjects with 1-Year mortality
| Variables | Total (n = 1334) |
Non death (n = 923) |
Death (n = 411) |
Statistic | P |
|---|---|---|---|---|---|
| Age, Mean ± SD | 71.57 ± 15.03 | 68.57 ± 15.10 | 78.31 ± 12.47 | t=-12.30 | < 0.001 |
| RBC, Mean ± SD | 4.12 ± 0.70 | 4.20 ± 0.67 | 3.92 ± 0.75 | t = 6.57 | < 0.001 |
| WBC, Mean ± SD | 10.54 ± 4.70 | 10.12 ± 4.22 | 11.47 ± 5.51 | t=-4.44 | < 0.001 |
| Platelet, Mean ± SD | 224.73 ± 82.42 | 226.27 ± 79.81 | 221.26 ± 88.00 | t = 0.99 | 0.324 |
| Hemoglobin, Mean ± SD | 12.25 ± 2.16 | 12.54 ± 2.04 | 11.60 ± 2.26 | t = 7.26 | < 0.001 |
| Sodium, Mean ± SD | 139.12 ± 3.93 | 139.15 ± 3.72 | 139.05 ± 4.38 | t = 0.39 | 0.694 |
| Creatinine, Mean ± SD | 1.11 ± 0.97 | 1.04 ± 0.94 | 1.27 ± 1.01 | t=-3.85 | < 0.001 |
| Triglyceride, Mean ± SD | 132.26 ± 167.07 | 124.64 ± 92.72 | 149.37 ± 266.44 | t=-1.83 | 0.067 |
| Glucose, Mean ± SD | 137.54 ± 66.59 | 129.79 ± 49.84 | 154.94 ± 91.60 | t=-5.23 | < 0.001 |
| Height, Mean ± SD | 168.09 ± 11.08 | 169.10 ± 11.10 | 165.80 ± 10.72 | t = 5.14 | < 0.001 |
| Weight, Mean ± SD | 79.11 ± 22.20 | 81.99 ± 23.00 | 72.62 ± 18.77 | t = 7.83 | < 0.001 |
| BMI, Mean ± SD | 27.81 ± 6.57 | 28.47 ± 6.76 | 26.32 ± 5.89 | t = 5.59 | < 0.001 |
| SOFA, M (Q₁, Q₃) | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | 1.00 (0.00, 3.00) | Z=-7.77 | < 0.001 |
| APSIII, M (Q₁, Q₃) | 35.00 (27.00, 47.00) | 32.00 (25.00, 42.00) | 45.00 (34.00, 57.00) | Z=-12.98 | < 0.001 |
| SAPSII, M (Q₁, Q₃) | 31.00 (25.00, 39.00) | 28.00 (22.00, 35.00) | 39.00 (33.00, 47.00) | Z=-16.69 | < 0.001 |
| OASIS, M (Q₁, Q₃) | 31.00 (25.00, 36.00) | 28.00 (24.00, 33.00) | 36.00 (31.00, 41.00) | Z=-14.13 | < 0.001 |
| GCS Score, M (Q₁, Q₃) | 3.00 (0.00, 10.00) | 0.00 (0.00, 10.00) | 3.00 (0.00, 15.00) | Z=-6.62 | < 0.001 |
| Gender, n (%) | χ²=6.55 | 0.010 | |||
| Female | 683 (51.20) | 451 (48.86) | 232 (56.45) | ||
| Male | 651 (48.80) | 472 (51.14) | 179 (43.55) | ||
| Race, n (%) | χ²=9.85 | 0.043 | |||
| Asian | 37 (2.77) | 26 (2.82) | 11 (2.68) | ||
| Black | 110 (8.25) | 71 (7.69) | 39 (9.49) | ||
| Other | 424 (31.78) | 273 (29.58) | 151 (36.74) | ||
| Spanish | 30 (2.25) | 23 (2.49) | 7 (1.70) | ||
| White | 733 (54.95) | 530 (57.42) | 203 (49.39) | ||
| Diabetes, n (%) | χ²=3.09 | 0.079 | |||
| No | 993 (74.44) | 700 (75.84) | 293 (71.29) | ||
| Yes | 341 (25.56) | 223 (24.16) | 118 (28.71) | ||
| Paraplegia, n (%) | χ²=8.40 | 0.004 | |||
| No | 438 (32.83) | 326 (35.32) | 112 (27.25) | ||
| Yes | 896 (67.17) | 597 (64.68) | 299 (72.75) | ||
| Renal Disease, n (%) | χ²=41.92 | < 0.001 | |||
| No | 1120 (83.96) | 815 (88.30) | 305 (74.21) | ||
| Yes | 214 (16.04) | 108 (11.70) | 106 (25.79) | ||
| Respiratory Failure, n (%) | χ²=107.25 | < 0.001 | |||
| No | 1082 (81.11) | 817 (88.52) | 265 (64.48) | ||
| Yes | 252 (18.89) | 106 (11.48) | 146 (35.52) | ||
| Congestive Heart Failure, n (%) | χ²=36.72 | < 0.001 | |||
| No | 1009 (75.64) | 742 (80.39) | 267 (64.96) | ||
| Yes | 325 (24.36) | 181 (19.61) | 144 (35.04) |
t: t-test, Z: Mann-Whitney test, χ²: Chi-square test
SD: standard deviation, M: Median, Q₁: 1st Quartile, Q₃: 3st Quartile
Multivariable Cox proportional hazard models
Multivariable Cox proportional hazard models analyzing the mortality risk associated with the Tyg and Tyg-BMI groups across different time intervals (30-day, 90-day, 180-day, and 1-year mortality). The analysis presents HR and intervals CI for different quartiles (Q1 to Q4) within both the Tyg and Tyg-BMI groups. Q1 serves as a reference category, with HR values for Q2, Q3, and Q4 representing increased or decreased risk of mortality relative to this baseline. For the 30-day mortality: Tyg Group Q4 shows a significant hazard ratio of 1.85 (HR: 1.85, CI: 1.33–2.58, p < 0.001), indicating a substantial increase in mortality risk compared to Q1. The Tyg BMI Group similarly reveals lower HRs in Q2 and Q3, indicating a decreased risk compared to Q1. For the 90-day mortality: The patterns observed are consistent, with higher quartiles generally reflecting increased mortality risk in the Tyg Group, particularly in Q4 (HR: 2.30). For the 180-day and 1-year mortality, the Tyg Group again shows statistically significant hazard ratios in higher quartiles, pointing towards compounded risks over time. (Table 2). Forest plots in Additional file 4: Fig. S1–8 depict the stratified analyzes of TyG, TyG-BMI and ACM. stratified analyses revealed that significant correlation between TyG index and all-cause mortality was more likely to occur among individuals who were < 60 years of age.
Table 2.
Multivariable Cox proportional hazard models for TYG and TYG-BMI in different time periods
| Variables | Model1 | Model2 | Model3 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |||
| 30-day mortality | ||||||||
| Tyg Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 1.01 (0.70 ~ 1.47) | 0.950 | 1.12 (0.77 ~ 1.64) | 0.555 | 1.10 (0.75 ~ 1.61) | 0.627 | ||
| Q3 | 1.00 (0.69 ~ 1.46) | 0.997 | 1.34 (0.91 ~ 1.99) | 0.141 | 1.30 (0.87 ~ 1.95) | 0.195 | ||
| Q4 | 1.85 (1.33 ~ 2.58) | < 0.001 | 2.30 (1.56 ~ 3.39) | < 0.001 | 2.17 (1.43 ~ 3.30) | < 0.001 | ||
| Tyg BMI Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.71 (0.51 ~ 0.99) | 0.045 | 0.64 (0.45 ~ 0.90) | 0.011 | 0.69 (0.46 ~ 1.03) | 0.069 | ||
| Q3 | 0.60 (0.42 ~ 0.85) | 0.004 | 0.61 (0.42 ~ 0.88) | 0.008 | 0.69 (0.42 ~ 1.15) | 0.159 | ||
| Q4 | 0.73 (0.53 ~ 1.02) | 0.067 | 0.72 (0.49 ~ 1.05) | 0.089 | 0.90 (0.43 ~ 1.87) | 0.783 | ||
| 90-day mortality | ||||||||
| Tyg Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.99 (0.72 ~ 1.37) | 0.953 | 1.10 (0.79 ~ 1.53) | 0.571 | 1.08 (0.77 ~ 1.50) | 0.665 | ||
| Q3 | 1.00 (0.72 ~ 1.38) | 0.991 | 1.38 (0.98 ~ 1.94) | 0.063 | 1.34 (0.94 ~ 1.89) | 0.103 | ||
| Q4 | 1.73 (1.30 ~ 2.32) | < 0.001 | 2.29 (1.63 ~ 3.21) | < 0.001 | 2.15 (1.49 ~ 3.09) | < 0.001 | ||
| Tyg BMI Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.67 (0.50 ~ 0.90) | 0.007 | 0.62 (0.46 ~ 0.83) | 0.002 | 0.67 (0.48 ~ 0.95) | 0.025 | ||
| Q3 | 0.59 (0.44 ~ 0.80) | < 0.001 | 0.60 (0.44 ~ 0.82) | 0.001 | 0.69 (0.45 ~ 1.08) | 0.104 | ||
| Q4 | 0.61 (0.46 ~ 0.83) | 0.001 | 0.62 (0.44 ~ 0.87) | 0.005 | 0.81 (0.42 ~ 1.53) | 0.512 | ||
| 180-day mortality | ||||||||
| Tyg Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 1.02 (0.75 ~ 1.38) | 0.896 | 1.15 (0.84 ~ 1.56) | 0.381 | 1.12 (0.82 ~ 1.53) | 0.462 | ||
| Q3 | 1.05 (0.78 ~ 1.42) | 0.736 | 1.48 (1.08 ~ 2.04) | 0.015 | 1.43 (1.04 ~ 1.98) | 0.029 | ||
| Q4 | 1.68 (1.27 ~ 2.21) | < 0.001 | 2.25 (1.63 ~ 3.11) | < 0.001 | 2.12 (1.50 ~ 2.99) | < 0.001 | ||
| Tyg BMI Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.65 (0.49 ~ 0.85) | 0.002 | 0.60 (0.45 ~ 0.80) | < 0.001 | 0.65 (0.47 ~ 0.90) | 0.009 | ||
| Q3 | 0.58 (0.44 ~ 0.77) | < 0.001 | 0.59 (0.44 ~ 0.79) | < 0.001 | 0.68 (0.45 ~ 1.02) | 0.062 | ||
| Q4 | 0.61 (0.46 ~ 0.81) | < 0.001 | 0.62 (0.45 ~ 0.85) | 0.003 | 0.80 (0.44 ~ 1.45) | 0.453 | ||
| 1-year mortality | ||||||||
| Tyg Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.97 (0.73 ~ 1.29) | 0.832 | 1.10 (0.82 ~ 1.47) | 0.523 | 1.08 (0.80 ~ 1.45) | 0.617 | ||
| Q3 | 1.00 (0.75 ~ 1.33) | 0.994 | 1.42 (1.05 ~ 1.91) | 0.024 | 1.37 (1.01 ~ 1.87) | 0.045 | ||
| Q4 | 1.58 (1.22 ~ 2.06) | < 0.001 | 2.13 (1.57 ~ 2.89) | < 0.001 | 2.02 (1.45 ~ 2.80) | < 0.001 | ||
| Tyg BMI Group | ||||||||
| Q1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||||
| Q2 | 0.72 (0.56 ~ 0.93) | 0.012 | 0.67 (0.52 ~ 0.88) | 0.003 | 0.73 (0.53 ~ 0.98) | 0.039 | ||
| Q3 | 0.57 (0.43 ~ 0.74) | < 0.001 | 0.57 (0.43 ~ 0.76) | < 0.001 | 0.65 (0.44 ~ 0.96) | 0.031 | ||
| Q4 | 0.59 (0.45 ~ 0.77) | < 0.001 | 0.58 (0.43 ~ 0.79) | < 0.001 | 0.74 (0.41 ~ 1.32) | 0.308 | ||
HR: Hazard Ratio, CI: Confidence Interval
Model1: Crude
Model2: Adjust: Gender, Age, Race
Model3: Adjust: Gender, Age, Race, BMI, Respiratory Failure, Diabetes, Congestive Heart Failure, Paraplegia, Renal Disease
K‒M survival analysis curves and hazard ratio for TYG index
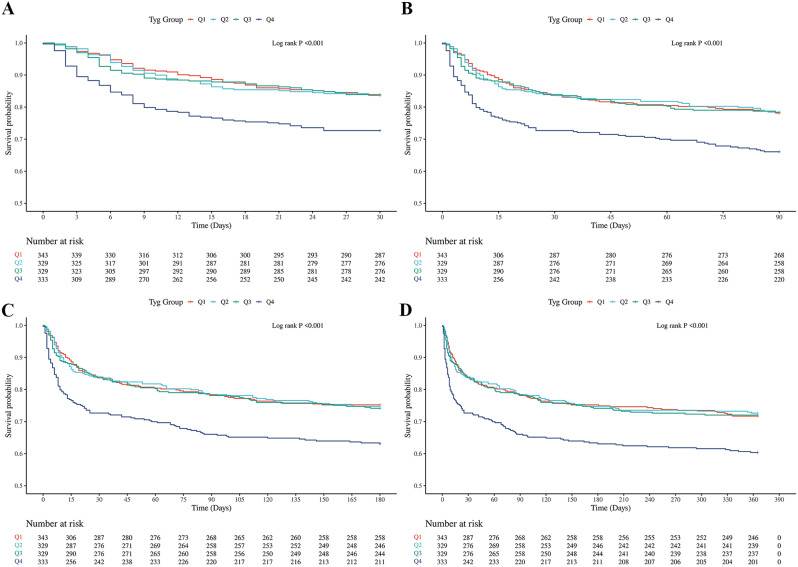
The Kaplan-Meier survival analysis revealed significant differences in ACM at 30-day, 90-day, 180-day, and 1-year intervals among patients stratified by TyG index quartiles. As illustrated in Fig. 1, patients in the highest TyG quartile exhibited substantially lower long-term survival rates compared to those in the lower quartiles. All log-rank P-values were less than 0.001, demonstrating statistically significant differences across all time points. Upon further examination of the survival curves, it is evident that the TyG index serves as a potential independent risk factor for both short-term and long-term mortality in critically ill patients with ischemic stroke. The separation of survival curves at 30-day, 90-day, 180-day, and 1-year time points underscores a consistent trend: patients with a higher TyG index experienced markedly higher cumulative mortality. This trend persisted throughout the 1-year follow-up, suggesting that the TyG index has a prolonged impact on patient outcomes.
Fig. 1.
K‒M survival analysis curves and cumulative incidence with TyG index for 30-day, 90-day, 180-day, and 1-year mortality in critically ill patients with ischemic stroke. A:30-day, B:90-day, C:180-day, D:1-year
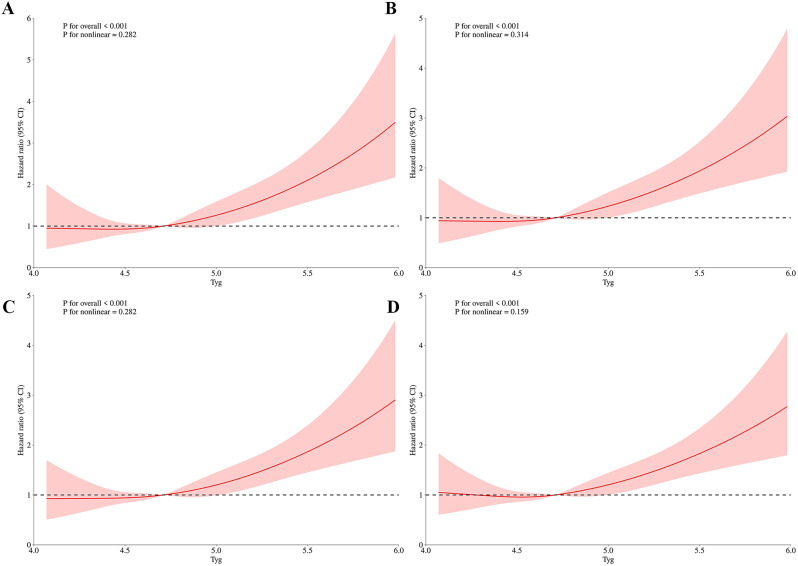
The RCS analyses revealed a positive linear relationship between the TyG index and ACM, indicating that the risk of death increases exponentially with higher TyG levels. The P values indicated in the figure demonstrate no significant non-linearity for ACM, with P values of 0.282 for the 30-day, 0.314 for the 90-day, 0.282 for the 180-day, and 0.159 for the 1-year follow-up. The figure that the threshold for the TyG index is identified at 4.71. Below this threshold, the risk of mortality remains relatively stable. However, once the TyG index exceeds 4.71, there is an exponential increase in the risk of mortality. (Fig. 2)
Fig. 2.
The TyG index hazard ratio for 30-day, 90-day, 180-day, and 1-year mortality in critically ill patients with ischemic stroke. A:30-day, B:90-day, C:180-day, D:1-year
K‒M survival analysis curves and hazard ratio for TYG-BMI index
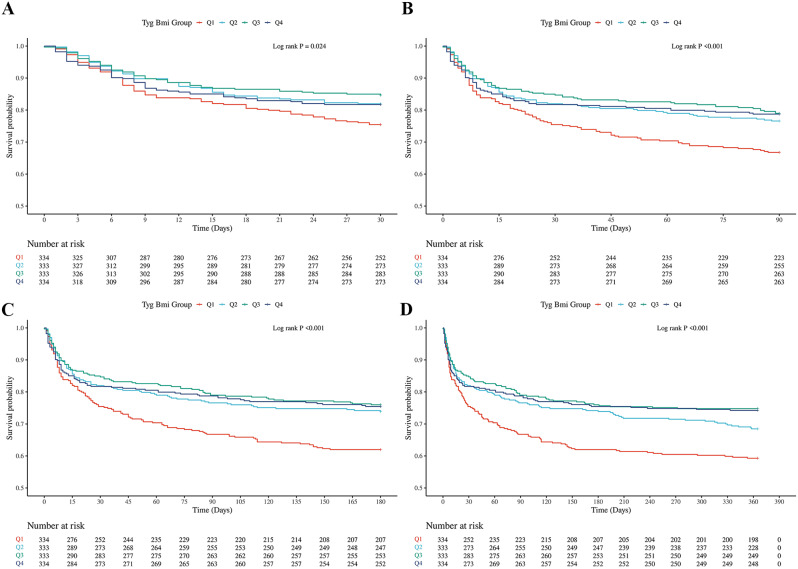
The Kaplan-Meier (K-M) survival analysis of the TyG-BMI index revealed distinct differences in all-cause mortality (ACM) across 30-day, 90-day, 180-day, and 1-year intervals, depending on the TyG-BMI quartiles. In contrast to the TyG index findings, patients in the lowest TyG-BMI quartile exhibited significantly lower long-term survival rates than those in higher quartiles, with log-rank P-values of 0.024, < 0.001, < 0.001, and < 0.001 at the 30-day, 90-day, 180-day, and 1-year marks, respectively. This pattern is clearly depicted in Fig. 3, which illustrates the cumulative incidence of mortality over time. The divergence in survival rates between the TyG and TyG-BMI indices suggests that the combination of the TyG index and BMI introduces a more nuanced layer of risk stratification for critically ill patients with ischemic stroke. Notably, the inverse relationship observed in the TyG-BMI quartile analysis, where the lowest quartile correlates with poorer outcomes, contrasts sharply with the TyG index results, indicating that BMI may modify the impact of the TyG index on survival outcomes.
Fig. 3.
K‒M survival analysis curves and cumulative incidence with TyG-BMI index for 30-day, 90-day, 180-day, and 1-year mortality in critically ill patients with ischemic stroke. A:30-day, B:90-day, C:180-day, D:1-year
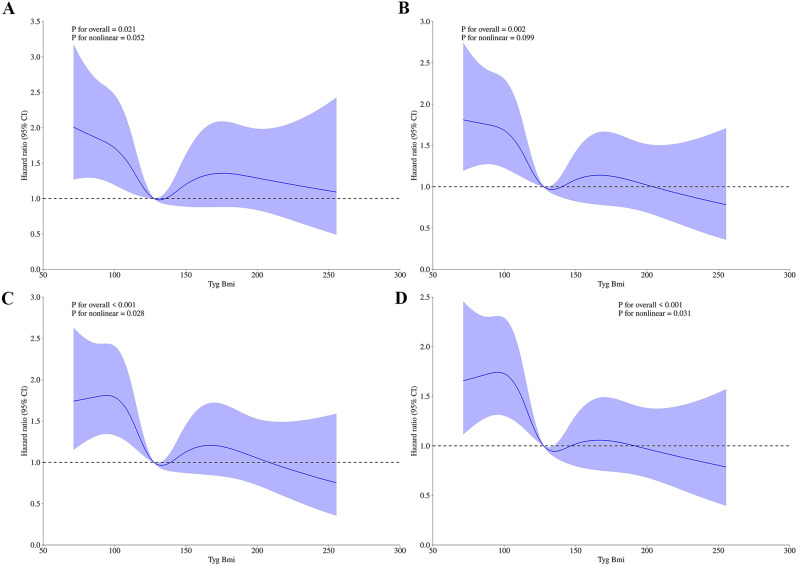
Figure 4 illustrating the TyG-BMI index hazard ratio for mortality at different time intervals in critically ill patients with ischemic stroke. The time intervals specified include 30-day, 90-day, 180-day, and 1-year mortality. Each part of the figure is labeled, with “A” corresponding to the 30-day, “B” to the 90-day, “C” to the 180-day, and “D” to the 1-year mortality hazard ratios. The use of Hazard ratios indicates a quantitative analysis of the risk associated with the TyG-BMI index over these specified periods. The ACM analyzes demonstrated significant nonlinear relationships (P = 0.028 for 180-day, P = 0.031 for 1-year).
Fig. 4.
The TyG-BMI index hazard ratio for 30-day, 90-day, 180-day, and 1-year mortality in critically ill patients with ischemic stroke. A:30-day, B:90-day, C:180-day, D:1-year
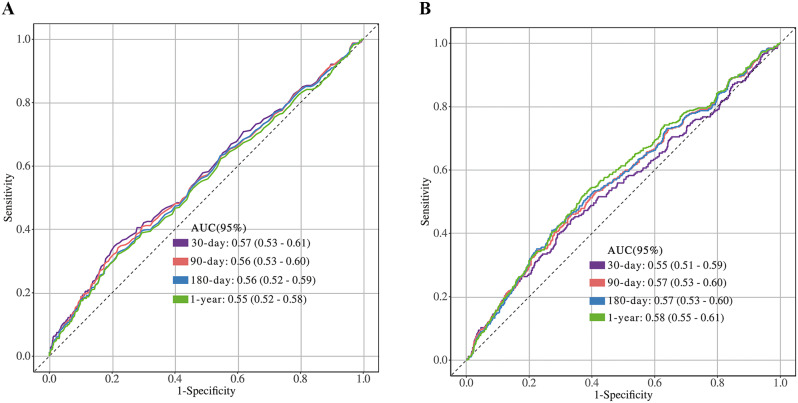
ROC curve analysis of TyG and TyG-BMI
Figure 5 illustrates the predictive performance of TYG and TYG-BMI for mortality in critically ill patients with ischemic stroke across various follow-up periods. The results indicate that the AUC values of TYG for predicting mortality at 30 days, 90 days, 180 days, and 1 year were 0.57 (0.53–0.61), 0.56 (0.53–0.60), 0.56 (0.52–0.59), and 0.55 (0.52–0.58), respectively. In comparison, the AUC values of TYG-BMI for predicting mortality at 30 days, 90 days, 180 days, and 1 year were 0.55 (0.51–0.59), 0.57 (0.53–0.60), 0.57 (0.53–0.60), and 0.58 (0.55–0.61), respectively. The ROC curve analysis revealed that the TyG index had superior predictive power for short-term mortality (30-day and 90-day), while the TyG-BMI index outperformed in predicting long-term mortality (180-day and 1-year). The detailed prediction results illustrated in Additional file 5, Table S7. The results of univariate logistic regression are presented in Additional file 3, Table S3-S6. These findings highlight the differential prognostic value of each index depending on the follow-up period.
Fig. 5.
The ROC curves of the TyG and TyG-BMI index as a marker to predict 30-day, 90-day, 180-day and 1-year mortality. A: TyG, B: TyG-BMI
Discussion
This study provides valuable insights into the prognostic utility of the TyG and TyG-BMI indices in critically ill ischemic stroke patients. The primary finding suggests that both indices serve as significant predictors of mortality, though their predictive strengths differ across short- and long-term outcomes. Specifically, the TyG index alone was more strongly associated with short-term mortality, while the TyG-BMI index demonstrated superior performance in predicting long-term mortality. This differential impact underscores the complexity of metabolic factors involved in mortality risk among this vulnerable patient population.
These results align with previous studies and meta-analyses investigating the role of the TyG index in ischemic stroke and other cardiometabolic disorders. A recent meta-analysis by Zhong et al. (2023) highlighted the association between the TyG index and arterial stiffness, a marker of cardiovascular risk, underscoring the predictive value of TyG in cardiovascular conditions [22]. In the context of ischemic stroke, TyG has been shown to reflect insulin resistance, which exacerbates early mortality risks by contributing to acute metabolic disturbances, including myocardial infarction or arrhythmias, commonly seen in these patients [23]. Moreover, the TyG index has been associated with poor outcomes in conditions like heart failure (HF), obstructive sleep apnea (OSA), atrial fibrillation (AF), and diabetes, reinforcing its role as a predictor of adverse outcomes in various cardiometabolic disorders [22].
In contrast, the superior performance of the TyG-BMI index in predicting long-term mortality suggests that obesity-related metabolic burden plays a more critical role in the prolonged survival of ischemic stroke patients. This finding is consistent with previous research linking obesity and its associated metabolic disturbances to chronic inflammation, endothelial dysfunction, and sustained insulin resistance, all of which contribute to poorer long-term outcomes [24]. The TyG-BMI index, combining insulin resistance and obesity, may provide a more comprehensive picture of the metabolic burden on stroke survivors, helping to predict long-term complications such as recurrent strokes, chronic heart failure, and progressive atherosclerosis, all of which increase long-term mortality risk [25]. These findings align with previous studies showing that obesity is a key determinant of long-term health outcomes in stroke survivors.
Interestingly, the RCS analysis demonstrated that while the TyG-BMI index did not exhibit a significant nonlinear relationship with short-term mortality, a notable nonlinear relationship was observed for long-term mortality. This suggests that the association between TyG-BMI levels and mortality is more complex, potentially influenced by additional factors not accounted for in this analysis [26]. In contrast, the TyG index exhibited a more linear relationship, suggesting a more consistent predictive capacity for short-term outcomes. This finding could be explained by the more immediate impact of insulin resistance on cardiovascular and metabolic function in the acute phase of ischemic stroke.
The clinical utility of these findings is substantial. The TyG index, with its strong association with short-term mortality, could serve as a valuable tool for early risk identification in critically ill ischemic stroke patients. Early recognition of high TyG levels could prompt the initiation of aggressive metabolic management strategies in the ICU, such as glucose control and anti-inflammatory interventions [27]. Conversely, the TyG-BMI index, which better predicts long-term mortality, could be integrated into long-term care plans, helping clinicians identify patients at higher risk for late complications and tailoring interventions such as weight management, anti-obesity treatments, and cardiovascular monitoring [28].
The strengths of this study lie in its large sample size, the use of both TyG and TyG-BMI indices, and its focus on critically ill ischemic stroke patients, a population with unique metabolic disturbances. Our findings contribute to the growing body of evidence supporting the role of these indices in risk stratification. Additionally, the application of advanced statistical methods, such as restricted cubic spline analysis, provides a deeper understanding of the nonlinear relationships between these indices and mortality outcomes [29].
The limitations of this study should be acknowledged. First, although the sample size was relatively large, the cohort was derived predominantly from a single institution, potentially limiting the generalizability of the findings to broader and more diverse populations [30]. Second, the retrospective design of the study precludes the establishment of definitive causal relationships between the TyG and TyG-BMI indices and mortality outcomes [31]. Third, while we accounted for several known confounders, additional factors such as concurrent infections, renal dysfunction, and other critical complications were not comprehensively evaluated. Moreover, the potential impact of medication regimens and therapeutic interventions on patient outcomes was not fully explored. These unmeasured variables may have influenced the observed associations.
Future research should aim to address these limitations by validating the findings in larger, multicenter cohorts and incorporating a broader range of clinical variables. Prospective studies are particularly needed to elucidate the causal pathways linking metabolic indices to mortality and to investigate the interactions between these indices and other complications, including infections, kidney injury, and pharmacological treatments. Such efforts could refine the understanding of these prognostic tools and enhance their application in clinical practice.
Conclusion
This finding suggests both the TyG and TyG-BMI indices serve as valuable predictors of mortality in critically ill ischemic stroke patients. However, significant differences were observed across the various follow-up periods. Based on the distinct characteristics of these two indicators, future research should focus on the selective integration of TyG and TyG-BMI indices into clinical risk assessment models, tailored to the metabolic profiles of ischemic stroke patients in the ICU. This approach could enhance the precision of mortality risk stratification and optimize patient management strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express their gratitude to the participants and staff of the MIMIC IV 3.0 database for their invaluable contributions to this study.
Abbreviations
- IS
Ischemic stroke
- RCS
Restricted cubic spline
- ICU
Intensive care unit
- TyG
Triglyceride-glucose
- ACM
All-cause mortality
- MIMIC-IV
Medical Information Mart for Intensive Care
- TG
Triglyceride
- FBG
Fasting blood glucose
- SQL
Structured Query Language
- APSIII
Acute Physiology Score III
- SAPS-II
Simplified Acute Physiology Score II
- OASIS
Oxford Acute Severity of Illness Score
- SOFA
Sepsis-related Organ Failure Assessment score
- CI
Confidence interval
- HR
Hazard ratio
- BMI
Body mass index
- WBC
White blood cell
- RBC
Red blood cell
- HDL
High-Density Lipoprotein
- ICUs
Intensive Care Units
- ROC
Receiver Operating Characteristic
- AUC
Area Under the Curve
- HF
Heart Failure
- OSA
Obstructive Sleep Apnea
- AF
Atrial Fibrillation
Author contributions
Conception and design: Yufan. Pu and Jiang. Xu; Administrative support: Jiang. Xu and Xuejing. Li; Collection and assembly of data: Yufan. Pu and Ying. Wang; Data analysis and interpretation: Yufan. Pu and Na. Xing; Manuscript writing: All authors; Final approval of manuscript: All authors.
Funding
This work was supported by the National Natural Science Foundation of China (82105004).
Data availability
The datasets that were used and evaluated in this study can be obtained from https://physionet.org/content/mimiciv/0.4/.
Declarations
Ethics approval and consent to participate
The study was performed according to the guidelines of the Helsinki Declaration. The use of the MIMIC-IV database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The data is publicly available (in the MIMIC-IV database), therefore, the ethical approval statement and the requirement for informed consent were waived for this study.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yufan Pu and Na Xing contributed equally to this work.
References
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke[J]. World Neurosurg. 2011;76(6):S85–90. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Norrving B, Mensah GA. Global burden of stroke[J]. Circul Res. 2017;120(3):439–48. [DOI] [PubMed] [Google Scholar]
- 3.Makris K, Haliassos A, Chondrogianni M, et al. Blood biomarkers in ischemic stroke: potential role and challenges in clinical practice and research[J]. Crit Rev Clin Lab Sci. 2018;55(5):294. [DOI] [PubMed] [Google Scholar]
- 4.Robba C, van Dijk EJ, van der Jagt M. Acute ischaemic stroke and its challenges for the intensivist[J]. Eur Heart J Acute Cardiovasc Care. 2022;11(3):258–68. [DOI] [PubMed] [Google Scholar]
- 5.Bevers MB, Kimberly WT. Critical care management of acute ischemic stroke[J]. Curr Treat Options Cardiovasc Med. 2017;19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montellano FA, Ungethüm K, Ramiro L, et al. Role of blood-based biomarkers in ischemic stroke prognosis: a systematic review[J]. Stroke. 2021;52(2):543–51. [DOI] [PubMed] [Google Scholar]
- 7.Tao LC, Xu J, Wang T, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations[J]. Cardiovasc Diabetol. 2022;21(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding X, Wang X, Wu J, et al. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies[J]. Cardiovasc Diabetol. 2021;20:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin JL, Yang J, Song XJ, et al. Triglyceride-glucose index and health outcomes: an umbrella review of systematic reviews with meta-analyses of observational studies[J]. Cardiovasc Diabetol. 2024;23(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding PF, Zhang HS, Wang J, et al. Insulin resistance in ischemic stroke: mechanisms and therapeutic approaches[J]. Front Endocrinol. 2022;13:1092431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley SA, Spring KJ, Beran RG, et al. Role of diabetes in stroke: recent advances in pathophysiology and clinical management[J]. Diab/Metab Res Rev. 2022;38(2):e3495. [DOI] [PubMed] [Google Scholar]
- 12.Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases[J]. Circul Res. 2020;126(11):1477–500. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Ren B, Tian Y et al. Association of the TyG index with prognosis in surgical intensive care patients: data from the MIMIC-IV[J]. Cardiovasc Diabetol, 2024, 23. [DOI] [PMC free article] [PubMed]
- 14.Liang S, Wang C, Zhang J, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170. 10.1186/s12933-023-01906-4. Published 2023 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim J, Kim J, Koo SH, et al. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: an analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey[J]. PLoS ONE. 2019;14(3):e0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsayed S, Othman M. The effect of body mass index (BMI) on the mortality among patients with stroke[J]. Eur J Mol Clin Med. 2021;8(4):181–7. [Google Scholar]
- 17.Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association[J]. Circulation. 2016;134(23):e535–78. [DOI] [PubMed] [Google Scholar]
- 18.Johnson A, Bulgarelli L, Pollard T et al. Mimic-iv[J]. PhysioNet. Available online at: https://physionet.org/content/mimiciv/1.0/(accessed August 23, 2021), 2020: 49–55.
- 19.Pölkki A, Pekkarinen PT, Takala J, et al. Association of sequential organ failure assessment (SOFA) components with mortality[J]. Acta Anaesthesiol Scand. 2022;66(6):731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC, White IR, Lee DS, van Buuren S. Missing Data in Clinical Research: a tutorial on multiple imputation. Can J Cardiol. 2021;37(9):1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai W, Xu J, Wu X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:138. 10.1186/s12933-023-01864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong H, Shao Y, Guo G, et al. Association between the triglyceride-glucose index and arterial stiffness: a meta-analysis[J]. Medicine. 2023;102(10):e33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Hu T, Li R et al. An Innovative Metabolic Index for Insulin Resistance Correlates with Early Neurological Deterioration Following Intravenous Thrombolysis in Minor Acute Ischemic Stroke Patients[J]. Heliyon, 2024. [DOI] [PMC free article] [PubMed]
- 24.Ferenc K, Jarmakiewicz-Czaja S, Sokal-Dembowska A, et al. Common denominator of MASLD and some non-communicable Diseases[J]. Curr Issues Mol Biol. 2024;46(7):6690–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghoorah K, Campbell P, Kent A, et al. Obesity and cardiovascular outcomes: a review[J]. Eur Heart Journal: Acute Cardiovasc Care. 2016;5(1):77–85. [DOI] [PubMed] [Google Scholar]
- 26.Hou Z, Pan Y, Yang Y, et al. An analysis of the potential relationship of triglyceride glucose and body mass index with stroke prognosis[J]. Front Neurol. 2021;12:630140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanathan G, Jagadeesha SN. Prediction of coronary artery disease using artificial intelligence–a systematic literature review[J]. Int J Health Sci Pharm (IJHSP). 2023;7(1):1–32. [Google Scholar]
- 28.Huo RR, Liao Q, Zhai L, et al. Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: a nationwide prospective cohort study[J]. Cardiovasc Diabetol. 2024;23(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherford MJ, Crowther MJ, Lambert PC. The use of restricted cubic splines to approximate complex hazard functions in the analysis of time-to-event data: a simulation study[J]. J Stat Comput Simul. 2015;85(4):777–93. [Google Scholar]
- 30.Song JW, Chung KC. Observational studies: cohort and case-control studies[J]. Plast Reconstr Surg. 2010;126(6):2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Kattan MW. Cohort studies: design, analysis, and reporting[J]. Chest. 2020;158(1):S72–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets that were used and evaluated in this study can be obtained from https://physionet.org/content/mimiciv/0.4/.