Abstract
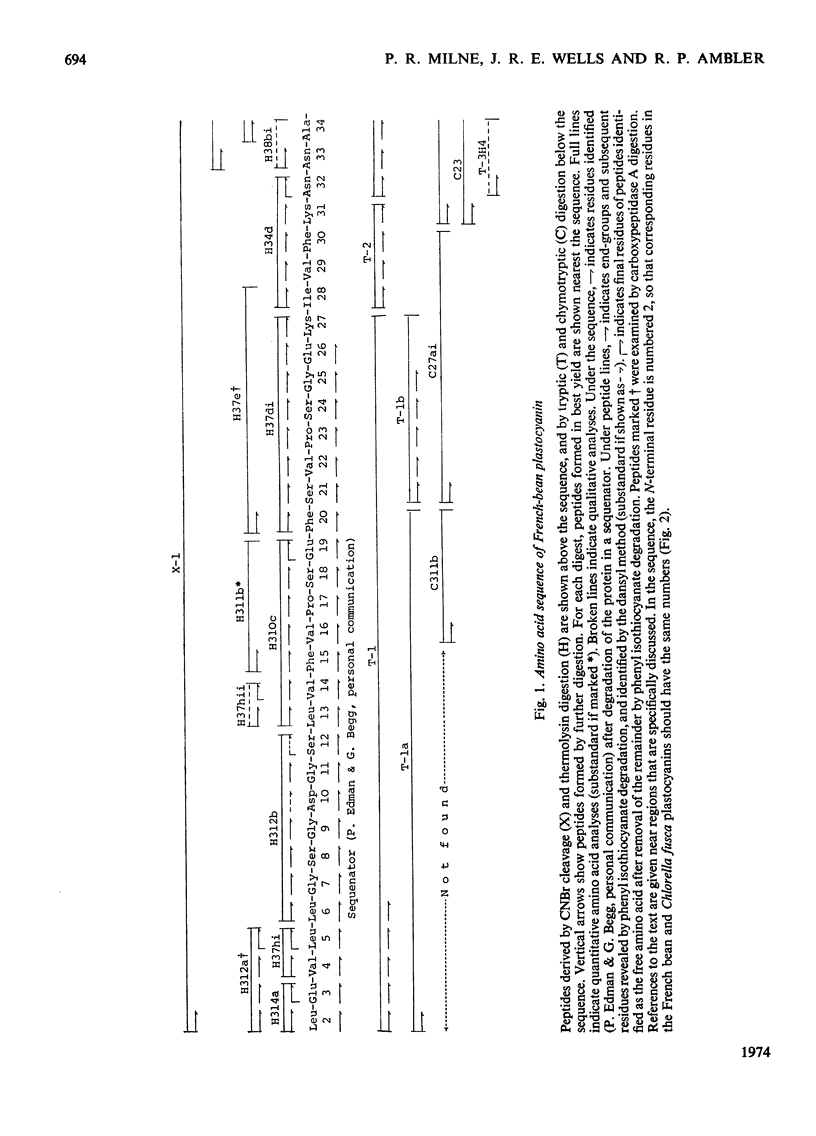
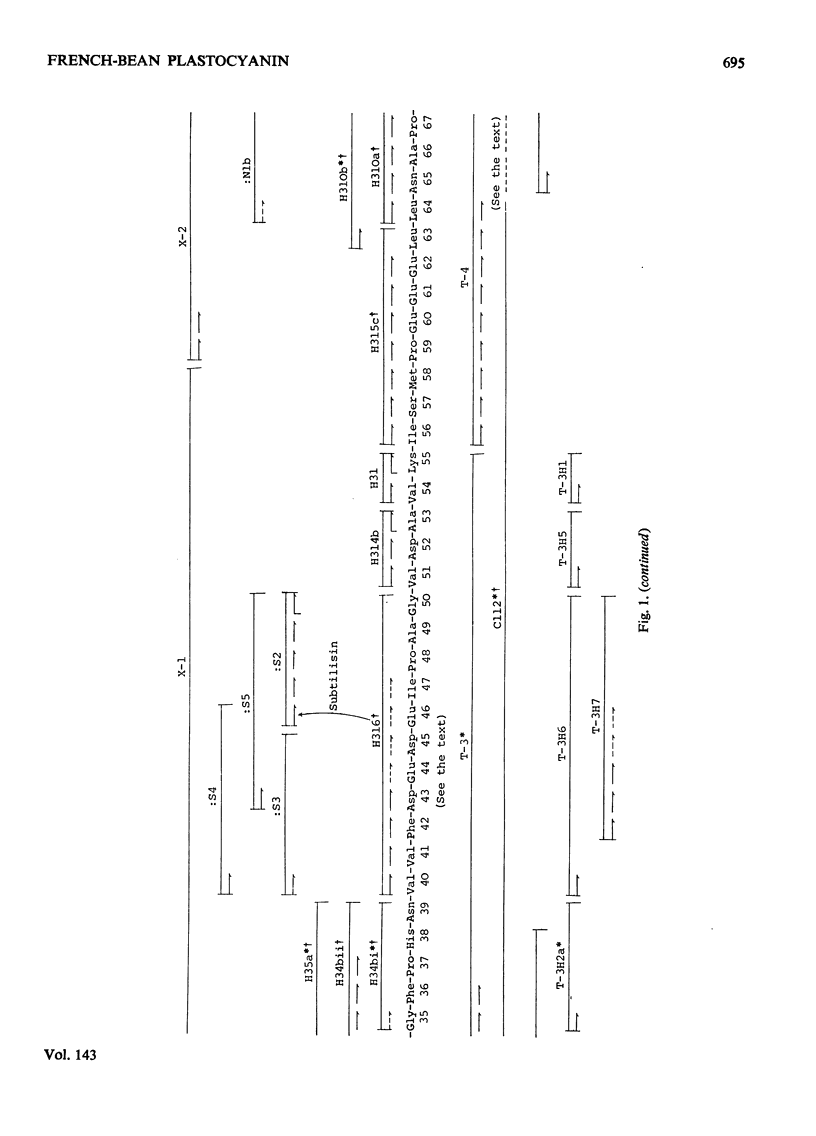
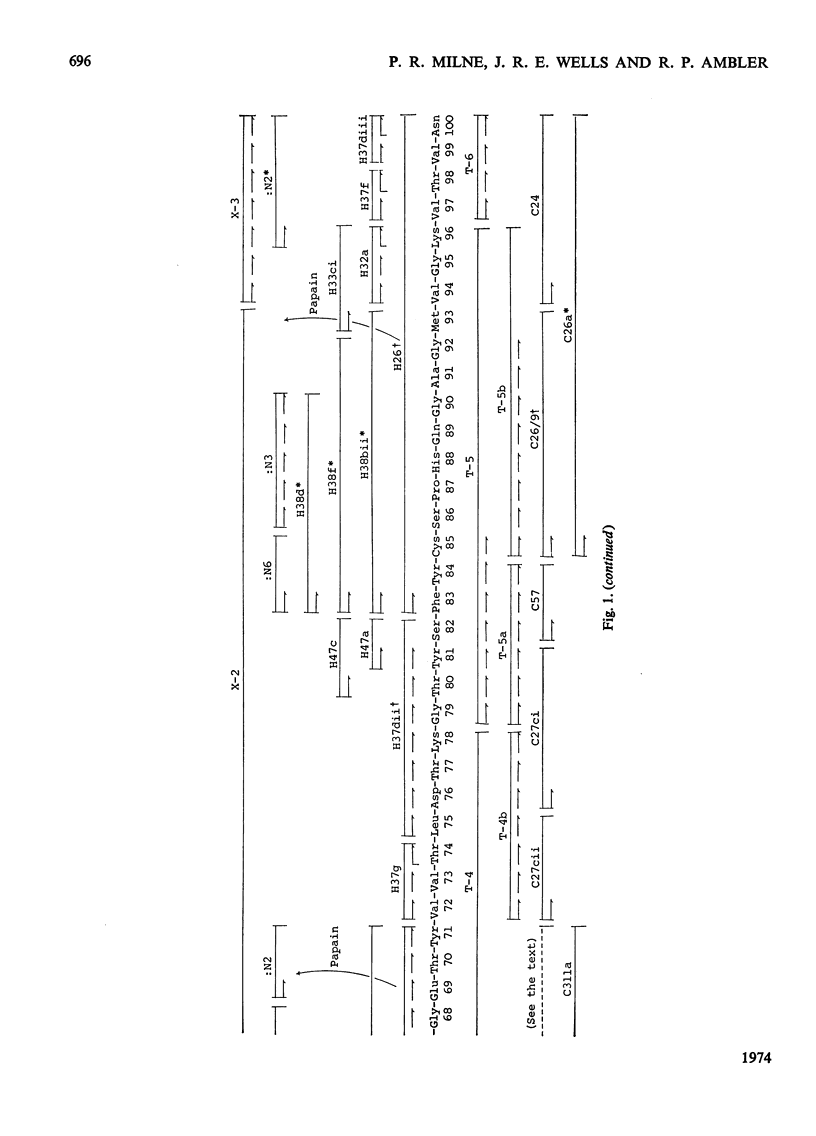
The amino acid sequence of the plastocyanin from French bean (Phaseolus vulgaris) was determined. The protein consists of a single polypeptide chain of 99 residues, and the sequence was determined by characterization of CNBr, tryptic, chymotryptic and thermolysin peptides. When the sequence is compared with that from the plastocyanin of the unicellular green alga Chlorella fusca, the French-bean protein shows the deletion of the N-terminal residue, a two residue insertion and 53 identical residues. Detailed evidence for the sequence of the protein has been deposited as Supplementary Publication SUP 50037 (16pp., 1 microfiche) at the British Library (Lending Division) (formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies may be obtained on the terms given in Biochem. J. (1973) 131, 5.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson L. E., Vänngård T. Evidence of a specific copper(II) in human ceruloplasmin as a binding site for inhibitory anions. Biochim Biophys Acta. 1970 Feb 17;200(2):247–257. doi: 10.1016/0005-2795(70)90168-6. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K., McSwain B. D., Tsujimoto H. Y., Knaff D. B. Evidence from chloroplast fragments for three photosynthetic light reactions. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1404–1409. doi: 10.1073/pnas.67.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K. The photochemical systems of photosynthesis. Adv Enzymol Relat Areas Mol Biol. 1968;30:1–79. doi: 10.1002/9780470122754.ch1. [DOI] [PubMed] [Google Scholar]
- Carey W. F., Wells J. R. Phaseolain. A plant carboxypeptidase of unique specificity. J Biol Chem. 1972 Sep 10;247(17):5573–5579. [PubMed] [Google Scholar]
- Finazzi-Agrò A., Rotilio G., Avigliano L., Guerrieri P., Boffi V., Mondovì B. Environment of copper in Pseudomonas fluorescens azurin: fluorometric approach. Biochemistry. 1970 Apr 28;9(9):2009–2014. doi: 10.1021/bi00811a023. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Dus K., de Klerk H., Kamen M. D. Comparative study of physicochemical properties of two c-type cytochromes of Rhodospirillum molischianum. Biochemistry. 1970 Apr 28;9(9):1991–1996. doi: 10.1021/bi00811a021. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. IV. Purification and Properties of Plastocyanin. Plant Physiol. 1966 Dec;41(10):1637–1642. doi: 10.1104/pp.41.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATOH S. A new copper protein from Chlorella ellisoidea. Nature. 1960 May 14;186:533–534. [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- KATOH S., SUGA I., SHIRATORI I., TAKAMIYA A. Distribution of plastocyanin in plants, with special reference to its localization in chloroplasts. Arch Biochem Biophys. 1961 Jul;94:136–141. doi: 10.1016/0003-9861(61)90020-0. [DOI] [PubMed] [Google Scholar]
- KATOH S., TAKAMIYA A. NATURE OF COPPER-PROTEIN BINDING IN SPINACH PLASTOCYANIN. J Biochem. 1964 Apr;55:378–387. doi: 10.1093/oxfordjournals.jbchem.a127898. [DOI] [PubMed] [Google Scholar]
- Keil-Dlouhá V., V, Zylber N., Imhoff J. -M., Tong N. -T., Keil B. Proteolytic activity of pseudotrypsin. FEBS Lett. 1971 Sep 1;16(4):291–295. doi: 10.1016/0014-5793(71)80373-3. [DOI] [PubMed] [Google Scholar]
- Kelly J., Ambler R. P. The amino acid sequence of plastocyanin from Chlorella fusca. Biochem J. 1974 Dec;143(3):681–690. doi: 10.1042/bj1430681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightbody J. J., Krogmann D. W. Isolation and properties of plastocyanin from Anabaena variabilis. Biochim Biophys Acta. 1967 May 9;131(3):508–515. doi: 10.1016/0005-2728(67)90010-2. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. Two forms of copper (II) in fungal laccase. Biochim Biophys Acta. 1968 Feb 1;156(1):67–76. doi: 10.1016/0304-4165(68)90105-0. [DOI] [PubMed] [Google Scholar]
- Milne P. R., Wells J. R. Structural and molecular weight studies on the small copper protein, plastocyanin. J Biol Chem. 1970 Apr 10;245(7):1566–1574. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The plastocyanin content of chloroplasts from some higher plants estimated by a sensitive enzymatic assay. Biochim Biophys Acta. 1970 Aug 4;216(1):192–199. doi: 10.1016/0005-2728(70)90170-2. [DOI] [PubMed] [Google Scholar]
- Ramshaw J. A., Brown R. H., Scawen M. D., Boulter D. Higher plant plastocyanin. Biochim Biophys Acta. 1973 Apr 20;303(2):269–273. doi: 10.1016/0005-2795(73)90357-7. [DOI] [PubMed] [Google Scholar]
- Ramshaw J. A., Scawen M. D., Bailey C. J., Boulter D. The amino acid sequence of plastocyanin from Solanum tuberosum L. (potato). Biochem J. 1974 Jun;139(3):583–592. doi: 10.1042/bj1390583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rist G. H., Hyde J. S., Vänngård T. Electron-Nuclear Double Resonance of a Protein That Contains Copper: Evidence for Nitrogen Coordination to Cu(II) in Stellacyanin. Proc Natl Acad Sci U S A. 1970 Sep;67(1):79–86. doi: 10.1073/pnas.67.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Wells J. R. Purification and properties of a proteolytic enzyme from French beans. Biochem J. 1965 Oct;97(1):228–235. doi: 10.1042/bj0970228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kouchkovsky Y., Fork D. C. A POSSIBLE FUNCTIONING IN VIVO OF PLASTOCYANIN IN PHOTOSYNTHESIS AS REVEALED BY A LIGHT-INDUCED ABSORBANCE CHANGE. Proc Natl Acad Sci U S A. 1964 Aug;52(2):232–239. doi: 10.1073/pnas.52.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]



