Abstract
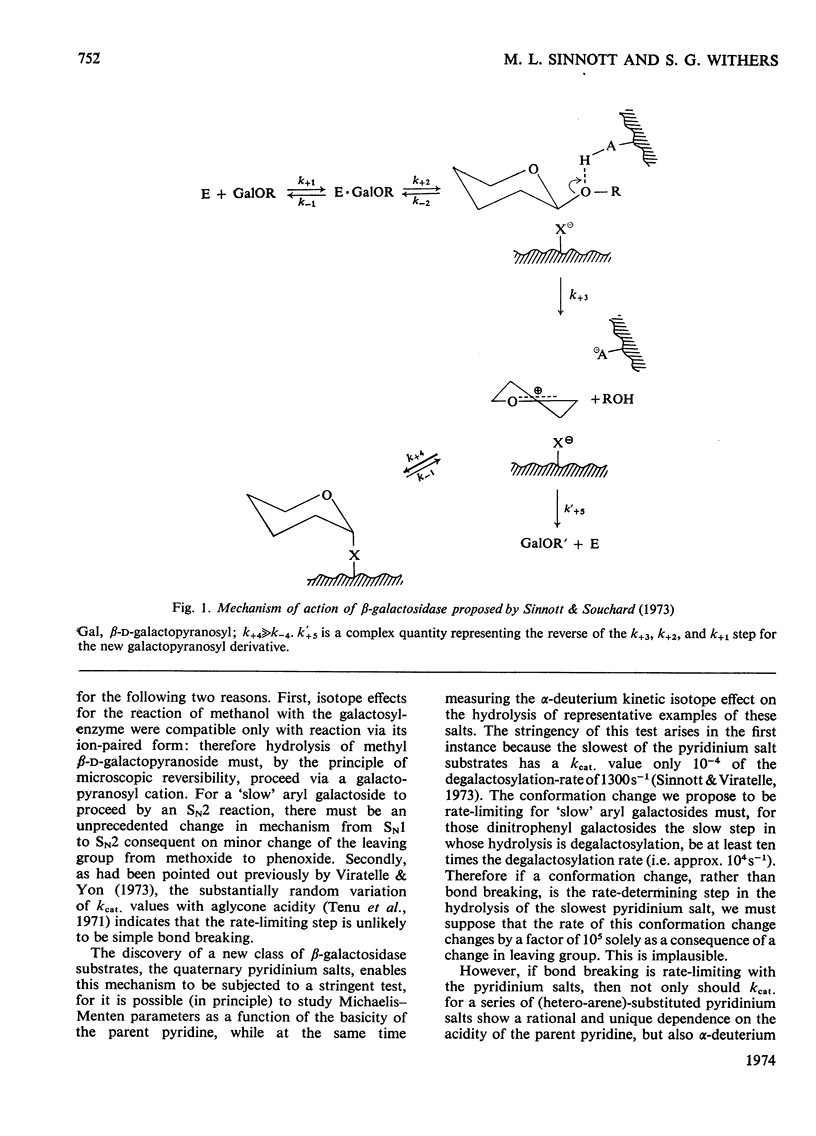
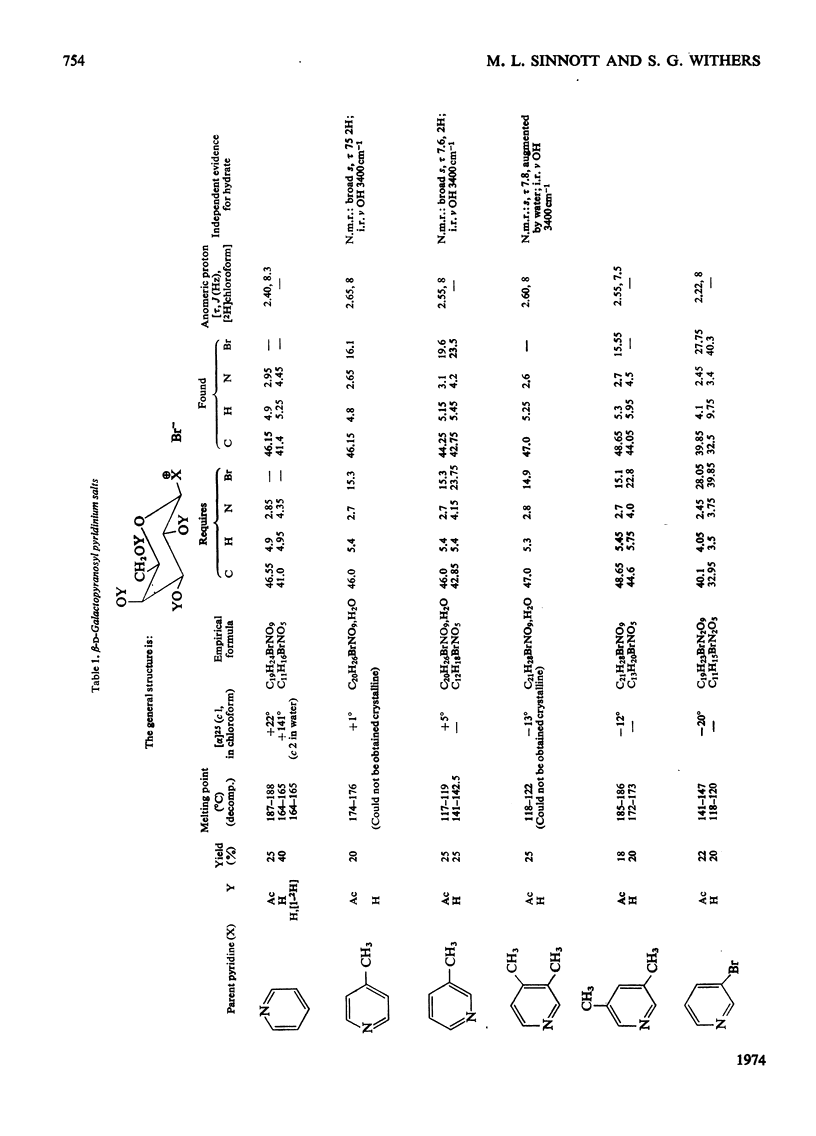
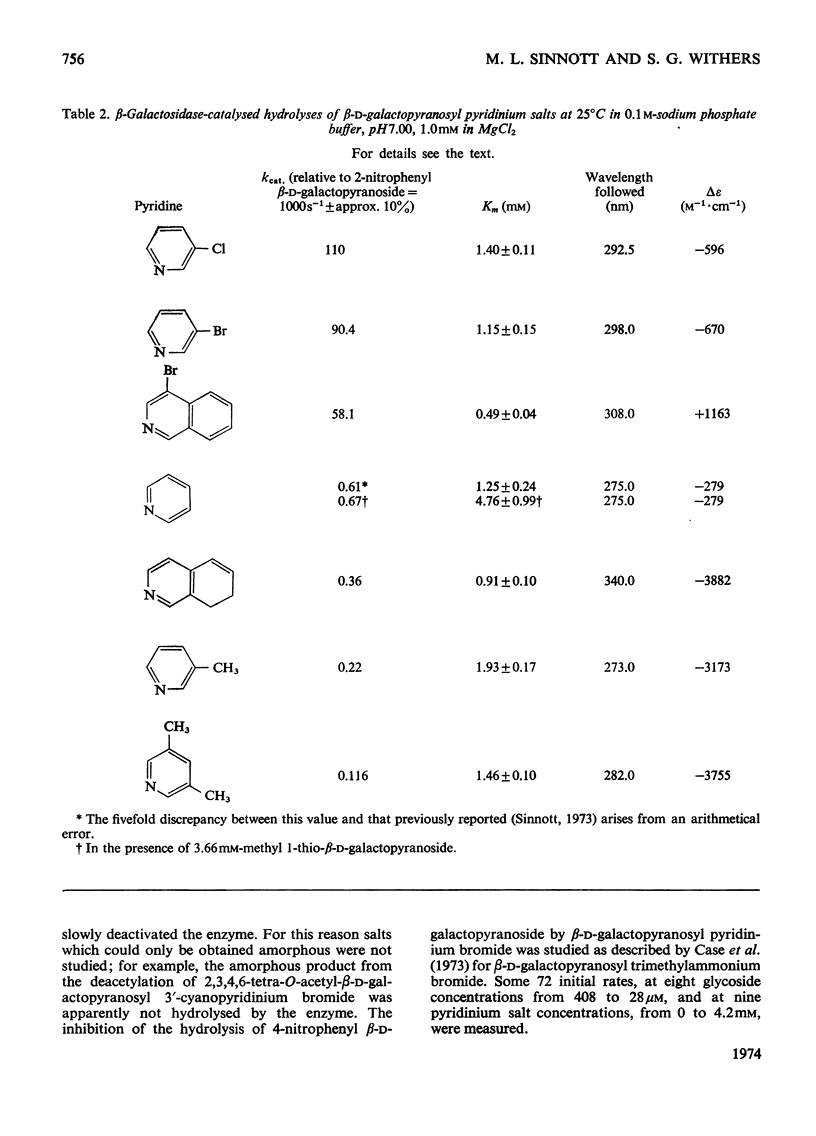
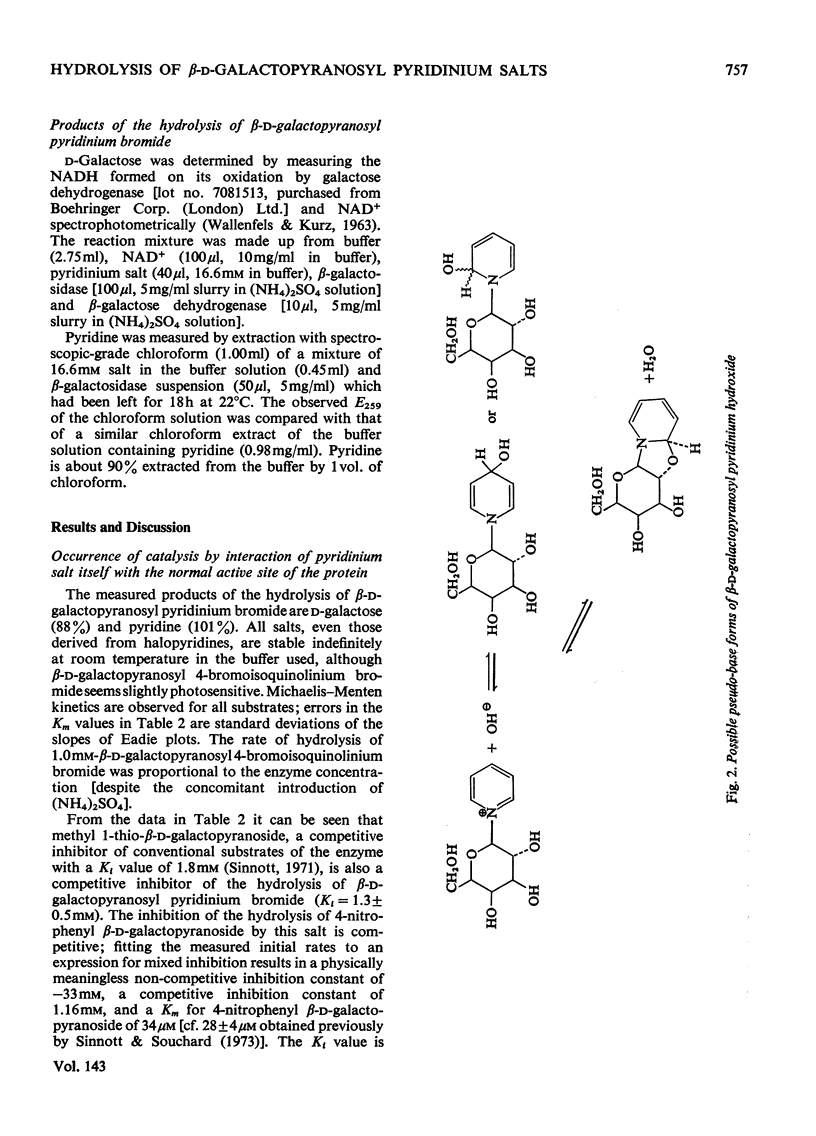
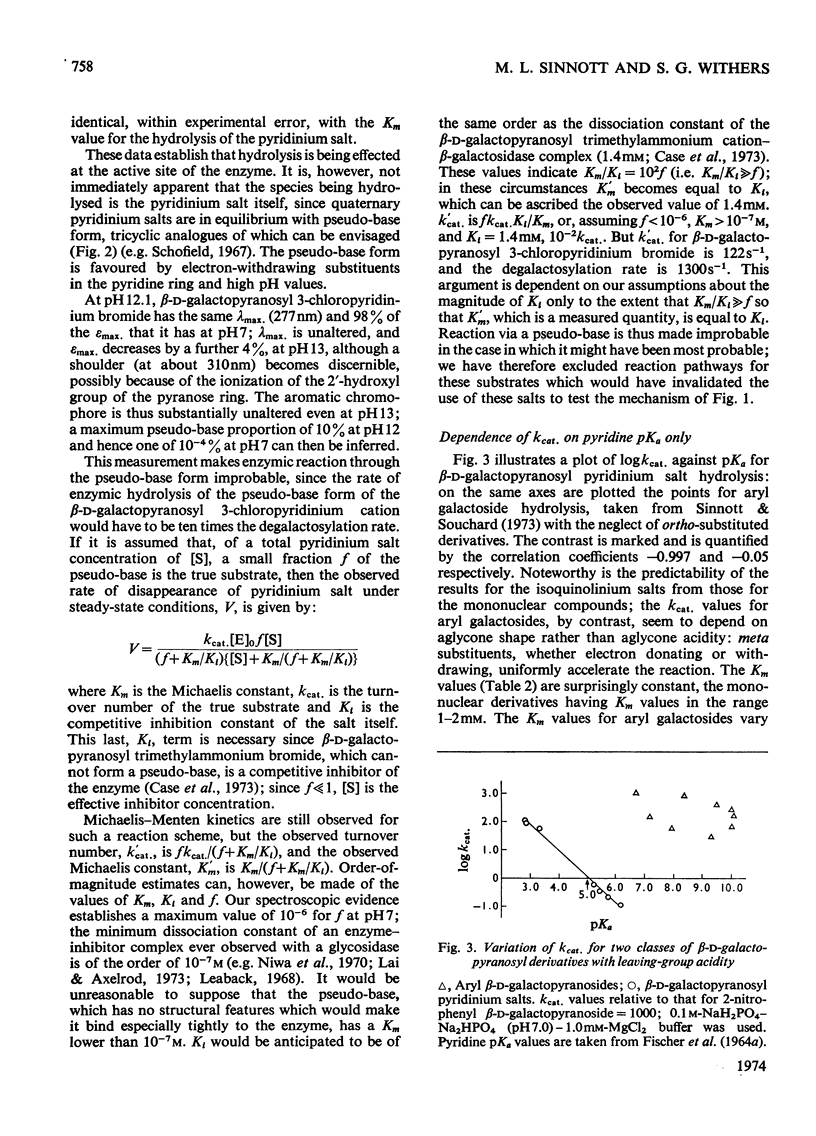
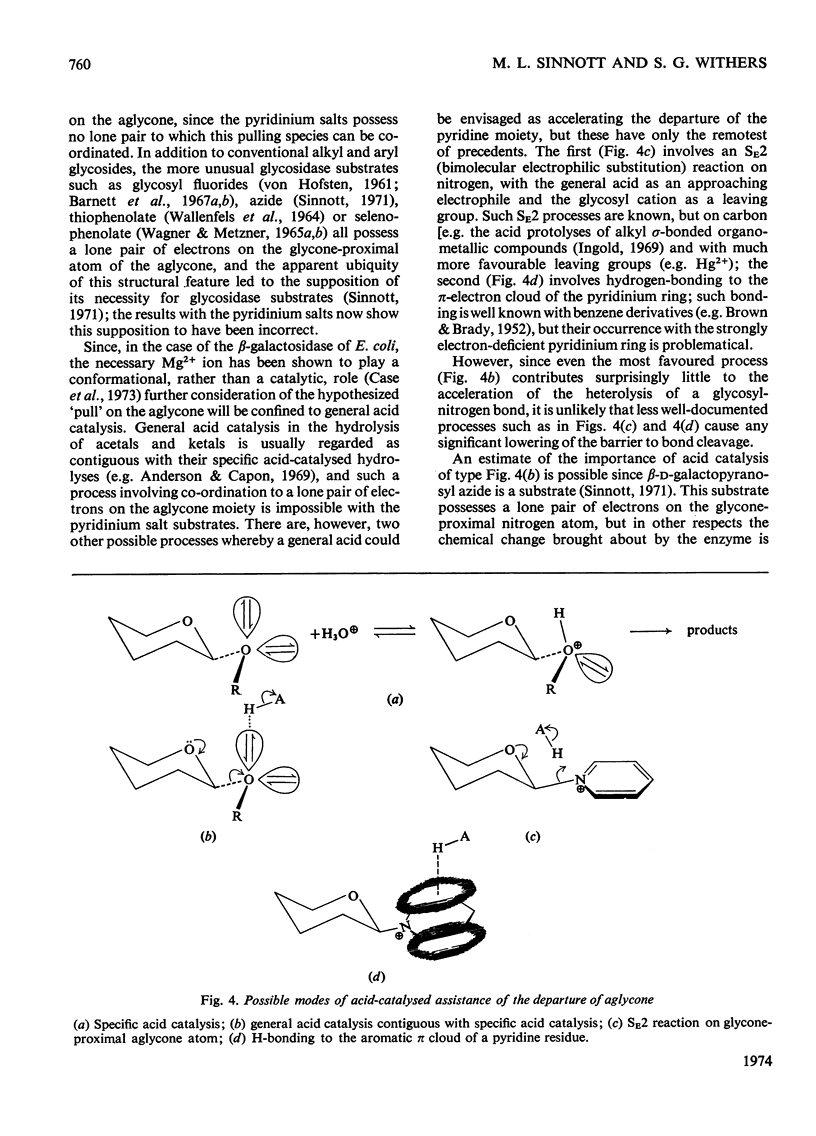
1. β-d-Galactopyranosyl pyridinium salts are well-behaved substrates for the β-galactosidase of Escherichia coli, catalysis occurring by the interaction of the salt itself with the normal active site of the protein. 2. logkcat. values for seven such salts show a linear relationship (correlation coefficient=−0.997) with the pKa of the parent pyridine. 3. The β-d-galactopyranosyl derivatives of pyridine and 4-bromoisoquinoline exhibit α-deuterium kinetic isotope effects of 1.136±0.040 and 1.187±0.046 on their enzymic hydrolysis, indicating formation of a galactopyranosyl cation in the rate-limiting step. 4. This behaviour of the pyridinium salts contrasts with the behaviour of aryl galactosides and this contrast can be accommodated by the β-galactosidase mechanism of Sinnott & Souchard (1973). 5. The α-deuterium kinetic isotope effect for the hydrolysis of β-d-galactopyranosyl azide is 1.098±0.033; comparison of the kcat. value of the azide with that of a pyridinium salt of the same aglycone pKa enables a maximum factor of 70 to be ascribed to the acceleration of the departure of azide by intracomplex general acid catalysis. 6. The possibility of the rate-limiting process in the glycosidase-catalysed hydrolysis of aryl glycosides being a conformation change is considered for a number of glycosidases where correlations of kcat. with aglycone acidity, reported in the literature, have been unsuccessful.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Jarvis W. T., Munday K. A. Enzymic hydrolysis of the carbon-fluorine bond of alpha-D-glucosyl fluoride by rat intestinal mucosa. Localization of intestinal maltase. Biochem J. 1967 Jun;103(3):699–704. doi: 10.1042/bj1030699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J. E., Jarvis W. T., Munday K. A. The hydrolysis of glycosyl fluorides by glycosidases. Biochem J. 1967 Nov;105(2):669–672. doi: 10.1042/bj1050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon B. The mechanism of glycosidase action. Biochimie. 1971;53(2):145–149. doi: 10.1016/s0300-9084(71)80045-7. [DOI] [PubMed] [Google Scholar]
- Case G. S., Sinnott M. L., Tenu J. P. The role of magnesium ions in beta-galactosidase hydrolyses. Studies on charge and shape of the beta-galactopyranosyl binding site. Biochem J. 1973 May;133(1):99–104. doi: 10.1042/bj1330099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Sable H. Z. Enzymes of pentose biosynthesis. II. Evidence that the proposed control of glucose 6-phosphate dehydrogenase by reduced diphosphopyridine nucleotide is an instrumental artifact. J Biol Chem. 1973 Apr 25;248(8):2815–2817. [PubMed] [Google Scholar]
- Dahlquist F. W., Rand-Meir T., Raftery M. A. Application of secondary alpha-deuterium kinetic isotope effects to studies of enzyme catalysis. Glycoside hydrolysis by lysozyme and beta-glucosidase. Biochemistry. 1969 Oct;8(10):4214–4221. doi: 10.1021/bi00838a045. [DOI] [PubMed] [Google Scholar]
- HALL A. N., HOLLINGSHEAD S., RYDON H. N. The influence of structure on the hydrolysis of substituted phenyl alpha-D-glucosides by alpha-glucosidase. Biochem J. 1962 Aug;84:390–394. doi: 10.1042/bj0840390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Lai H. Y., Axelrod B. 1-Aminoglycosides, a new class of specific inhibitors of glycosidases. Biochem Biophys Res Commun. 1973 Sep 18;54(2):463–468. doi: 10.1016/0006-291x(73)91443-5. [DOI] [PubMed] [Google Scholar]
- Leaback D. H. On the inhibition of beta-N-acetyl-D-glucosaminidase by 2-acetamido-2-deoxy-D-glucono-(1-5)-lactone. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1025–1030. doi: 10.1016/0006-291x(68)90132-0. [DOI] [PubMed] [Google Scholar]
- NATH R. L., RYDON H. N. The influence of structure on the hydrolysis of substituted phenyl beta-D-glucosides by emulsin. Biochem J. 1954 May;57(1):1–10. doi: 10.1042/bj0570001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L. -galactosidase-catalysed hydrolysis of -D-galactopyranosyl azide. Biochem J. 1971 Dec;125(3):717–719. doi: 10.1042/bj1250717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L., Souchard I. J. The mechanism of action of beta-galactosidase. Effect of aglycone nature and -deuterium substitution on the hydrolysis of aryl galactosides. Biochem J. 1973 May;133(1):89–98. doi: 10.1042/bj1330089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott M. L., Viratelle O. M. The effect of methanol and dioxan on the rates of the beta-galactosidase-catalysed hydrolyses of some beta-D-galactrophyranosides: rate-limiting degalactosylation. The ph-dependence of galactosylation and degalactosylation. Biochem J. 1973 May;133(1):81–87. doi: 10.1042/bj1330081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Hiromi K., Ono S. Substituent effect on the hydrolyses of phenyl -maltosides catalyzed by saccharifying -amylase from Bacillus subtilis. J Biochem. 1971 Oct;70(4):595–601. doi: 10.1093/oxfordjournals.jbchem.a129676. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Viratelle O. M., Garnier J., Yon J. pH dependence of the activity of beta-galactosidase from Escherichia coli. Eur J Biochem. 1971 Jun 11;20(3):363–370. doi: 10.1111/j.1432-1033.1971.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Viratelle O. M., Yon J. M. Nucleophilic competition in some -galactosidase-catalyzed reactions. Eur J Biochem. 1973 Feb 15;33(1):110–116. doi: 10.1111/j.1432-1033.1973.tb02661.x. [DOI] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]
- Wagner G., Metzner R. Beiträge zur enzymatischen, sauren und alkalischen Spaltung von beta-D-Thioglucosden von Mercapto-pi-Mangel-Heterocyclen und von beta-D-Thioglucosiden und beta-D-Selenoglucosiden von Nitrothiophenolen und Nitroselenophenolen. Pharmazie. 1965 Dec;20(12):752–757. [PubMed] [Google Scholar]
- Wang C. C., Touster O. Studies of catalysis by -glucuronidase. The effect of structure on the rate of hydrolysis of substituted phenyl- -d-glucopyranosiduronic acids. J Biol Chem. 1972 May 10;247(9):2650–2656. [PubMed] [Google Scholar]
- von HOFSTEN Fluoro-D-galactosides as substrates and inducers of the beta-galactosidase of Escherichia coli. Biochim Biophys Acta. 1961 Mar 18;48:159–163. doi: 10.1016/0006-3002(61)90527-3. [DOI] [PubMed] [Google Scholar]


