Abstract
Tissierella praeacuta (T. praeacuta)is a gram-variable obligate anaerobe. In this case report, we describe the first documented case of T. praeacuta bacteremia in a patient with sepsis resulting from lower extremity cellulitis without concomitant osteomyelitis. During the inpatient course, the patient was treated with IV vancomycin, cefepime, and ertapenem, in addition to surgical debridement and incision and drainage of his foot wound. The patient was discharged to a skilled care nursing facility on ertapenem with significant clinical improvement.
Keywords: gram-variable, lower extremity cellulitis, matrix-assisted laser desorption/ionization-time of flight, severe sepsis, tissierella praeacuta
Introduction
Tissierella praeacuta was first described as a gram-variable, non-spore-forming, obligate anaerobe, and rod-shaped bacterium discovered in 1908 by P. H. Tissier [1,2]. It is found commonly in the human gastrointestinal tract and environmental sources such as soil [3]. It is a rare cause of infection in humans, with very few documented case reports in medical literature involving brain abscess [4], hepatic abscess, pyonephrosis [2], pyometra [5], chronic sacral wounds, decubitus ulcers [3], pseudoarthrosis of the femur, osteomyelitis [1], and gas gangrene of the eyelid [6]. Identifying T. praeacuta can be challenging, as conventional methods are widely used in most labs. It may require newer technology, such as matrix-assisted laser desorption-ionization-time of flight (MALDI-TOF) and 16s RNA sequencing. In this case report, we describe the first documented case of T. praeacuta bacteremia in a patient with sepsis resulting from lower extremity cellulitis, confirmed by MALDI-TOF.
Case presentation
A 78-year-old male former smoker with a past medical history of chronic obstructive pulmonary disease, hypertension, diet-controlled type 2 diabetes mellitus, and peripheral arterial disease complicated by right above-knee amputation presented to the emergency department for dizziness aggravated by positional changes. He reported no history of vision change, headache, recent fall, or trauma. He denied having shortness of breath, chest pain, fever, chills, dysuria, hematuria, or drug abuse. His vital signs were notable for mild tachycardia. The labs were remarkable for leukocytosis with a white cell count of (>25.5 109/L) with left shift and elevated high sensitivity troponin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CPR).
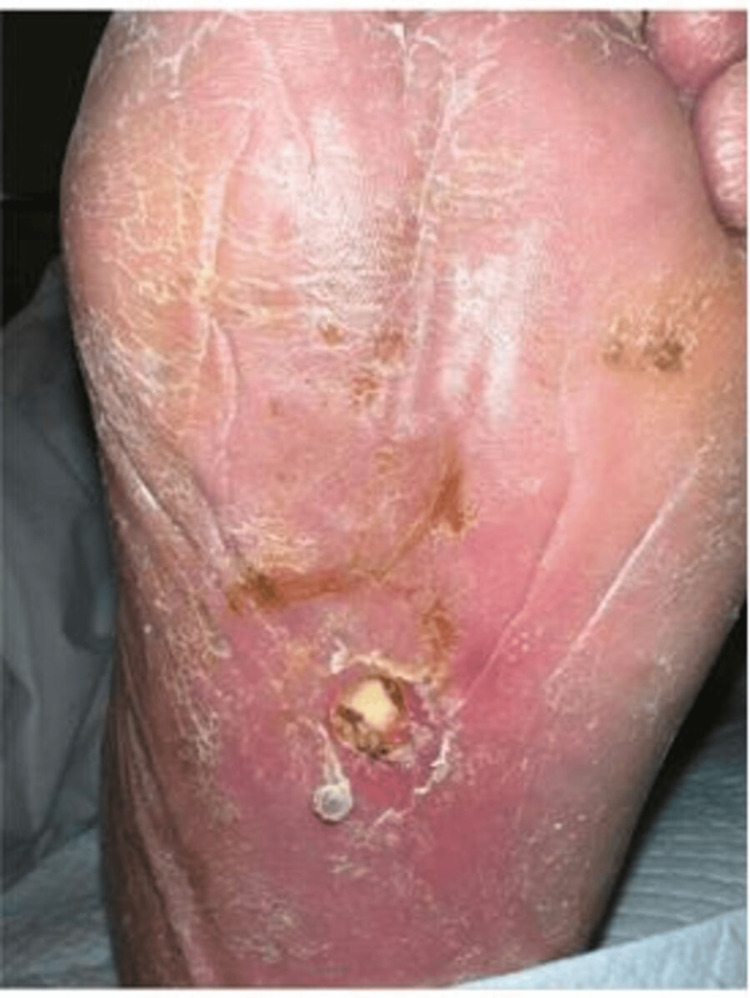
Left lower extremity examination was remarkable for erythema of the left foot extending up to the knee. An ulcer was present on the plantar aspect of the left foot, measuring 1 cm x 1 cm x 0.4 cm, with purulent discharge, a fibrotic base, and exposed plantar fascia (Figure 1). A small amount of seropurulent discharge was noted, along with peri-wound erythema and edema surrounding the wound borders. The presentation was consistent with sepsis due to left lower extremity cellulitis. The MRI of the foot was concerning for sinus tract and abscess formation but was negative for osteomyelitis. Debridement of the wound was followed by incision and drainage. The wound culture was positive for Streptococcus pyogenes. Anaerobic blood culture was positive post-incubation (Figure 2). Gram staining revealed gram-positive, rod-shaped organisms. Therefore, subcultures of the broth were performed onto blood agar incubated at 37°C, chocolate agar incubated at 37°C in 5% CO2, and blood agar incubated in an anaerobic atmosphere for 48 h. Bacterial growth was observed only on the anaerobic plate. Identification of the strain using Rapid ANA (ThermoFisher Scientific, Waltham, MA, USA) was inconclusive. The specimen was sent out for MALDI-TOF, revealing T. praeacuta as an offending agent. During the inpatient course, the patient was treated with IV vancomycin, cefepime, and ertapenem. The patient was ultimately discharged to a skilled nursing facility with six weeks of IV ertapenem therapy.
Figure 1. Plantar ulcer. Ulcer identified during examination on the left foot before drainage. Cellulitis is a source of infection.
Figure 2. Sheep Blood Agar Plate. T. praeacuta colonies on blood agar captured at 48 hours post-incubation.
Discussion
Cellulitis is common in the middle to older population. The incidence of cellulitis is about 200 cases per 100,000 patients per year [7]. The most common cause of cellulitis is the beta-hemolytic streptococci family, most commonly group Streptococcus or Streptococcus pyogenes [8,9]. Staphylococcus aureus, including methicillin-resistant strains, is a less common cause [9]. Other less common causes of cellulitis include Haemophilus influenzae type b, clostridia and non-spore-forming anaerobes, Streptococcus pneumoniae, and Neisseria meningitidis [10-14]. The clinical significance of identifying correct etiologic agents causing disease and directing targeted antimicrobial therapy cannot be overstated, as appropriate antibiotic treatment helps decrease the duration of hospital stay and prevents the emergence of newer multidrug-resistant organisms [15].
The Tissierella genus is associated with five species: T. praeacuta, T. carlieri, T. creatinine, T. creatinophila, and T. pigra [3]. Of these, only T. praeacuta is known to cause clinically relevant infection in humans [1,2]. T. praecuta has been described as a causative organism in brain abscesses [4], hepatic abscesses, pyonephrosis [2], pyometra [5], chronic sacral wounds, decubitus ulcers [3], pseudoarthrosis of the femur [2], osteomyelitis [1], and gas gangrene of the eyelid [6].
T. praeacuta is now used synonymously with Clostridium hastiforme (C. hastiforme). Clostridium stains gram-positive, particularly in the early stages of growth, whereas T. praeacuta has been described as a gram-negative organism in most case reports previously. Despite this discrepancy in Gram staining, 16S rRNA gene sequences displayed 99.9 % similarity in a study conducted by Farrow et al [16]. Moreover, Gram staining and spore formation are not used as a basis of relatedness [16]. Alternatively, Bae et al. [17] found T. praeacuta to be Gram-positive, which was confirmed by a parallel confirmatory KOH test to rule out false negative results using the technique used by Power [18]. DNA-DNA relatedness was estimated at 96.5 %, which was well above the suggested 70% needed to establish strains belonging to the same species. T. praeacuta and C. hastiforme were also found to be identical based on their biochemical characteristics. It was thus concluded that C. hastiforme is a later synonym of T. praeacuta [17].
Identification of the organism at the bench requires growth-dependent methods such as culturing colonies on blood agar, subjecting them to aerotolerance testing and gram staining, and biochemical methods such as Rapid ANA II testing and VITEK [17,19,20]. The growth-dependent system of identifying anaerobic organisms, while reliable and convenient, can be a time-consuming process. Of these, gram staining can yield false negative results typically with gram-variable organisms or due to over-decolorizing for prolonged periods or by using old cultures. Rapid ANA is used to identify organisms through biochemical-dependent methods rapidly, but it has limitations, particularly in identifying anaerobes [21]. Rapid ANA is a four-hour kit test that identifies anaerobes by utilizing an assay of preformed enzymes against chromogenic substrates. The biochemical profile generates a six-digit code, which is then interpreted either from the code book or the manufacturer's database. A comparison study by Hussain et al. [21] between the growth-dependent system and Rapid ANA correctly identified only 71% of the clostridia species (an anaerobe). The panels containing the clostridia strains were found to be challenging to interpret.
T. praeacuta can be identified by using newer techniques such as MALDI-TOF and mass spectrometry (MS) [3,22]. However, the database needs to contain the mass spectrum of this strain [3]. Molecular techniques such as identification by 16S rRNA sequencing can also be used for inconclusive cases [2]. T. praeacuta is known to have antibiotic sensitivity to beta-lactams, chloramphenicol, rifampin, and metronidazole [2,3].
Conclusions
This is the first documented case report of gram-positive T. praeacuta bacteremia in a patient with sepsis resulting from lower extremity cellulitis without concomitant osteomyelitis, confirmed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS). Although rare, T. praeacuta is well characterized and has a complex history of bacterial taxonomy and nomenclature classification, being most recently characterized as synonymous with C. hastiforme. Due to many laboratories' policies to limit the workup of gram-negative rods, it is possible that T. praeacuta and C. hastiforme are under-reported. Additionally, the gram-variable nature of T. praeacuta can cause misdiagnosis. Rapid ANA results can be challenging to interpret for anaerobes and may be inconclusive, as was the case in our case. Newer techniques, such as the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF), can aid in diagnosing rare cases such as T. praeacuta, emphasizing the importance of reporting such cases to build the database that contains the mass spectrum of this strain. Molecular testing with 16S rRNA sequencing can also be helpful in successfully identifying this rare organism.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Gopal Kumar, Sahar Iqbal, Fnu Raja, Thessicar Antoine-Reid
Acquisition, analysis, or interpretation of data: Gopal Kumar, Thessicar Antoine-Reid
Drafting of the manuscript: Gopal Kumar, Sahar Iqbal, Fnu Raja, Thessicar Antoine-Reid
Critical review of the manuscript for important intellectual content: Gopal Kumar, Fnu Raja, Thessicar Antoine-Reid
References
- 1.Tissiarella praeacuta bacteremia, a rare complication of osteomyelitis. Gill M, Bofinger J, Glaser A. IDCases. 2022;27:0. doi: 10.1016/j.idcr.2022.e01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infections caused by Tissierella praeacuta: a report of two cases and literature review. Caméléna F, Pilmis B, Mollo B, Hadj A, Le Monnier A, Mizrahi A. Anaerobe. 2016;40:15–17. doi: 10.1016/j.anaerobe.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Tissierella praeacuta infection in the setting of chronic sacral wounds. Yang J, Gilbert D, Meece L, Afroze A. Cureus. 2022;14:0. doi: 10.7759/cureus.23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anaerobic brain abscess following chronic suppurative otitis media in a child from Uganda. Cox K, Al-Rawahi G, Kollmann T. Can J Infect Dis Med Microbiol. 2009;20:0–3. doi: 10.1155/2009/407139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clostridium hastiforme bacteraemia secondary to pyometra in a 64-year-old woman. Ørum M, Fuglsang-Damgaard D, Nielsen HL. BMJ Case Rep. 2017;2017:0. doi: 10.1136/bcr-2016-218084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eyelid gas gangrene. Lyon DB, Lemke BN. Ophthalmic Plast Reconstr Surg. 1989;5:212–215. doi: 10.1097/00002341-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Cellulitis incidence in a defined population. Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL. Epidemiol Infect. 2006;134:293–299. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cellulitis: a review. Raff AB, Kroshinsky D. JAMA. 2016;316:325–337. doi: 10.1001/jama.2016.8825. [DOI] [PubMed] [Google Scholar]
- 9.Association of athlete's foot with cellulitis of the lower extremities: diagnostic value of bacterial cultures of ipsilateral interdigital space samples. Semel JD, Goldin H. Clin Infect Dis. 1996;23:1162–1164. doi: 10.1093/clinids/23.5.1162. [DOI] [PubMed] [Google Scholar]
- 10.Clinical practice. Cellulitis. Swartz MN. N Engl J Med. 2004;350:904–912. doi: 10.1056/NEJMcp031807. [DOI] [PubMed] [Google Scholar]
- 11.Clinical syndromes associated with adult pneumococcal cellulitis. Parada JP, Maslow JN. Scand J Infect Dis. 2000;32:133–136. doi: 10.1080/003655400750045213. [DOI] [PubMed] [Google Scholar]
- 12.Acute cellulitis: an unusual manifestation of meningococcal disease. Porras MC, Martínez VC, Ruiz IM, Encinas PM, Fernandez MT, García J, Martín Martín LC. Scand J Infect Dis. 2001;33:56–59. doi: 10.1080/003655401750064086. [DOI] [PubMed] [Google Scholar]
- 13.Pneumococcal soft-tissue infections: a problem deserving more recognition. Patel M, Ahrens JC, Moyer DV, DiNubile MJ. Clin Infect Dis. 1994;19:149–151. doi: 10.1093/clinids/19.1.149. [DOI] [PubMed] [Google Scholar]
- 14.Bacteremic pneumococcal cellulitis compared with bacteremic cellulitis caused by Staphylococcus aureus and Streptococcus pyogenes. Capdevila O, Grau I, Vadillo M, Cisnal M, Pallares R. Eur J Clin Microbiol Infect Dis. 2003;22:337–341. doi: 10.1007/s10096-003-0945-z. [DOI] [PubMed] [Google Scholar]
- 15.Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Llor C, Bjerrum L. Ther Adv Drug Saf. 2014;5:229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phylogenetic evidence that the gram-negative nonsporulating bacterium Tissierella (Bacteroides) praeacuta is a member of the Clostridium subphylum of the gram-positive bacteria and description of Tissierella creatinini sp. nov. Farrow JA, Lawson PA, Hippe H, Gauglitz U, Collins MD. Int J Syst Bacteriol. 1995;45:436–440. doi: 10.1099/00207713-45-3-436. [DOI] [PubMed] [Google Scholar]
- 17.Clostridium hastiforme is a later synonym of Tissierella praeacuta. Bae JW, Park JR, Chang YH, Rhee SK, Kim BC, Park YH. Int J Syst Evol Microbiol. 2004;54:947–949. doi: 10.1099/ijs.0.63068-0. [DOI] [PubMed] [Google Scholar]
- 18.Efficacy of the Ryu nonstaining KOH technique for rapidly determining gram reactions of food-borne and waterborne bacteria and yeasts. Powers EM. Appl Environ Microbiol. 1995;61:3756–3758. doi: 10.1128/aem.61.10.3756-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Multilocus analysis reveals diversity in the genus Tissierella: description of Tissierella carlieri sp. nov. in the new class Tissierellia classis nov. Alauzet C, Marchandin H, Courtin P, et al. Syst Appl Microbiol. 2014;37:23–34. doi: 10.1016/j.syapm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Clinical evaluation of the RapID-ANA II panel for identification of anaerobic bacteria. Celig DM, Schreckenberger PC. J Clin Microbiol. 1991;29:457–462. doi: 10.1128/jcm.29.3.457-462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comparison of RapID-ANA and Minitek with a conventional method for biochemical identification of anaerobes. Hussain Z, Lannigan R, Schieven BC, Stoakes L, Kelly T, Groves D. Diagn Microbiol Infect Dis. 1987;7:69–72. doi: 10.1016/0732-8893(87)90073-3. [DOI] [PubMed] [Google Scholar]
- 22.How MALDI-TOF mass spectrometry can aid the diagnosis of hard-to-identify pathogenic bacteria-the rare and the unknown. Kostrzewa M, Nagy E, Schröttner P, Pranada AB. Expert Rev Mol Diagn. 2019;19:667–682. doi: 10.1080/14737159.2019.1643238. [DOI] [PubMed] [Google Scholar]