Abstract
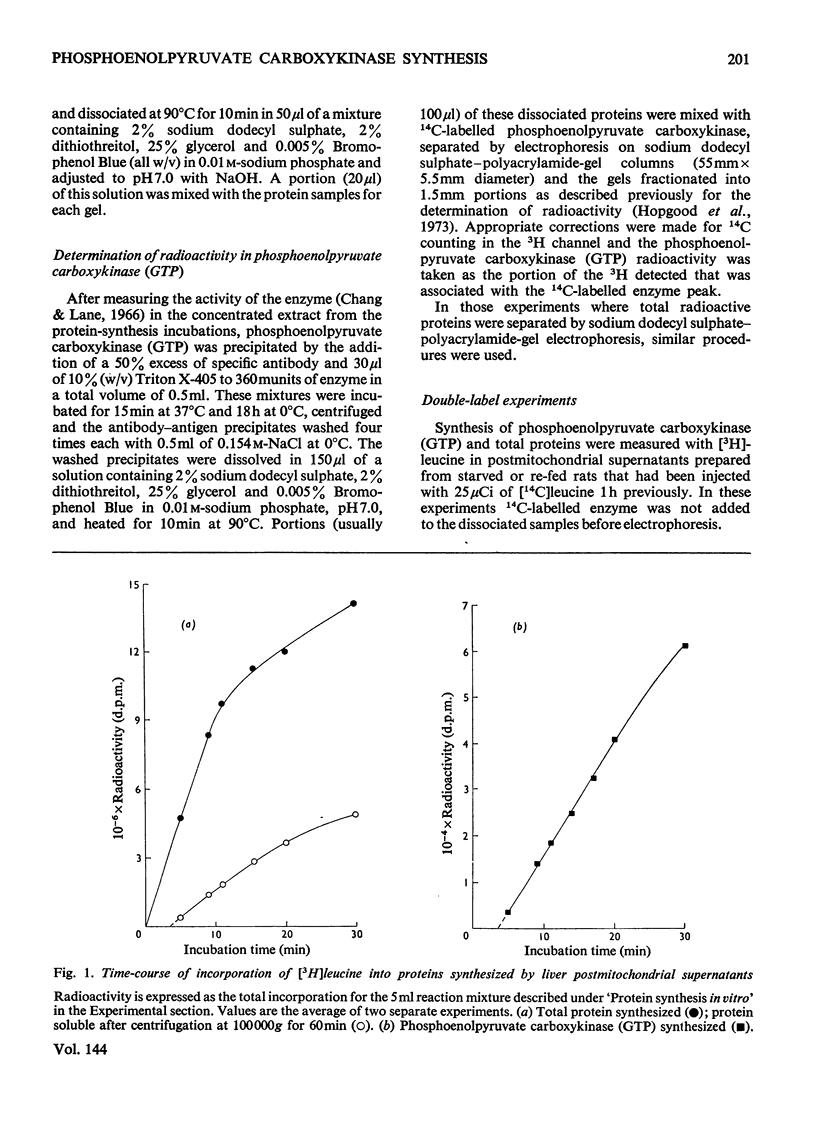
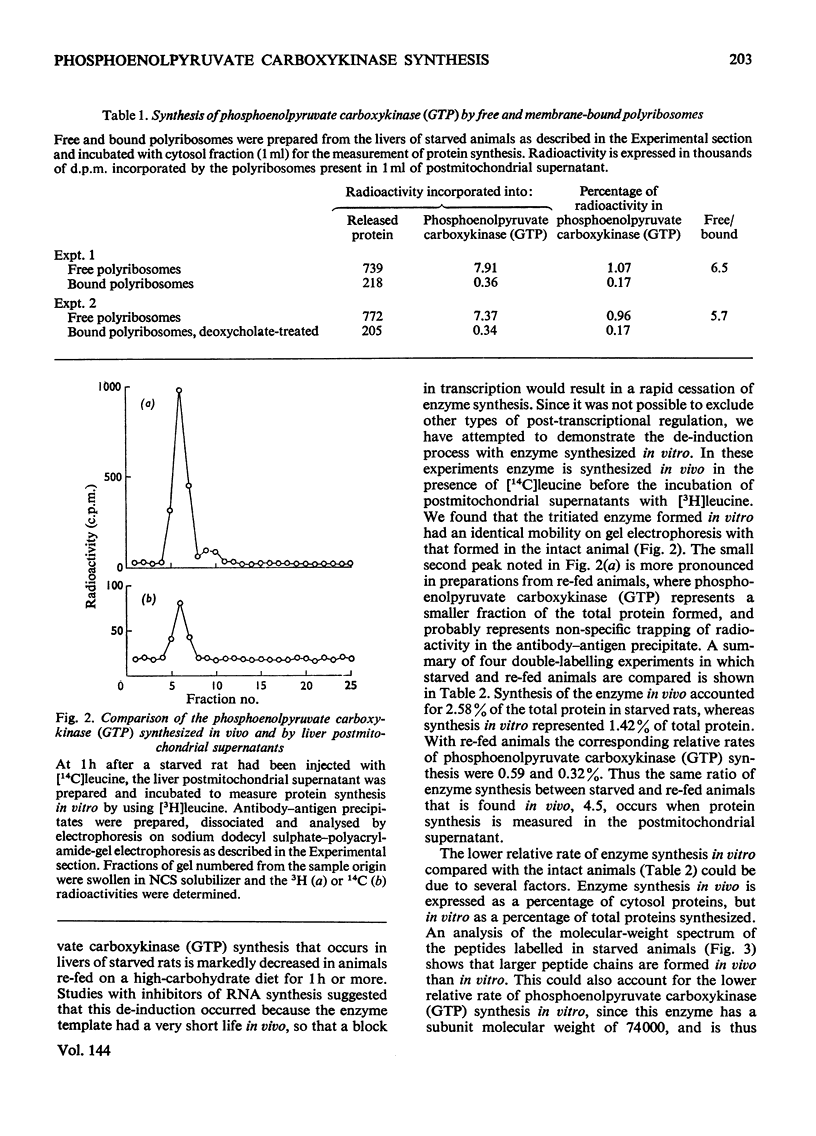
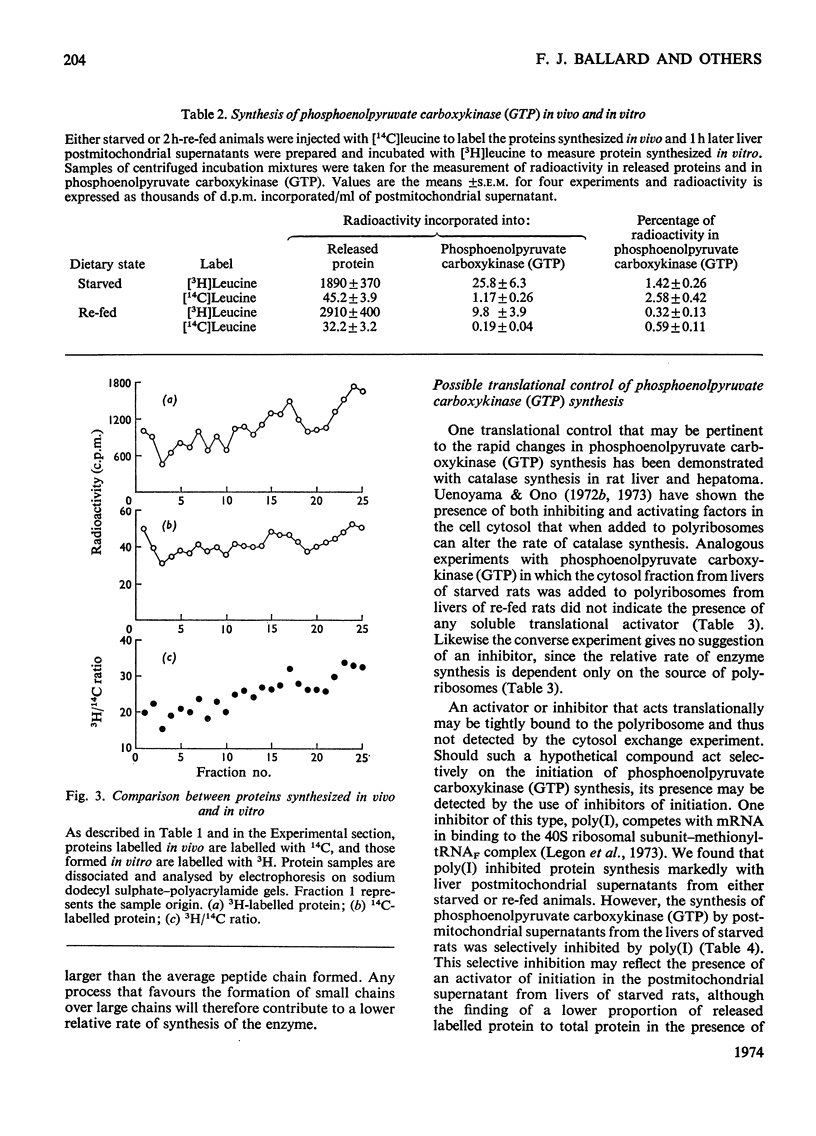
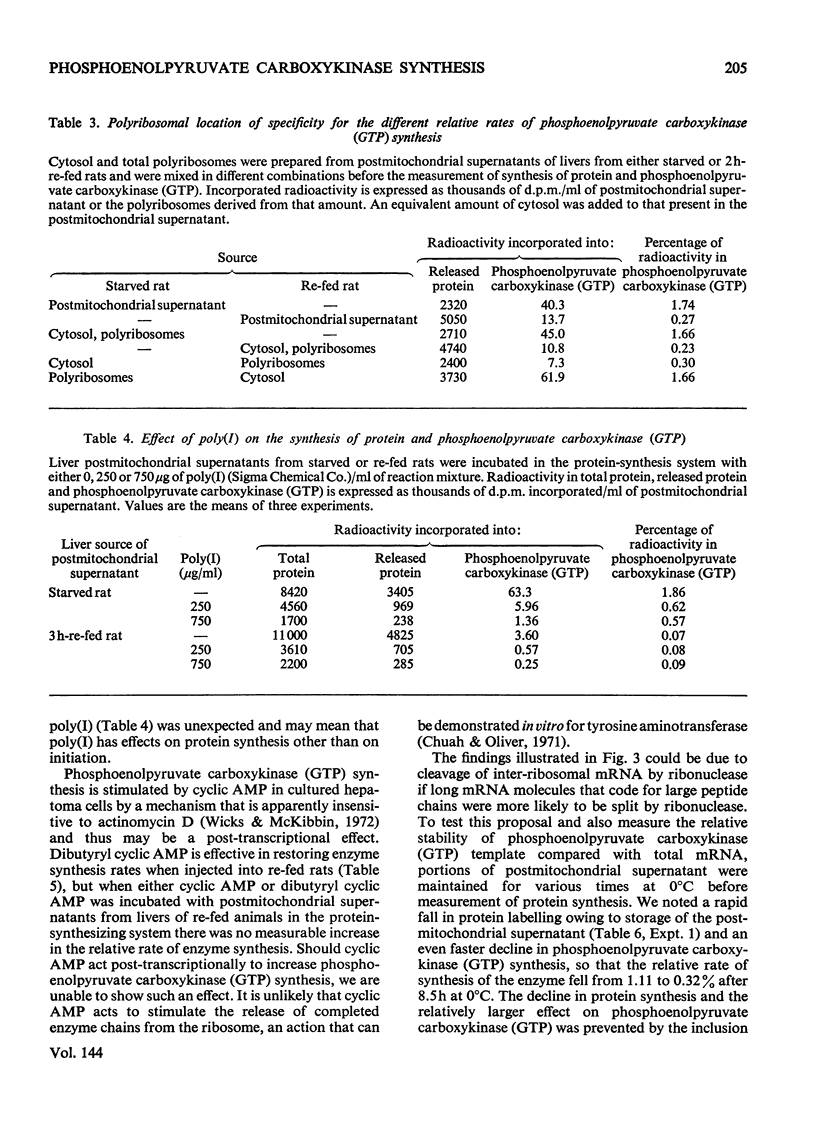
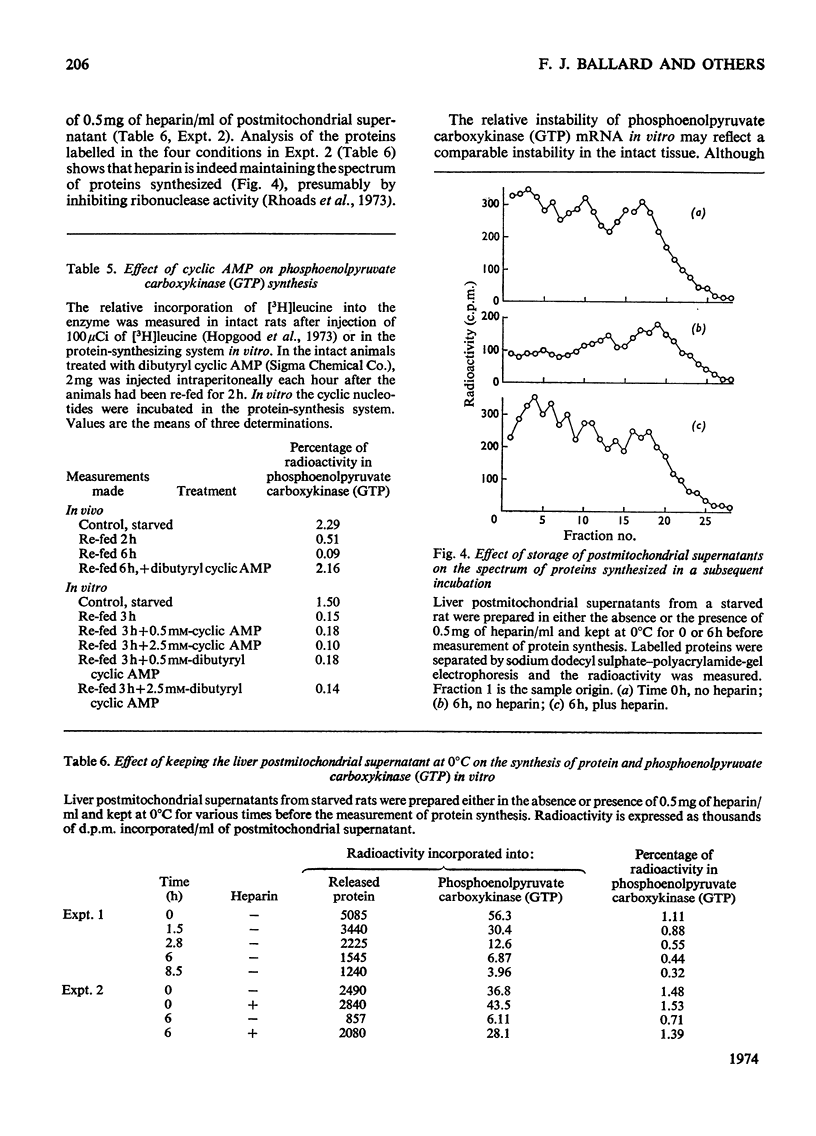
1. Phosphoenolpyruvate carboxykinase (GTP) (EC 4.1.1.32) was synthesized by postmitochondrial supernatants of rat liver in the presence of appropriate salts, an energy supply and [3H]leucine. Synthesis of enzyme released from polyribosomes was detected by immunoprecipitation with specific antibody followed by electrophoresis of the dissolved antibody–antigen precipitates on sodium dodecyl sulphate–polyacrylamide gels in the presence of a 14C-labelled enzyme marker. 2. Enzyme synthesis in vitro occurs predominantly on free rather than bound polyribosomes. 3. Starved animals in which de-induction of phosphoenolpyruvate carboxykinase (GTP) had been initiated by re-feeding for 2h had a markedly decreased rate of enzyme synthesis, whether the measurements were made after injection of radioactive leucine into the intact animal or if synthesis was determined in vitro. 4. The low rate of enzyme synthesis by liver polyribosomes from re-fed animals was not due to the absence of soluble factors, nor could it be increased by the addition of cyclic AMP to the protein synthesis system. 5. Phosphoenolpyruvate carboxykinase (GTP) synthesis in vitro is diminished relative to total protein synthesis when the postmitochondrial supernatant is kept at 0°C for several hours before measurement of protein synthesis. Since this effect is blocked by heparin, it is probably caused by selective ribonuclease attack on enzyme mRNA. 6. De-induction of phosphoenolpyruvate carboxykinase (GTP) is tentatively explained as being due to a transcriptional block in specific mRNA synthesis, followed by rapid degradation of existing message.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Hanson R. W. Purification of phosphoenolpyruvate carboxykinase from the cytosol fraction of rat liver and the immunochemical demonstration of differences between this enzyme and the mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Oct 25;244(20):5625–5630. [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F. Phosphopyruvate carboxylase induction by L-tryptophan. Effects on synthesis and degradation of the enzyme. Biochem J. 1973 Oct;136(2):259–264. doi: 10.1042/bj1360259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hopgood M. F., Reshef L., Hanson R. W. Degradation of phosphoenolpyruvate carboxykinase (guanosine triphosphate) in vivo and in vitro. Biochem J. 1974 Jun;140(3):531–538. doi: 10.1042/bj1400531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. P., Beck G., Wong K. Y., Tomkins G. M. Synthesis of inducible tyrosine aminotransferase in a cell-free extract from cultured hepatoma cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3615–3619. doi: 10.1073/pnas.69.12.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL P. N., GREENGARD O., KERNOT B. A. Studies on the synthesis o serum albumin by the isolated microsome fraction from rat liver. Biochem J. 1960 Jan;74:107–117. doi: 10.1042/bj0740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y., Pitot H. C. Localization of polysome-bound albumin and serine dehydratase in rat liver cell fractions. J Cell Biol. 1973 Oct;59(1):28–44. doi: 10.1083/jcb.59.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W., Attardi G. Stability of cytoplasmic messenger RNA in HeLa cels. Proc Natl Acad Sci U S A. 1973 Jan;70(1):115–119. doi: 10.1073/pnas.70.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Nakagawa H. Cold-adaptation. II. Effect of thyroxine on phosphoenolpyruvate carboxykinase in rat liver in normal and cold environments. J Biochem. 1972 Jan;71(1):125–131. doi: 10.1093/oxfordjournals.jbchem.a129733. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Palmiter R. D., Schimke R. T. Regulation of protein synthesis in chick oviduct. 3. Mechanism of ovalbumin "superinduction" by actinomycin D. J Biol Chem. 1973 Mar 10;248(5):1502–1512. [PubMed] [Google Scholar]
- Philippidis H., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. The initial synthesis of proteins during development. Phosphoenolpyruvate carboxylase in rat liver at birth. Biochem J. 1972 Mar;126(5):1127–1134. doi: 10.1042/bj1261127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- SHRAGO E., LARDY H. A., NORDLIE R. C., FOSTER D. O. METABOLIC AND HORMONAL CONTROL OF PHOSPHOENOLPYRUVATE CARBOXYKINASE AND MALIC ENZYME IN RAT LIVER. J Biol Chem. 1963 Oct;238:3188–3192. [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-1-C14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol. 1960 Jul;7:619–630. doi: 10.1083/jcb.7.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1304–1308. doi: 10.1073/pnas.71.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Post-transcriptional regulation of catalase synthesis in rat liver and hepatoma: factors activating and inhibiting catalase synthesis. J Mol Biol. 1973 Mar 15;74(4):439–452. doi: 10.1016/0022-2836(73)90038-7. [DOI] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Synthesis of albumin by the free polyribosomes in 5123 hepatoma. Biochim Biophys Acta. 1972 Sep 29;281(1):124–129. doi: 10.1016/0005-2787(72)90194-3. [DOI] [PubMed] [Google Scholar]
- Uenoyama K., Ono T. Translation regulation of catalase synthesis in Morris 5123TC hepatoma. Int J Cancer. 1972 May 15;9(3):608–617. doi: 10.1002/ijc.2910090318. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Wicks W. D., Lewis W., McKibbin J. B. Induction of phosphoenolpyruvate carboxykinase by N 6 , O 2 '-dibutyryl cyclic AMP in rat liver. Biochim Biophys Acta. 1972 Mar 30;264(1):177–185. doi: 10.1016/0304-4165(72)90129-8. [DOI] [PubMed] [Google Scholar]
- Wicks W. D., McKibbin J. B. Evidence for translational regulation of specific enzyme synthesis by N 6 , O 2' -dibutyryl cyclic AMP in hepatoma cell cultures. Biochem Biophys Res Commun. 1972 Jul 11;48(1):205–211. doi: 10.1016/0006-291x(72)90364-6. [DOI] [PubMed] [Google Scholar]



