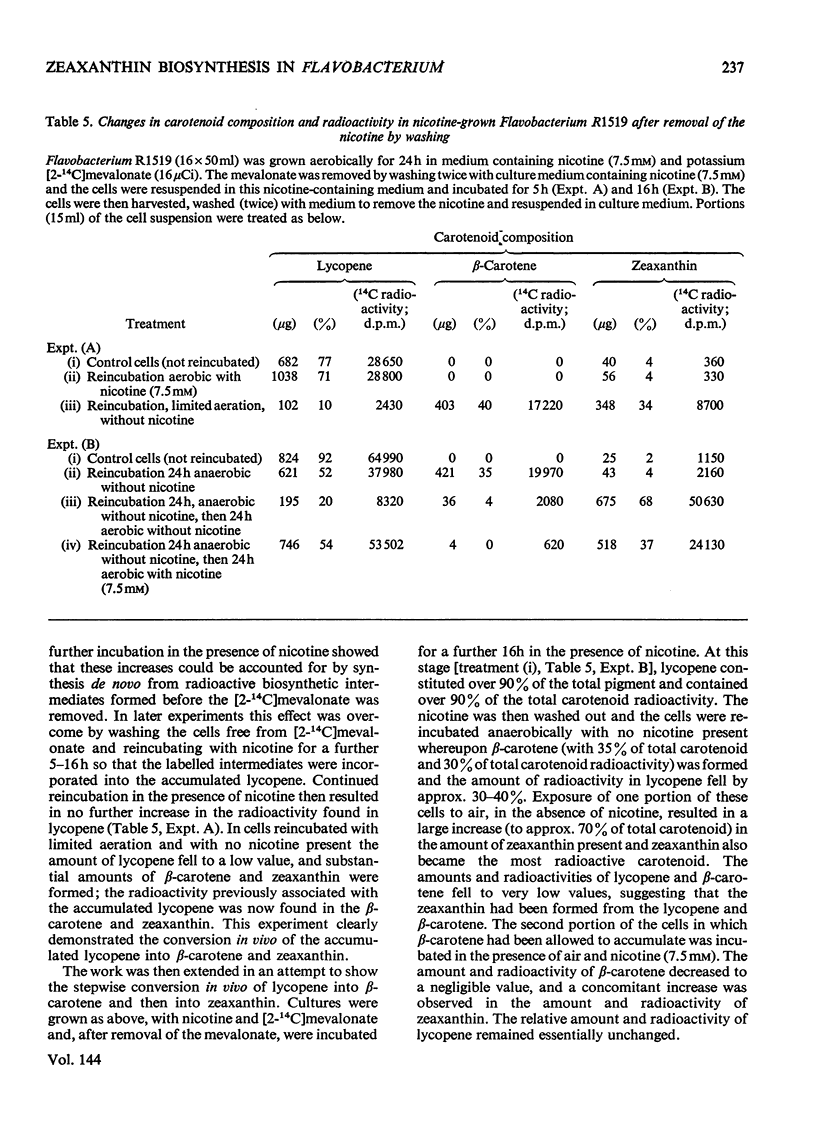
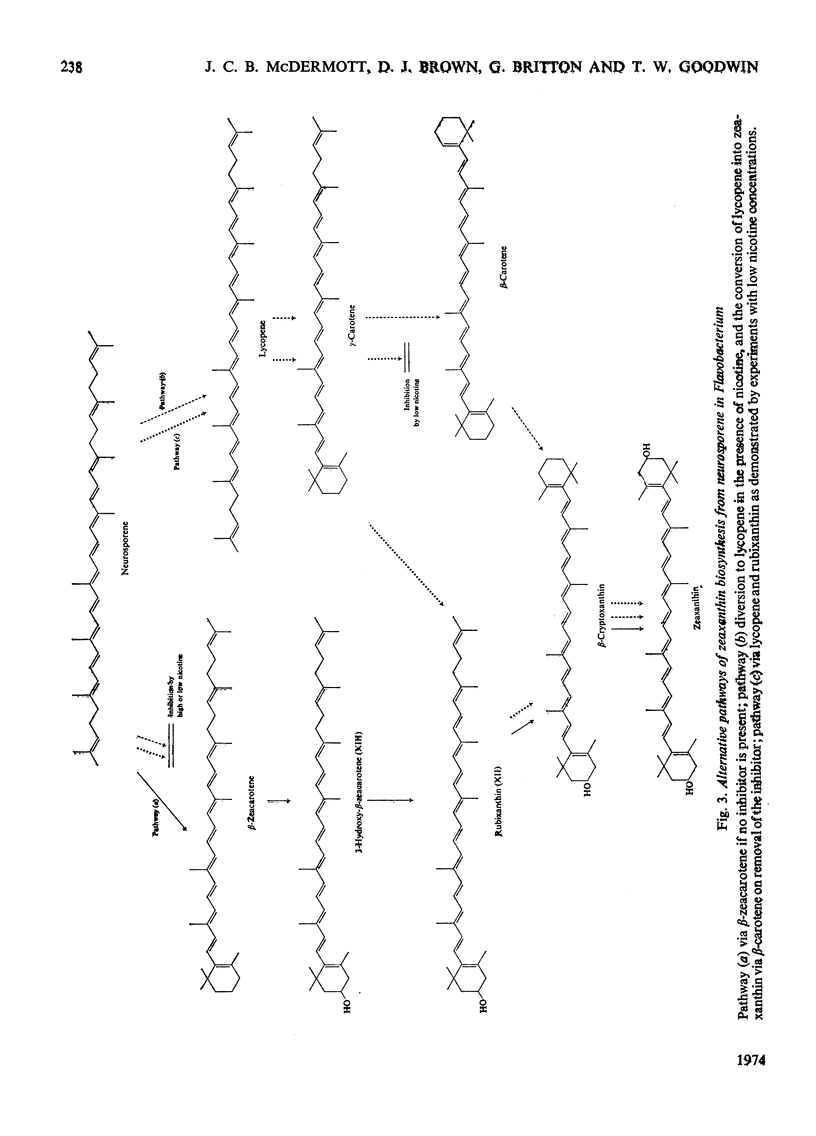
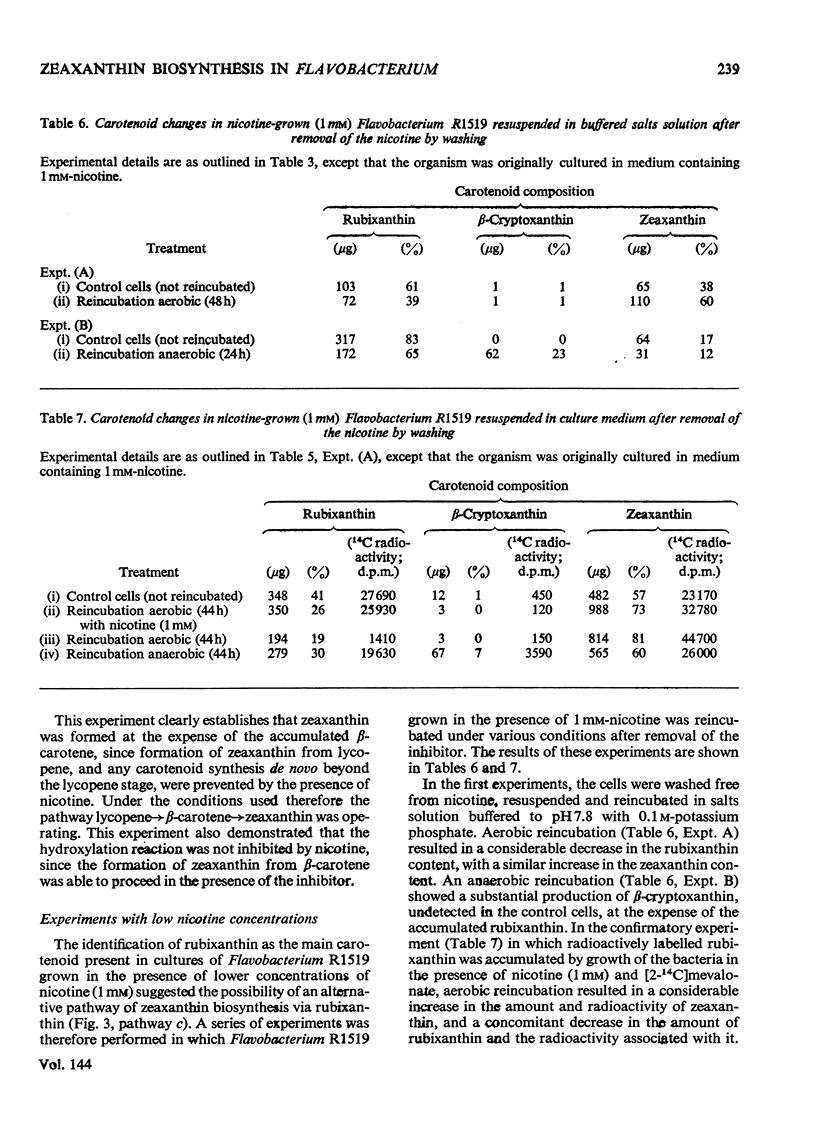
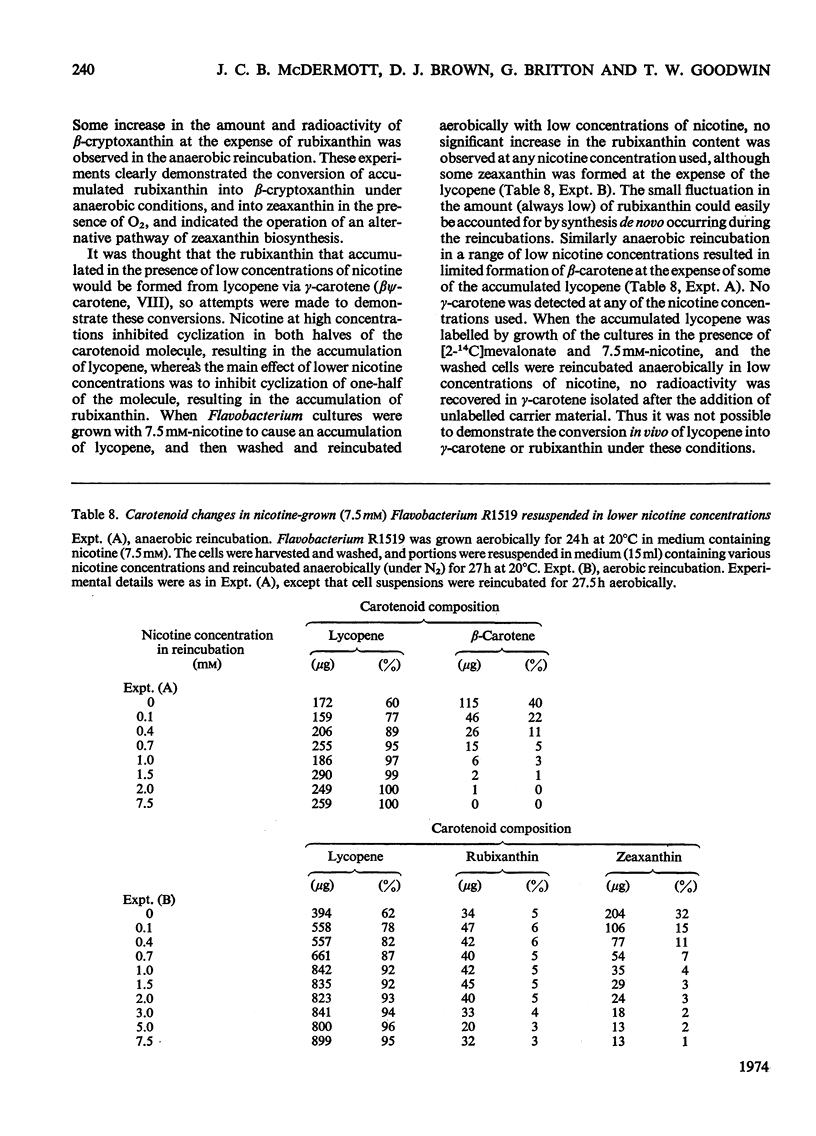
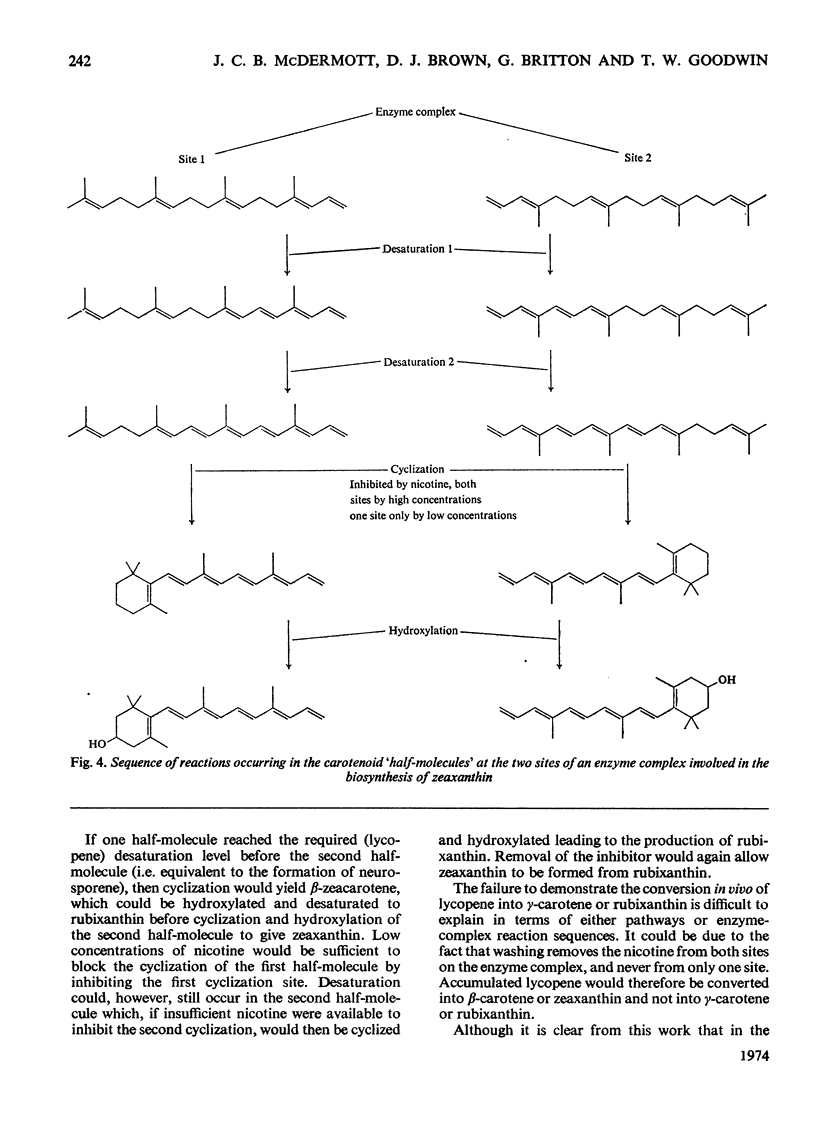
Abstract
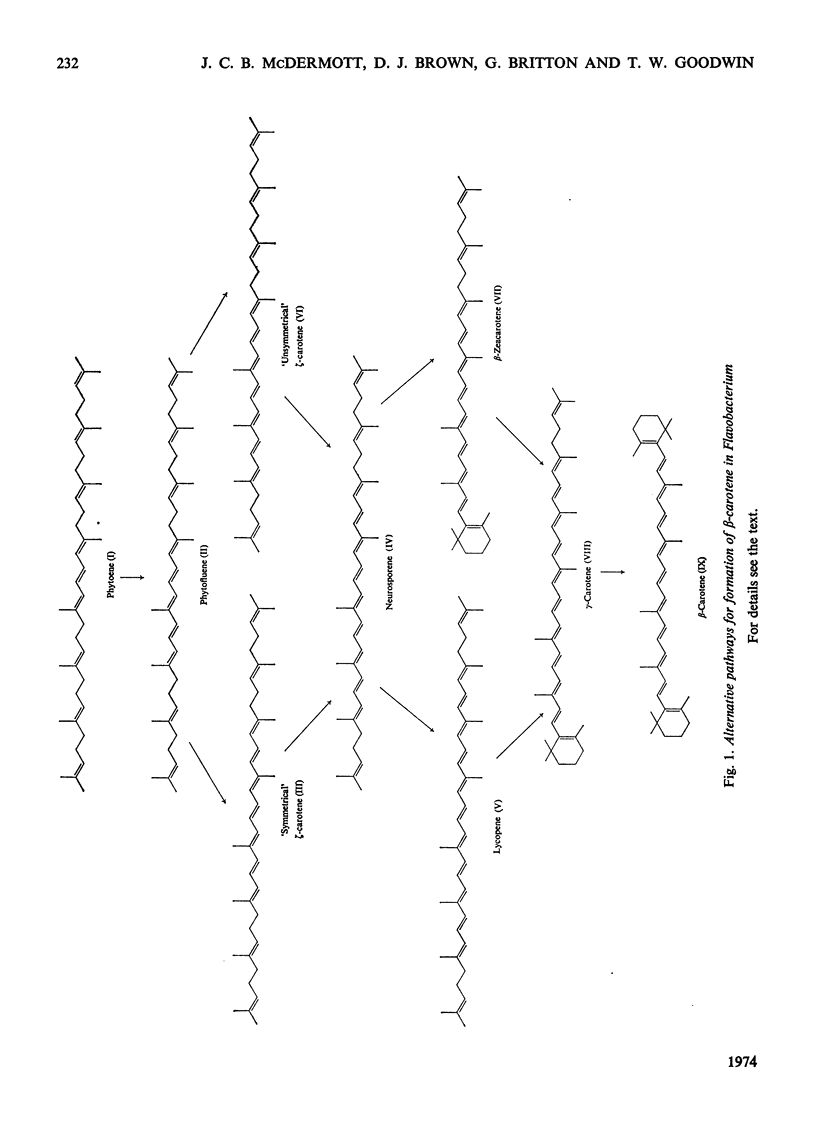
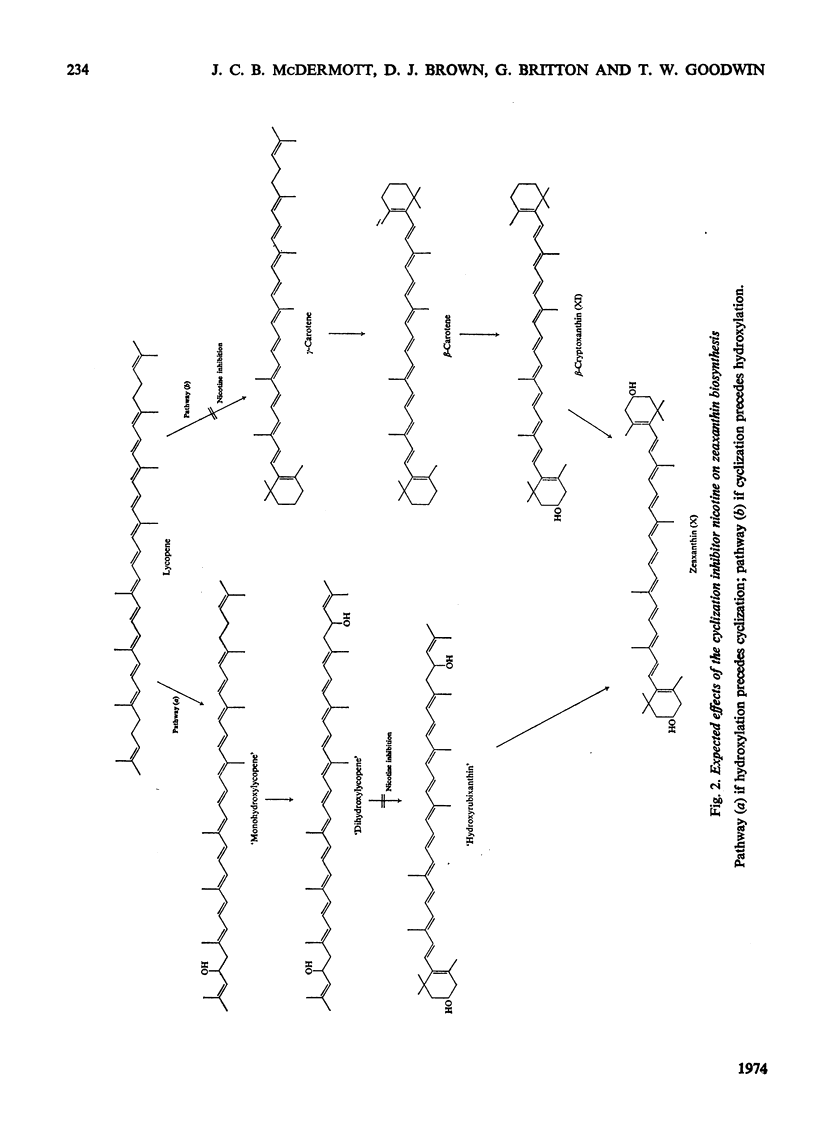
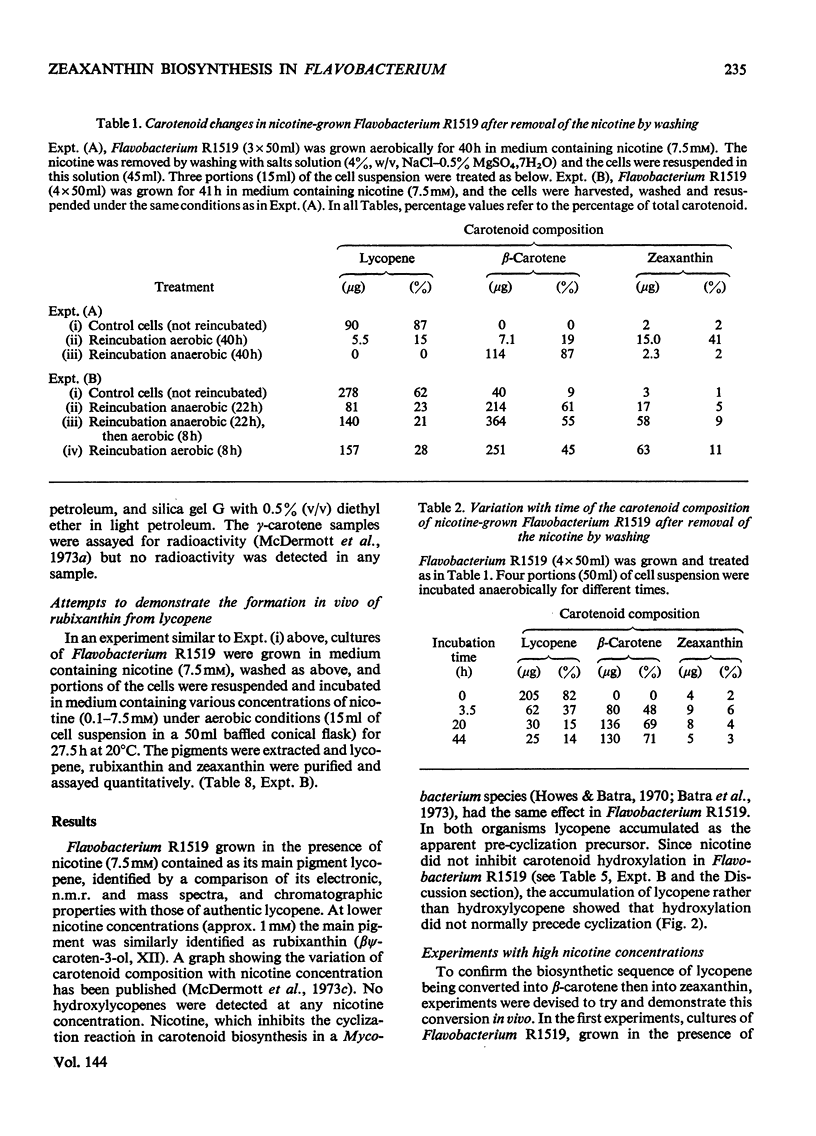
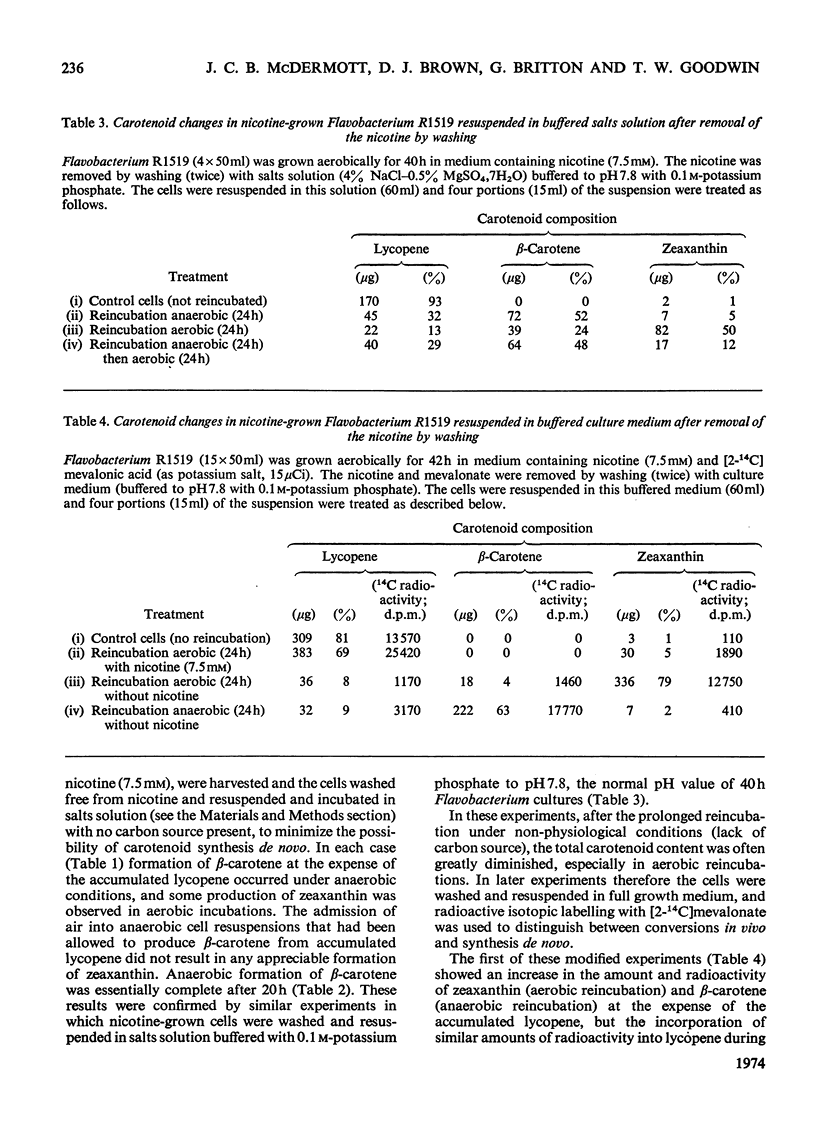
In Flavobacterium R1519, nicotine blocks zeaxanthin biosynthesis by specifically inhibiting the cyclization reaction. Lycopene (at high nicotine concentrations, e.g. 7.5mm) and rubixanthin (at low nicotine concentration, e.g. 1mm) replace zeaxanthin as the main carotenoid. On removal of the nicotine lycopene is converted into β-carotene under anaerobic conditions and into zeaxanthin in the presence of O2. The conversion in vivo of β-carotene into zeaxanthin was also demonstrated. Cyclization (an anaerobic process) thus precedes hydroxylation (O2-requiring) in the biosynthesis of zeaxanthin. The conversion in vivo of rubixanthin into β-cryptoxanthin and into zeaxanthin was demonstrated, thus indicating the operation of alternative pathways of zeaxanthin biosynthesis. Several alternative biosynthetic pathways are considered and the results are also discussed in terms of reaction sequences of carotenoid `half-molecules'.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. J., Ash L., Kowerski R. C., Epstein W. W., Larsen B. R., Rilling H. C., Muscio F., Gregonis D. E. Prephytoene pyrophosphate. A new intermediate in the biosynthesis of carotenoids. J Am Chem Soc. 1972 May 3;94(9):3257–3259. doi: 10.1021/ja00764a073. [DOI] [PubMed] [Google Scholar]
- Barnes F. J., Qureshi A. A., Semmler E. J., Porter J. W. Prelycopersene pyrophosphate and lycopersene. Intermediates in carotene biosynthesis. J Biol Chem. 1973 Apr 25;248(8):2768–2773. [PubMed] [Google Scholar]
- Bartlett L., Klyne W., Mose W. P., Scopes P. M., Galasko G., Mallams A. K., Weedon B. C., Szabolcs J., Tóth G. Optical rotatory dispersion of carotenoids. J Chem Soc Perkin 1. 1969;18:2527–2544. doi: 10.1039/j39690002527. [DOI] [PubMed] [Google Scholar]
- CLAES H. Biosynthese von Carotinoiden bei Chlorella. V. Die Trennung von Licht- und Dunkelreaktionen bei der lichtabhängigen Xanthophyllsynthese von Chlorella. Z Naturforsch B. 1959 Jan;14B(1):4–7. [PubMed] [Google Scholar]
- Davies B. H. A novel sequence for phytoene dehydrogenation in Rhodospirillum rubrum. Biochem J. 1970 Jan;116(1):93–99. doi: 10.1042/bj1160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. H. Carotene biosynthesis in fungi. Pure Appl Chem. 1973;35(1):1–28. doi: 10.1351/pac197335010001. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Batra P. P. Accumulation of lycopene and inhibition of cyclic carotenoids in Mycobacterium in the presence of nicotine. Biochim Biophys Acta. 1970 Oct 27;222(1):174–179. doi: 10.1016/0304-4165(70)90362-4. [DOI] [PubMed] [Google Scholar]
- McDermott J. C., Ben-Aziz A., Singh R. K., Britton G., Goodwin T. W. Recent studies of carotenoid biosynthesis in bacteria. Pure Appl Chem. 1973;35(1):29–45. doi: 10.1351/pac197335010029. [DOI] [PubMed] [Google Scholar]
- McDermott J. C., Britton G., Goodwin T. W. Carotenoid biosynthesis in a Flavobacterium sp.: stereochemistry of hydrogen elimination in the desaturation of phytoene to lycopene, rubixanthin and zeaxanthin. Biochem J. 1973 Aug;134(4):1115–1117. doi: 10.1042/bj1341115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A. A., Barnes F. J., Porter J. W. Lycopersene and prelycopersene pyrophosphate. Intermediates in carotene biosynthesis. J Biol Chem. 1972 Oct 25;247(20):6730–6732. [PubMed] [Google Scholar]
- Qureshi A. A., Barnes F. J., Semmler E. J., Porter J. W. Biosynthesis of prelycopersene pyrophosphate and lycopersene by squalene synthetase. J Biol Chem. 1973 Apr 25;248(8):2755–2767. [PubMed] [Google Scholar]
- Williams R. J., Britton G., Charlton J. M., Goodwin T. W. The stereospecific biosynthesis of phytoene and polyunsaturated carotenes. Biochem J. 1967 Sep;104(3):767–777. doi: 10.1042/bj1040767. [DOI] [PMC free article] [PubMed] [Google Scholar]


