Abstract
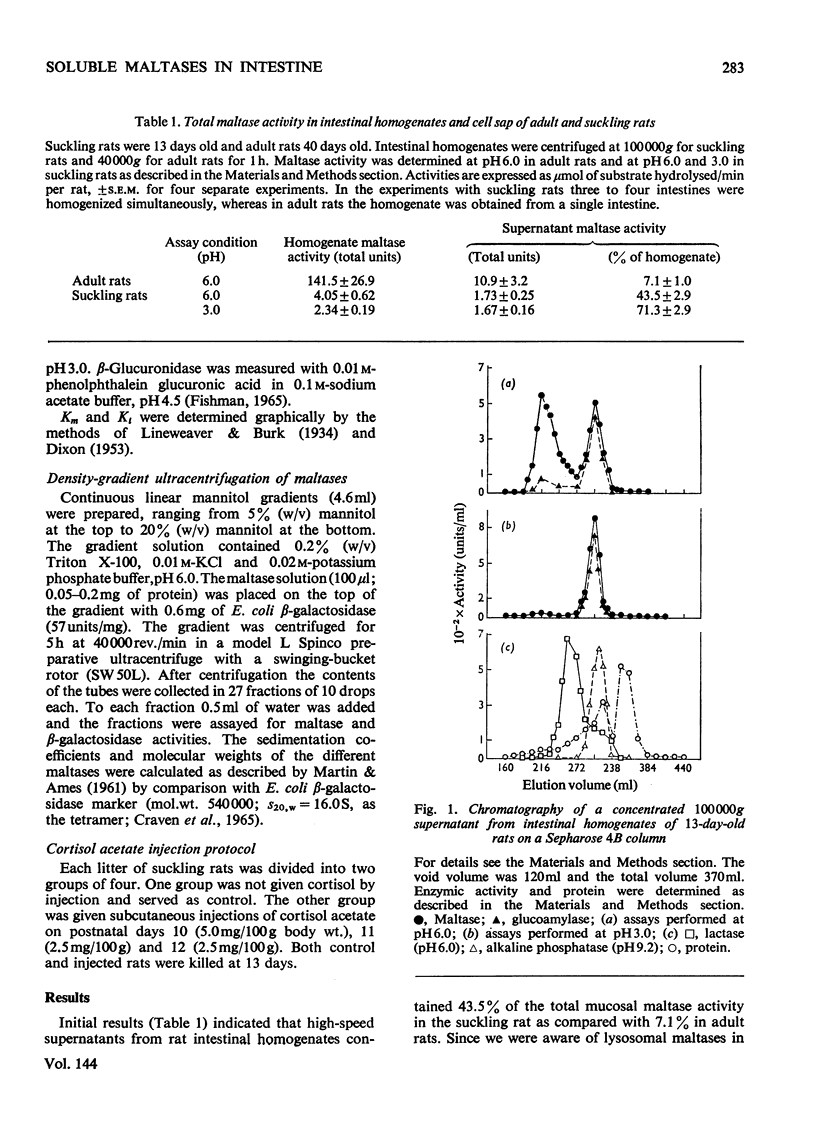
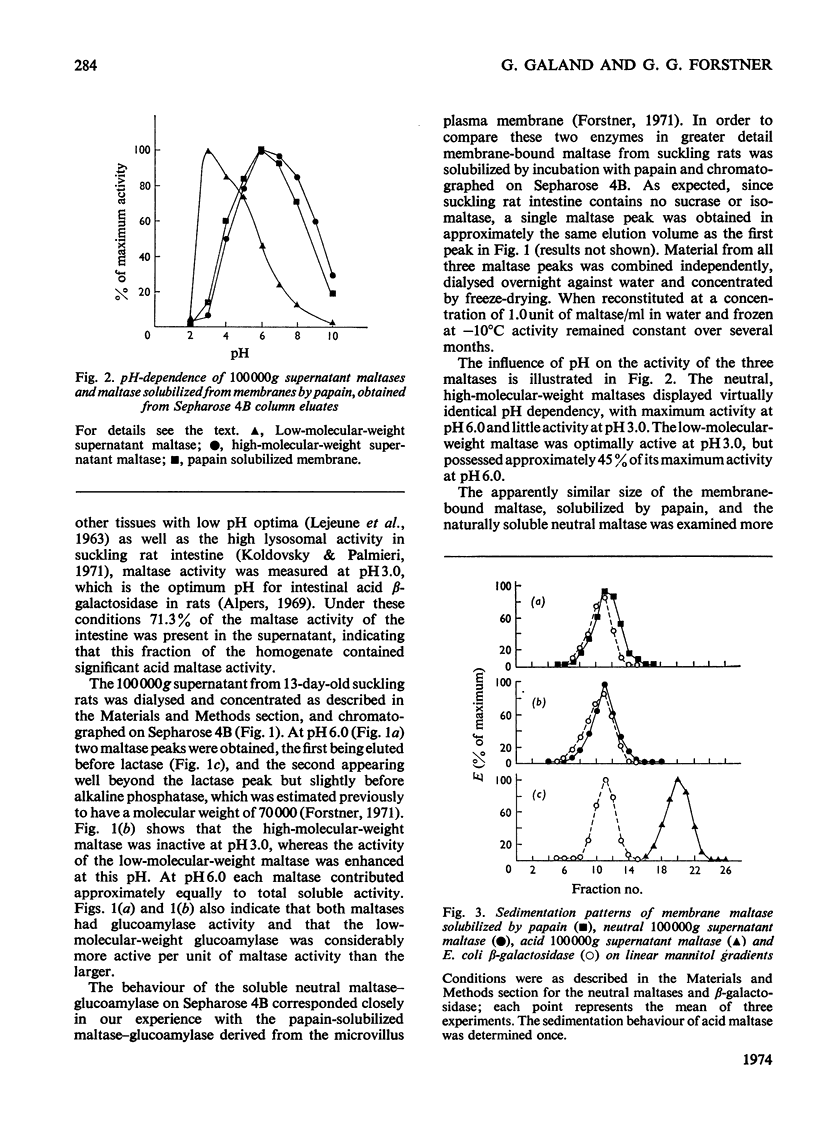
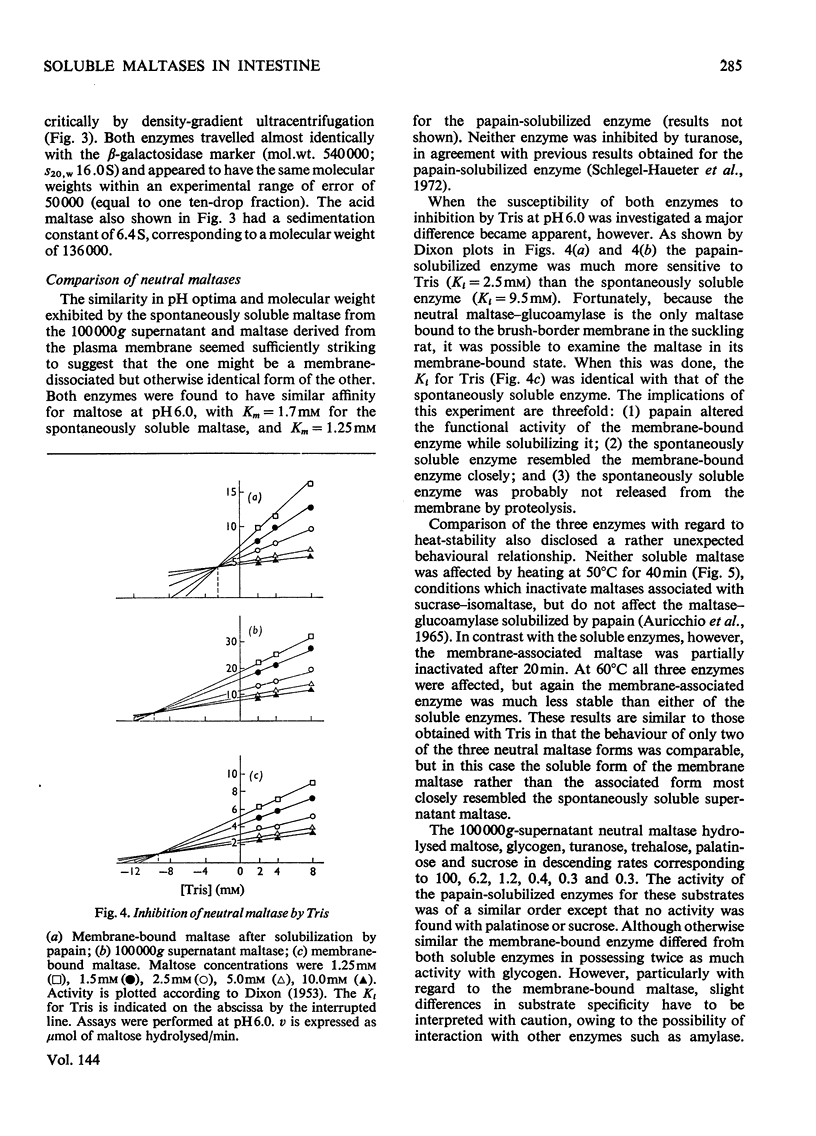
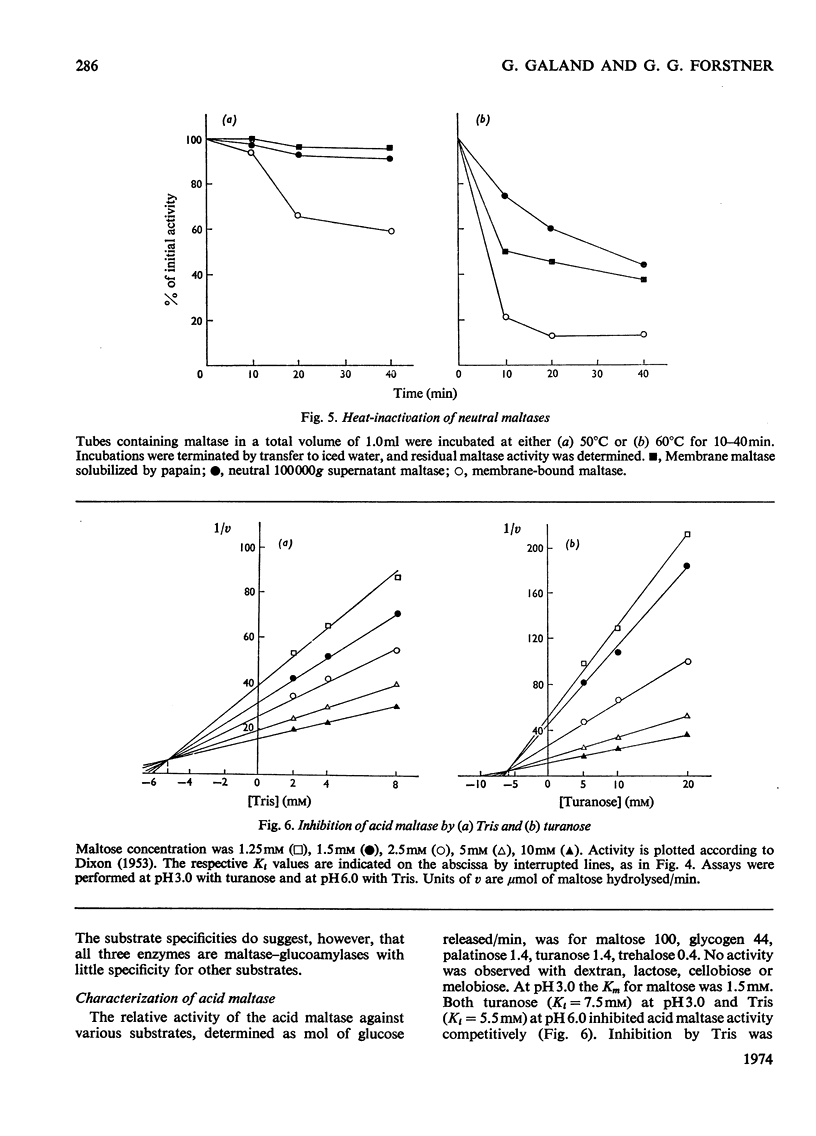
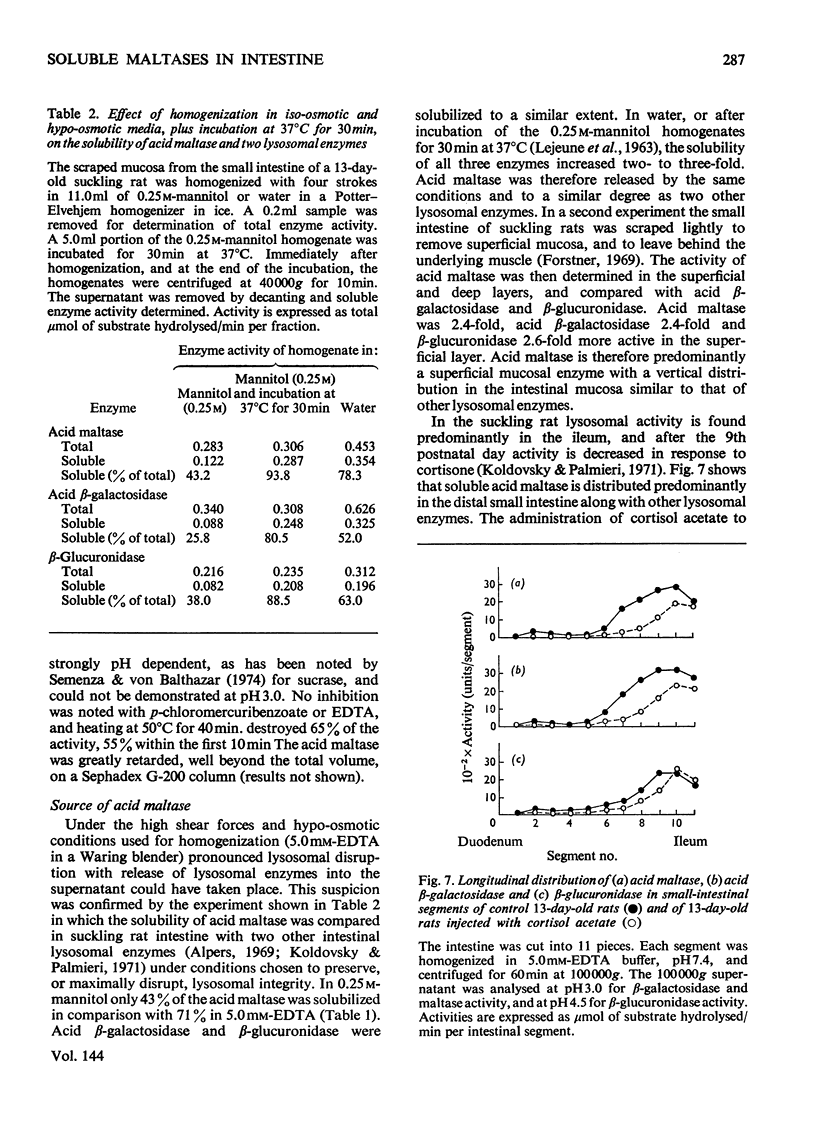
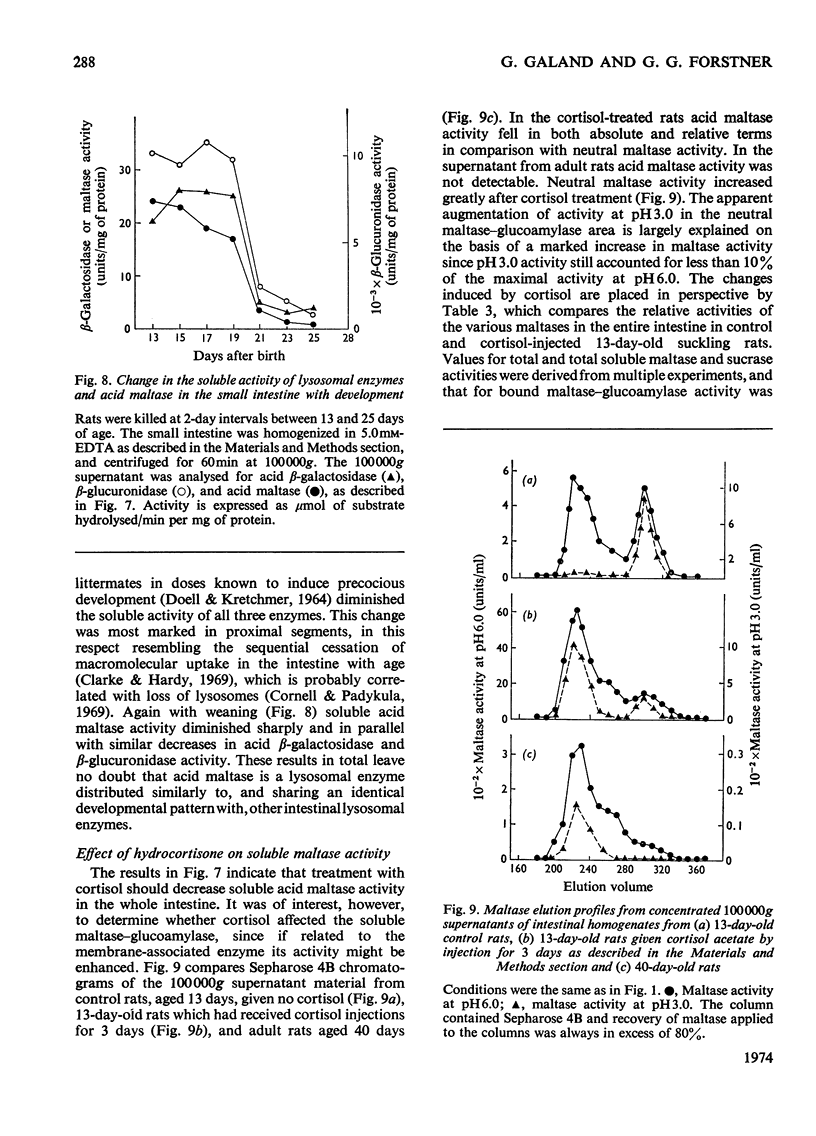
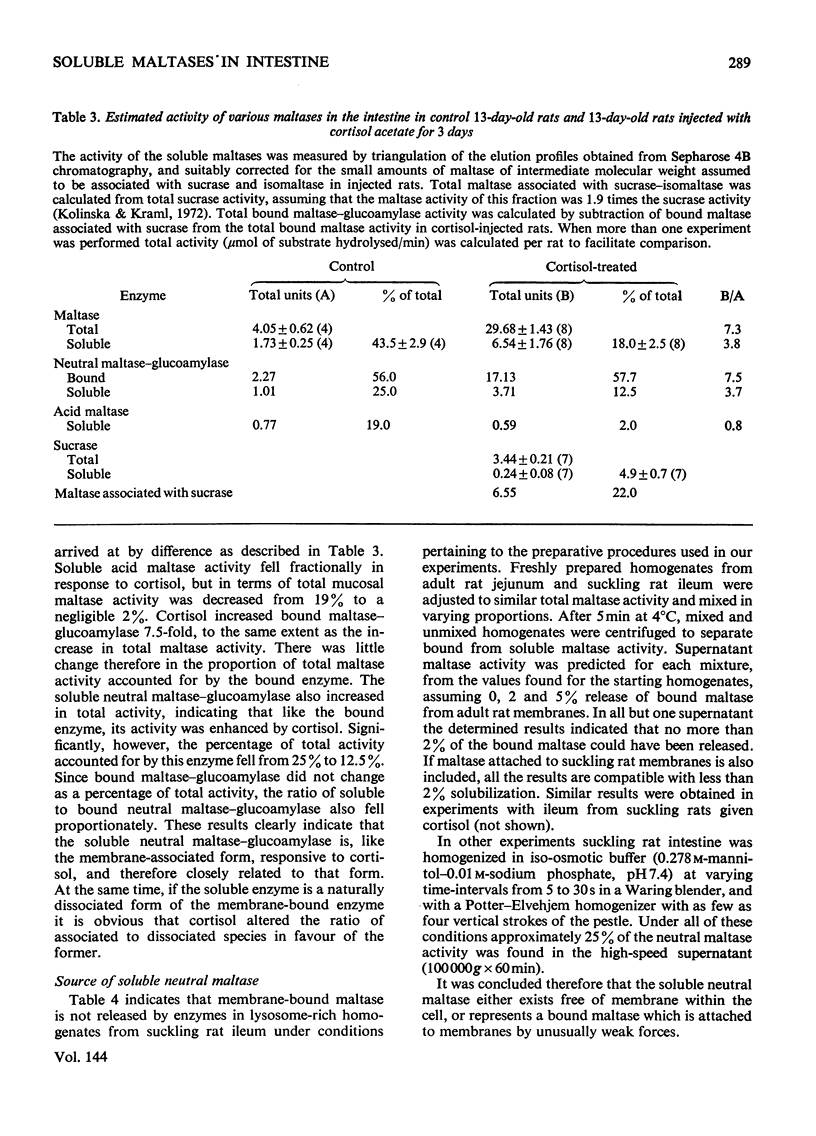
The 100000g supernatants from 13-day-old suckling-rat intestinal homogenates contained 43.5% of the total intestinal maltase activity, compared with 7.1% in weaned adult rats aged 40 days. The soluble maltase activity was separated on Sepharose 4B into two quantitatively equal fractions at pH6.0, one containing a maltase with a neutral pH optimum and the other a maltase with an acid pH optimum. The neutral maltase was shown to be a maltase–glucoamylase identical with membrane-bound maltase–glucoamylase in molecular weight, heat-sensitivity, substrate specificity, Km for maltose and Ki for Tris. The soluble enzyme was induced by cortisol, but the ratio of the soluble to bound enzyme fell during induction. Solubility of the neutral maltase was not accounted for by the action of endogenous proteinases under the preparative conditions used. It is postulated that the soluble neutral maltase is a membrane-dissociated form of the bound enzyme and that the relationship between these two forms is modulated by cortisol. The acid maltase generally resembled acid maltase of liver, muscle and kidney. It was shown to be a maltase–glucoamylase with optimal activity at pH3.0, and molecular weight of 136000 by density-gradient centrifugation. At pH3.0 its Km for maltose was 1.5mm. It was inhibited by turanose (Ki=7.5mm) and Tris (Ki=5.5mm) but not by p-chloromercuribenzoate or EDTA. Some 55% of its activity was destroyed by heating at 50°C for 10min. The acid maltase closely resembled β-glucuronidase and acid β-galactosidase in its distribution in the intestine, response to tissue homogenization in various media, and decrease in activity with cortisol treatment and weaning, indicating that it was a typical lysosomal enzyme concentrated in the ileum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURICCHIO S., DAHLQVIST A., SEMENZA G. SOLUBILIZATION OF THE HUMAN INTESTINAL DISACCHARIDASES. Biochim Biophys Acta. 1963 Aug 6;73:582–587. doi: 10.1016/0006-3002(63)90329-9. [DOI] [PubMed] [Google Scholar]
- AURICCHIO S., SEMENZA G., RUBINO A. MULTIPLICITY OF HUMAN INTESTINAL DISACCHARIDASES. II. CHARACTERIZATION OF THE INDIVIDUAL MALTASES. Biochim Biophys Acta. 1965 Mar 22;96:498–507. doi: 10.1016/0005-2787(65)90566-6. [DOI] [PubMed] [Google Scholar]
- Alpers D. H. Separation and isolation of rat and human intestinal beta-galactosidases. J Biol Chem. 1969 Mar 10;244(5):1238–1246. [PubMed] [Google Scholar]
- Alpers D. H., Solin M. The characterization of rat intestinal amylase. Gastroenterology. 1970 Jun;58(6):833–842. [PubMed] [Google Scholar]
- Angelini C., Engel A. G. Subcellular distribution of acid and neutral alpha-glucosidases in normal, acid maltase deficient, and myophosphorylase deficient human skeletal muscle. Arch Biochem Biophys. 1973 May;156(1):350–355. doi: 10.1016/0003-9861(73)90374-3. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Bruni C. B., Sica V. Further purification and characterization of the acid alpha-glucosidase. Biochem J. 1968 Jun;108(2):161–167. doi: 10.1042/bj1080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. S. Studies on the biogenesis of mitochondrial protein components in rat liver slices. J Biol Chem. 1968 Aug 10;243(15):4027–4033. [PubMed] [Google Scholar]
- Berger S. J., Sacktor B. Isolation and biochemical characterization of brush borders from rabbit kidney. J Cell Biol. 1970 Dec;47(3):637–645. doi: 10.1083/jcb.47.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Auricchio F., Covelli I. Acid alpha-D-glucosidase glucohydrolase from cattle liver. J Biol Chem. 1969 Sep 10;244(17):4735–4742. [PubMed] [Google Scholar]
- CLARK S. L., Jr The ingestion of proteins and colloidal materials by columnar absorptive cells of the small intestine in suckling rats and mice. J Biophys Biochem Cytol. 1959 Jan 25;5(1):41–50. doi: 10.1083/jcb.5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M., Hardy R. N. An analysis of the mechanism of cessation of uptake of macromolecular substances by the intestine of the young rat ('closure'). J Physiol. 1969 Sep;204(1):127–134. doi: 10.1113/jphysiol.1969.sp008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R., Padykula H. A. A cytological study of intestinal absorption in the suckling rat. Am J Anat. 1969 Jul;125(3):291–315. doi: 10.1002/aja.1001250304. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A., THOMSON D. L. SEPARATION AND CHARACTERIZATION OF TWO RAT-INTESTINAL AMYLASES. Biochem J. 1963 Nov;89:272–277. doi: 10.1042/bj0890272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. INTESTINAL INVERTASE: PRECOCIOUS DEVELOPMENT OF ACTIVITY AFTER INJECTION OF HYDROCORTISONE. Science. 1964 Jan 3;143(3601):42–44. doi: 10.1126/science.143.3601.42. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A., Telenius U. Column chromatography of human small-intestinal maltase, isomaltase and invertase activities. Biochem J. 1969 Jan;111(2):139–146. doi: 10.1042/bj1110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- Donaldson R. M., Jr, Small D. M., Robins S., Mathan V. I. Receptors for vitamin B12 related to ileal surface area and absorptive capacity. Biochim Biophys Acta. 1973 Jul 6;311(3):477–481. doi: 10.1016/0005-2736(73)90327-1. [DOI] [PubMed] [Google Scholar]
- Eggermont E. The hydrolysis of the naturally occurring alpha-glucosides by the human intestinal mucosa. Eur J Biochem. 1969 Jul;9(4):483–487. doi: 10.1111/j.1432-1033.1969.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Eichholz A. Studies on the organization of the brush border in intestinal epithelial cells. V. Subfractionation of enzymatic activities of the microvillus membrane. Biochim Biophys Acta. 1968 Aug;163(1):101–107. doi: 10.1016/0005-2736(68)90037-0. [DOI] [PubMed] [Google Scholar]
- Forstner G. G. Release of intestinal surface-membrane glycoproteins associated with enzyme activity by brief digestion with papain. Biochem J. 1971 Mar;121(5):781–789. doi: 10.1042/bj1210781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G. Surface sugar in the intestine. Am J Med Sci. 1969 Sep;258(3):172–180. doi: 10.1097/00000441-196909000-00004. [DOI] [PubMed] [Google Scholar]
- GITZELMANN R., DAVIDSON E. A., OSINCHAK J. DISACCHARIDASE OF RABBIT SMALL INTESTINE: INTRACELLULAR DISTRIBUTION, SOLUBILIZATION, PURIFICATION AND SPECIFICITY. Biochim Biophys Acta. 1964 Apr 6;85:69–81. doi: 10.1016/0926-6569(64)90168-3. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. I. Purification and properties of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1403–1415. doi: 10.1021/bi00808a015. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- Jones R. E. Intestinal absorption and gastro-intestinal digestion of protein in the young rat during the normal and cortisone-induced post-closure period. Biochim Biophys Acta. 1972 Aug 9;274(2):412–419. doi: 10.1016/0005-2736(72)90187-3. [DOI] [PubMed] [Google Scholar]
- Kelly J. J., Alpers D. H. Properties of human intestinal glucoamylase. Biochim Biophys Acta. 1973 Jul 5;315(1):113–122. doi: 10.1016/0005-2744(73)90135-6. [DOI] [PubMed] [Google Scholar]
- Koldovský O., Palmieri M. Cortisone-evoked decrease of acid -galactosidase, -glucuronidase, N-acetyl- -glucosaminidase and arylsulphatase in the ileum of suckling rats. Biochem J. 1971 Dec;125(3):697–701. doi: 10.1042/bj1250697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolínská J., Kraml J. Separation and characterization of sucrose-isomaltase and of glucoamylase of rat intestine. Biochim Biophys Acta. 1972 Sep 19;284(1):235–247. doi: 10.1016/0005-2744(72)90062-9. [DOI] [PubMed] [Google Scholar]
- Kolínská J., Semenza G. Studies on intestinal sucrase and on intestinal sugar transport. V. Isolation and properties of sucrase-isomaltase from rabbit small intestine. Biochim Biophys Acta. 1967 Sep 12;146(1):181–195. doi: 10.1016/0005-2744(67)90085-x. [DOI] [PubMed] [Google Scholar]
- LEJEUNE N., THINES-SEMPOUX D., HERS H. G. Tissue fractionation studies. 16. Intracellular distribution and properties of alpha-glucosidases in rat liver. Biochem J. 1963 Jan;86:16–21. doi: 10.1042/bj0860016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. II. Localization of disaccharide hydrolysis in the isolated brush border portion of intestinal epithelial cells. Biochim Biophys Acta. 1961 Sep 16;52:293–298. doi: 10.1016/0006-3002(61)90678-3. [DOI] [PubMed] [Google Scholar]
- Palmer T. N. The maltase, glucoamylase and transglucosylase activities of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):713–724. doi: 10.1042/bj1240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., ZIMBALATTI F., AURICCHIO S. INTESTINAL DISACCHARIDASE ACTIVITIES IN ADULT AND SUCKLING RATS. Biochim Biophys Acta. 1964 Nov 22;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]
- STEERS E., Jr, CRAVEN G. R., ANFINSEN C. B., BETHUNE J. L. EVIDENCE FOR NONIDENTICAL CHAINS IN THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2478–2484. [PubMed] [Google Scholar]
- Schlegel-Haueter S., Hore P., Kerry K. R., Semenza G. The preparation of lactase and glucoamylase of rat small intestine. Biochim Biophys Acta. 1972 Feb 28;258(2):506–519. doi: 10.1016/0005-2744(72)90242-2. [DOI] [PubMed] [Google Scholar]
- Semenza G., von Balthazar A. K. Steady-state kinetics of rabbit-intestinal sucrase. Kinetic mechanism, Na+ activation, inhibition by tris(hydroxymethyl)aminomethane at the glucose subsite. Eur J Biochem. 1974 Jan 3;41(1):149–162. doi: 10.1111/j.1432-1033.1974.tb03255.x. [DOI] [PubMed] [Google Scholar]
- Siddons R. C. Heat inactivation and sephadex chromatography of the small-intestine disaccharidases of the chick. Biochem J. 1970 Jan;116(1):71–78. doi: 10.1042/bj1160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan N., Radhakrishnan A. N. Studies on intestinal disaccharidases. IV. Heterogeneity of monkey intestinal maltases. Indian J Biochem. 1970 Mar;7(1):19–23. [PubMed] [Google Scholar]
- TORRES H. N., OLAVARRIA J. M. LIVER ALPHA-GLUCOSIDASES. J Biol Chem. 1964 Aug;239:2427–2434. [PubMed] [Google Scholar]
- Van Hoof F., Hue L., De Barsy T., Jacquemin P., Devos P., Hers H. G. Glycogen storage diseases. Biochimie. 1972;54(5):745–752. doi: 10.1016/s0300-9084(72)80177-9. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Preiser H., Woodley J. F., Crane R. K. An enriched microvillus membrane preparation from frozen specimens of human small intestine. Gastroenterology. 1972 Apr;62(4):572–582. [PubMed] [Google Scholar]
- de Barsy T., Jacquemin P., Devos P., Hers H. G. Rodent and human acid -glucosidase. Purification, properties and inhibition by antibodies. Investigation in type II glycogenosis. Eur J Biochem. 1972 Nov 21;31(1):156–165. doi: 10.1111/j.1432-1033.1972.tb02514.x. [DOI] [PubMed] [Google Scholar]


