Abstract
Objective
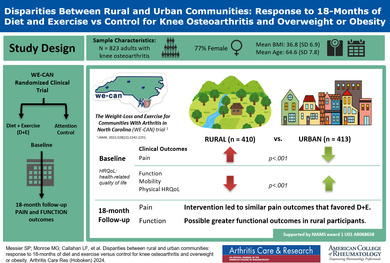
The study objective was to determine whether the clinical response of older adults with knee osteoarthritis and overweight or obesity to 18 months of diet and exercise (D + E) or attention control (C) interventions differed between participants from rural versus urban communities.
Methods
Participants were 823 older adults (mean age, 64.6 years; 77% women) with knee osteoarthritis and overweight or obesity who resided in rural (n = 410) and urban (n = 413) counties in North Carolina. All were enrolled in the Weight Loss and Exercise for Communities with Arthritis in North Carolina clinical trial that randomly assigned participants to either 18 months of D + E or C interventions. General linear models were used to examine differences in clinical outcomes between rural and urban groups after adjusting for covariates.
Results
The rural group had significant differences (P < 0.05) at baseline in clinical outcomes, education, comorbidities, medication use, and income compared with the urban dwellers. After adjusting for baseline differences, the group (rural or urban) by treatment (D + E or C) interactions for Western Ontario McMasters Universities Osteoarthritis Index (WOMAC) pain (rural: D + E – C = −0.63, 95% confidence interval [CI] −1.31 to 0.06; urban: D + E − C= −0.29, 95% CI −0.99 to 0.41; P = 0.50) and WOMAC function (rural: D + E − C = −4.60, 95% CI −6.89 to −2.31; urban: D + E − C = −1.38, 95% CI −3.73 to 0.94; P = 0.054) indicated that the groups responded similarly to the interventions.
Conclusion
Among participants with knee osteoarthritis and overweight or obesity, D + E compared to C led to similar pain outcomes in rural and urban dwellers that favored D + E. The possibility that there may be greater differential efficacy in functional outcomes among rural participants needs further study.

INTRODUCTION
Geographic location has a significant impact on health inequities. People residing in rural communities in the United States, Europe, and China have poorer health metrics compared with residents of urban communities including greater distances to sources of high‐quality food, lower walkability and presence of exercise facilities in neighborhoods, and a reduced availability of specialized health care. 1 , 2 , 3 , 4 , 5 Furthermore, health disparities in rural communities include lack of access to medical insurance and a high prevalence of obesity, a major risk factor for knee osteoarthritis (OA). As with obesity, knee OA also impacts rural communities disproportionately, hence disability may be higher than in urban communities. 6 , 7 , 8
SIGNIFICANCE & INNOVATIONS.
Adults with overweight or obesity and knee osteoarthritis who live in rural communities have more pain, poorer function, worse mobility, and poorer physical health–related quality of life at baseline than their urban counterparts.
Rural dwellers benefit from participating in long‐term behavioral diet and exercise interventions.
Diet plus exercise is an equally effective treatment in rural and urban settings.
Knee OA is the most common and persistent cause of mobility dependency and disability with prevalence estimated at >300 million people worldwide. 9 , 10 OA develops from a complex interaction of biomechanical and inflammatory disease pathways. Obesity is common to both pathways, resulting in increased mechanical joint stress and the release of proinflammatory cytokines and adipokines. 11 The prevalence of obesity in the United States has risen from 30% in 2000 to 42% in 2018. 12 In the population aged ≥60 years, the 71% prevalence of overweight and obesity is nearly 20% higher than in the population aged 20 to 39 years. 13 This coincides with the increased prevalence of knee OA among older adults living in rural and urban communities. 8
The combination of dietary therapy and exercise has level 1 evidence of efficacy in the treatment of knee OA. 14 , 15 , 16 , 17 Clinical trials involving weight loss in rural populations without diagnosed knee OA generally found positive results. 18 , 19 , 20 However, whether rural and urban adults with obesity and knee OA respond similarly to a behavioral diet and exercise (D + E) intervention is unknown. Hence, the premise of this study is that the response to 18 months of D + E in older adults with knee OA and overweight and obesity who live in rural communities is superior to a similar cohort that resides in urban communities. Twenty percent of older adults reside in rural communities; their responses to behavioral interventions should be examined when evaluating programs that could benefit older adults broadly. 21
PATIENTS AND METHODS
Study design
The Weight Loss and Exercise for Communities with Arthritis in North Carolina (WE‐CAN) trial was a Phase III, assessor‐blinded, three‐center (Forsyth County, NC; Haywood County, NC; and Johnston County, NC) randomized clinical trial with two parallel groups followed for 18 months. The study design included many pragmatic components, including a large sample size, broad inclusion criteria, patient‐centered outcomes, and settings in established community facilities rather than referral centers. The study protocol was reviewed and approved by the Human Subjects Committees of Wake Forest Health Sciences and The University of North Carolina at Chapel Hill and is in compliance with the terms and conditions set forth in the Declaration of Helsinki. The Wake Forest Health Sciences and The University of North Carolina at Chapel Hill Institutional Review Boards reviewed all research involving humans to ensure that participants were informed of all known risks posed by the research study and that these studies were conducted in accordance with the ethical standards put forward by the Belmont Report and federal, state, and local regulations and policies governing human research. All participants gave informed consent to participate in the study. The trial design and the main outcome paper have been published previously. 22 , 23
Participants
Participants were 823 ambulatory, community‐dwelling men and women with a body mass index (BMI) ≥27 kg/m2 who met the American College of Rheumatology (ACR) clinical criteria for knee OA of age ≥50 years, knee pain on most days of the week, and at least two of the following: stiffness <30 min/day, crepitus, bony tenderness, bony enlargement, and/or no palpable warmth. 24 Key exclusions were symptomatic coronary artery disease, type 1 diabetes, BMI <27 kg/m2, and failure to meet the ACR clinical criteria for knee OA. Enrollment occurred between May 2016 and August 2019. All participants could maintain their regular medications, including analgesics. Participants lived in North Carolina in two rural counties (n = 410), defined as a population density <500 people/square mile with open countryside (Haywood County, population density = 100 people/square mile; Johnston County, population density = 200 people/square mile), and one urban county (n = 413), defined as having an urban nucleus of ≥50,000 people, a core population of 1,000 people/square mile, and adjoining territory ≥500 people/square mile (Forsyth County, population density = 800 people/square mile). 25
Measurements
Self‐reported knee pain was measured using the Likert version of the Western Ontario McMasters Universities Osteoarthritis Index (WOMAC), 26 , 27 which assesses knee pain over the last 48 hours. 28 The total score ranges from 0 to 20 (higher scores indicate greater pain). The minimal clinically important difference (MCID) in WOMAC pain between groups is 2 on the 20‐point Likert scale. 29 The pain categories on a 0‐to‐20 scale are 2 to 8, mild; ≥8 to 14, moderate; and ≥14 to 20, severe. 30 WOMAC function assessed the degree of difficulty with activities of daily living in the last 48 hours with a total score range of 0 to 68. Higher scores indicated poorer function (MCID = 6), with a score of ≥21 indicating physical work limitations. 31 , 32 , 33 Six‐minute walk distance assessed the maximum distance a participant could walk along a standardized walkway in 6 minutes (MCID >30.5 m). 34
The 36‐item Short‐Form Health Survey (SF‐36) measures health‐related quality of life using two broad summary scores: physical and mental health scaled from 0 (worst) to 100 (best). 35 The MCID for each subscale is at least 10 points. 36 The Center for Epidemiologic Studies Depression Scale (CES‐D) 10 (range, 0–30) assessed depressive symptoms during the last week. Ten items are scored from 0 (rarely or none of the time) to 3 (most or almost all the time). 37 The range for internal consistency is 0.80 to 0.88 (Cronbach alpha). 38 Comparisons between rural and urban communities also included changes in BMI across the 18‐month intervention period.
Interventions
The community centers where interventions were conducted included a medical mall, recreation center, rural hospital community fitness center, YMCA, local gymnasium, church recreation facility, and a community healthy lifestyle program. Interventionists were hired within each county and the surrounding area, had at least a bachelor's degree in a health‐related field, and were subsequently trained by experienced coordinating center staff who tailored the instruction to the local facilities. The exercise component for the D + E group included 60‐minute sessions 3 days per week for 18 months at one of the designated community facilities.
For the first 6 months, a dietary plan included an energy‐restricted diet using 1 to 2 partial meal replacements (Lean Shakes, GNC) per day provided by the study with the option to incorporate one study‐provided meal replacement per day during months 7 to 18. The initial diet plan ensured an energy‐intake deficit of 800 to 1,000 kcal/day from the estimated energy expenditure (predicted resting metabolism calculated using the Owen equation 39 × 1.2 activity factor). An average of 200 kcal/day were expended with exercise for a total imbalance of at least 1,000 kcal/day. The lowest intake was 1,100 kcal for women and 1,200 kcal for men.
The attention control (C) group provided social interaction and evidence‐based nutrition and health education delivered in five 1‐hour, face‐to‐face group meetings at months 1, 3, 6, 9, and 15, and via informational packets and individual sessions via phone during alternate months. Adherence to the D + E, and C classes was defined as the number of sessions completed divided by the number scheduled.
Statistical analysis
Descriptive statistics, demographic data, and variables of interest were analyzed in SAS. We evaluated pairwise differences of each of the counties to determine if the two rural counties, Johnston and Haywood, were not statistically different enough from each other to be combined into a single category as the rural group. P values <0.0167 were considered significant, meaning that P values higher than this allow for the two counties’ data to be combined into a single category.
We used mean and 95% confidence intervals (CIs) to describe the rural and urban categories for each of the baseline variables and t‐tests and chi‐square tests to determine the statistical significance of rural‐urban comparisons. P values ≤0.05 were considered statistically significant. We estimated pairwise comparisons between rural and urban groups from a mixed model using a three‐way interaction between treatment (D + E and C), visit month (6, 12, and 18‐month follow‐ups) and rural/urban category, adjusted for sex, income, BMI, and the interaction between rural/urban and baseline outcome. The interaction effects were not prespecified and there was no adjustment for multiple comparisons; hence, these results should be considered exploratory.
The investigators support data sharing and will comply with all National Institutes of Health (NIH) guidelines as outlined in the NIH Data‐Sharing Policy and Implementation Guidance document. Any data‐sharing agreement will require that the data be used only for research purposes, no attempts will be made to identify individual participants, the data will be kept secure, the user will not distribute the data to other researchers, the user will return the files or destroy them once the project is completed, and the user will acknowledge the data source.
RESULTS
Pairwise differences in baseline features between the two rural counties (Haywood and Johnston) revealed no statistically significant differences (P > 0.0167); hence, the data for the two rural counties were combined into a single group. Baseline characteristics of the rural and urban groups are in Table 1. The rural group was slightly older, had a lower percentage of male participants, had less education, had fewer comorbidities, consumed more medications, and had a lower income than the urban group. The mean adherence rate for the diet classes for the D + E group was 80% for both the rural and urban groups; adherence to the exercise classes was 80% for the rural group and 70% for the urban group. Attendance to the C group's nutrition and health education sessions was 78% for both the rural and urban groups.
Table 1.
Descriptive mean (SD) baseline characteristics of the participants overall and by group (rural or urban)*
| Group | ||||
|---|---|---|---|---|
| Variable | Overall | Rural (n = 410) | Urban (n = 413) | P value |
| Age, mean (SD), years | 64.6 (7.8) | 65.2 (8.0) | 64.0 (7.5) | 0.027 |
| Male sex, n (%) | 186 (23) | 66 (16) | 120 (29) | <0.001 |
| Weight, mean (SD), kg | 100.9 (21.3) | 99.5 (19.5) | 102.3 (22.9) | 0.062 |
| BMI, mean (SD), kg/m2 | 36.8 (6.9) | 36.7 (6.6) | 36.8 (7.2) | 0.84 |
| Waist circumference, mean (SD), cm | 114.5 (15.5) | 114.1 (14.5) | 114.9 (16.5) | 0.43 |
| Education: bachelor's or more, n (%) | 374 (46) | 159 (39) | 215 (52) | <0.001 |
| Comorbid illness, n (%) | 2.03 (1.63) | 1.84 (1.57) | 2.22 (1.66) | <0.001 |
| Number of medications, mean (SD) | 8.2 (5.0) | 8.8 (5.3) | 7.6 (4.7) | <0.001 |
| Income ≥$75,000/year, n (%) | 232 (29.3) | 93 (23.3) | 139 (35.3) | <0.001 |
BMI, body mass index.
The mean WOMAC pain at baseline was significantly greater in the rural group compared with the urban group, 8.04 versus 7.02 (adjusted difference 1.02, 95% CI 0.59–1.46; P < 0.001) (Table 2; Figure 1). The rural group had significantly worse WOMAC function compared with the urban group (28.41 vs 23.49, adjusted difference 4.92, 95% CI 3.34–6.50; P < 0.001) (Table 2). Six‐minute walk distance was significantly shorter in the rural group (354.6 m vs 388.7 m, adjusted difference −34.1, 95% CI −46.6 to −21.5; P < 0.001). This coincided with significantly worse physical health–related quality of life (33.7 vs 36.4, adjusted difference −2.7, 95% CI −4.0 to −1.5; P < 0.001). Mental health–related quality of life was not significantly different between the rural and urban groups at baseline; however, the number of depressive symptoms was significantly greater in the rural group (5.8 vs 5.1, adjusted difference 0.8, 95% CI 0.1–1.5; P = 0.03).
Table 2.
Mean (SD) baseline clinical outcomes with pairwise comparisons between the rural and urban groups*
| Group, mean (SD) | ||||
|---|---|---|---|---|
| Outcome | Rural (n = 410) | Urban (n = 413) | Pairwise comparison, difference (95% CI) | P value |
| WOMAC pain (0–20) | 8.04 (3.16) | 7.02 (3.20) | 1.02 (0.59 to 1.46) | <0.001 |
| WOMAC function (0–68) | 28.41 (11.64) | 23.49 (11.41) | 4.92 (3.34 to 6.50) | <0.001 |
| Six‐minute walk, m | 354.6 (88.7) | 388.7 (94.3) | −34.1 (−46.6 to −21.5) | <0.001 |
| SF‐36 physical (0–100) | 33.7 (9.2) | 36.4 (7.2) | −2.7 (−4.0 to −1.5) | <0.001 |
| SF‐36 mental (0–100) | 54.3 (10.4) | 55.6 (9.6) | −1.2 (−2.6 to 0.1) | 0.07 |
| CES‐D (0–30) | 5.8 (5.2) | 5.1 (4.9) | 0.8 (0.1 to 1.5) | 0.03 |
CES‐D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; SF‐36, 36‐item Short‐Form Health Survey; WOMAC, Western Ontario McMasters Universities Osteoarthritis Index.
Figure 1.
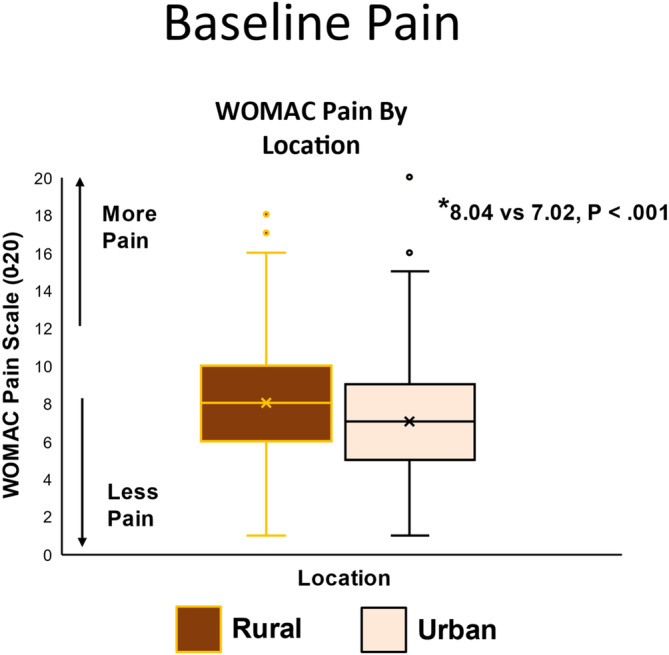
Box plots are shown in which the middle line represents the median value, the X the mean value, and the box represents the interquartile range. Whiskers extend to the most extreme observed values within the 1.5 times the interquartile range of the nearer quartile, and dots represent observed values outside the range. WOMAC, Western Ontario McMasters Universities Osteoarthritis Index (range 0 [no pain] to 20 [severe pain]).
The effect of the two interventions (D + E and C) on WOMAC knee pain and WOMAC function at 18‐month follow‐up was not statistically different (P > 0.05) between the rural and urban groups; the difference in WOMAC function was not significant (P interaction = 0.054) (Figure 2A and B). Six‐minute walk distance, SF‐36 physical, and the CES‐D at 18‐month follow‐up were not statistically different between the rural and urban groups (D + E − C, P interaction > 0.05; Table 3). The change in BMI across the 18‐month intervention period showed a pairwise difference between D + E and C for the rural group of 2.47 kg/m2 compared with a difference of 1.78 kg/m2 for the urban group, a 39% relative difference between the rural and urban cohorts (P = 0.15) (Figure 3).
Figure 2.
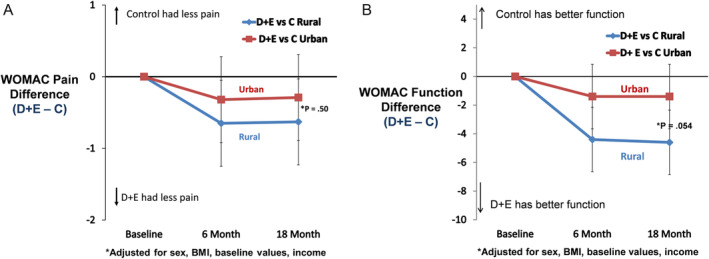
(A) Pairwise comparisons (95% confidence interval) between D + E and C in WOMAC pain for rural and urban communities. Negative values indicate that the D + E group had less pain than the C group. Positive values indicate that the C group had less pain than the D + E group. (B) Pairwise comparisons (95% confidence interval) between D + E and C in function for rural and urban communities. Negative values indicate that the D + E group had better function than the C group. Positive values indicate that the C group had better function than the D + E group. BMI, body mass index; C, attention control; D, diet; E, exercise; WOMAC, Western Ontario McMasters Universities Osteoarthritis Index.
Table 3.
Change at 18‐month follow‐up (18‐month follow‐up minus baseline) and mean 18‐month clinical outcomes with treatment comparisons (D + E − C) for the rural and urban groups*
| Rural | Urban | P value, group × treatment b | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Treatment | Change (95% CI) a | 18‐month mean | D + E – C (95% CI) | Change, (95% CI) | 18‐month mean | D + E – C (95% CI) | |
| WOMAC pain (0–20) | D + E | −2.63 (−3.13 to −2.13) | 4.71 | −0.63 (−1.31 to 0.06) | −2.08 (−2.59 to −1.57) | 5.26 | −0.29 (−0.99 to 0.41) | 0.50 |
| C | −2.00 (−2.54 to −1.47) | 5.34 | −1.79 (−2.30 to −1.28) | 5.55 | ||||
| WOMAC Function (0–68) | D + E | −10.00 (−11.70 to −8.30) | 15.15 | −4.60 (−6.89 to −2.31) | −7.51 (−9.22 to −5.79) | 17.64 | −1.38 (−3.73 to 0.94) | 0.054 |
| C | −5.40 (−7.20 to −3.60) | 19.75 | −6.13 (−7.83 to −4.24) | 19.03 | ||||
| Six‐minute walk, m | D + E | 47.8 (36.0 to 59.6) | 427.5 | 58.3 (32.0 to 64.6) | 37.8 (25.5 to 50.1) | 417.5 | 32.9 (15.7 to 50.1) | 0.20 |
| C | −0.5 (−13.1 to 12.0) | 379.2 | 4.8 (−7.8 to 17.5) | 384.5 | ||||
| SF‐36 physical (0–100) | D + E | 6.0 (4.6 to 7.4) | 41.4 | 4.2 (2.3 to 6.1) | 6.5 (5.1 to 7.9) | 41.9 | 3.2 (1.2 to 5.1) | 0.47 |
| C | 1.8 (0.3 to 3.3) | 37.2 | 3.3 (1.9 to 4.8) | 38.7 | ||||
| SF‐36 mental (0–100) | D + E | −0.5 (−1.9 to 0.9) | 55.1 | 1.1 (−0.7 to 3.0) | 0.1 (−1.3 to 1.5) | 55.7 | 1.1 (−0.8 to 3.1) | 0.99 |
| C | −1.6 (−3.1 to −0.2) | 53.9 | −1.0 (−2.4 to 0.3) | 54.5 | ||||
| CES‐D (0–30) | D + E | −1.2 (−1.9 to −0.5) | 3.8 | −0.9 (−1.8 to 0.1) | −0.7 (−1.4 to 0.1) | 4.3 | −0.4 (−1.4 to 0.7) | 0.50 |
| C | −0.4 (−1.1 to 0.4) | 4.6 | −0.3 (−1.0 to 0.5) | 4.7 | ||||
The group (rural or urban) by treatment (D + E or C) interactions revealed whether the two groups responded similarly or differently to the D + E and C interventions after adjusting for sex, income, body mass index, and baseline differences in the outcome. C, attention control; CES‐D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; D, diet; E, exercise; SF‐36, 36‐item Short‐Form Health Survey; WOMAC, Western Ontario McMasters Universities Osteoarthritis Index.
Change is calculated as the 18‐month follow‐up minus the baseline.
Estimated from a mixed model using a three‐way interaction between treatment (D + E or C), visit month, and group (rural or urban) adjusted by sex, income, body mass index, and baseline differences in the outcome.
Figure 3.
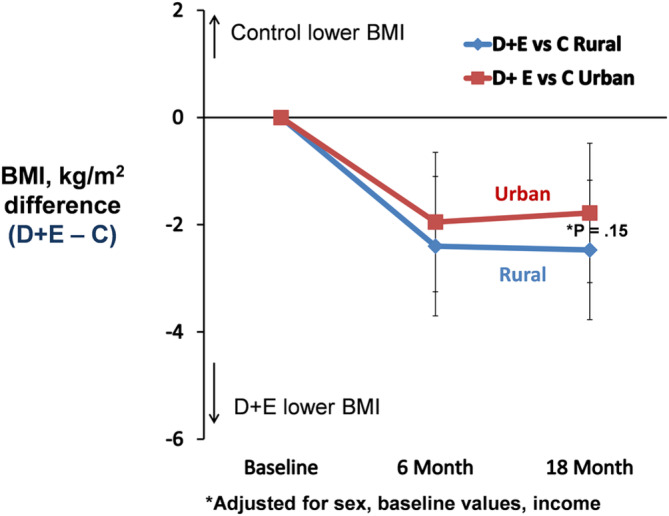
Pairwise comparisons (95% confidence interval) between D + E and C in BMI for rural and urban communities. Negative values indicate that the D + E group reduced BMI more than the C group. Positive values indicate that the C group reduced BMI more than the D + E group. BMI, body mass index; C, attention control; D, diet; E, exercise.
DISCUSSION
Rural participants had more pain, poorer function, worse mobility, and a poorer physical health–related quality of life at baseline than their urban counterparts. These baseline differences align with past reports that documented the inadequate access of rural areas to services related to a healthier lifestyle, including few health care specialists resulting in long wait times and long travel distances for medical appointments, the lack of grocery stores that stock healthy food choices at reasonable prices, low neighborhood walkability, and limited availability of exercise and recreational facilities managed by a skilled workforce. 2 What remained unknown was how patients with knee OA living in rural locations would respond to behavioral interventions relative to their urban counterparts. Despite initial differences between groups and the larger improvements in the rural group because of D + E, the response to D + E as compared with C was similar across the rural and urban groups. The only outcome that approached significance was WOMAC function (P = 0.054), wherein the rural group responded more positively to the D + E treatment relative to C. Taken together, these data suggest that the D + E intervention was equally effective in rural and urban settings.
The same pattern of worse baseline values for the rural group relative to the urban group but similar group values at follow‐up was also present for the C group, suggesting that some of the improvement in the rural cohort could be attributed to regression to the mean. Regardless of the mechanism involved, these data indicate that rural dwellers benefit from participating in a long‐term behavioral D + E trial and that the possibility of greater efficacy in rural settings needs further examination.
These results may not reflect how rural participants respond to other interventions. Hence, their inclusion in future behavioral clinical trials would better reflect the US population. Access to proper facilities and trained personnel, however, remains a serious concern in rural areas. Using telehealth to reach patients in remote areas may be one solution to this problem. 40 , 41 , 42
This study has several limitations. The statistical analyses adjusted for differences in annual income, our marker of socioeconomic status. 43 Other measures, such as social circumstances, availability of health care, number of health care providers, and distance to grocery stores with healthy choices, were not documented. 44 Our definition of rurality was based on population density (number of people per square mile). Availability of and travel time to health care services, a measure of remoteness, was not included in our analysis. 44 , 45
Among participants with knee OA and overweight or obesity, D + E compared with C led to equally effective outcomes in rural and urban dwellers that favored D + E. The possibility that there may be greater differential efficacy in functional outcomes among rural participants needs further study.
AUTHOR CONTRIBUTIONS
All authors contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Messier confirms that all authors have provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Declaration of Helsinki requirements.
Supporting information
Disclosure Form:
ACKNOWLEDGMENTS
The authors thank General Nutrition Centers for their generosity in supplying the Lean Shakes to us at no cost. They have not funded the study in any other way and have not contributed to and have no influence over study design, publications, or dissemination of results.
The WE‐CAN investigators wish to acknowledge the tremendous efforts of our staff in making this project come to fruition: Natalia Favoreto, Nathan Fiore, James Gerosa, Daniel Hamm, Ryan Hill, Elyse Howdershell, Erika Janssen, Monica Love, Chris Mygrant, Alex Nielson, Carol Patterson, Elena Wright, Caroline Wyrosdick, and project managers Betsy Hackney and Jovita Newman.
Clinicaltrials.gov Identifier: NCT02577549
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (grant 1‐U01‐AR‐068658‐01).
Author disclosures and graphical abstract are available at https://onlinelibrary.wiley.com/doi/10.1002/acr.25448.
REFERENCES
- 1. Stoller EP, Grzywacz JG, Quandt SA, et al. Calling the doctor: a qualitative study of patient‐initiated physician consultation among rural older adults. J Aging Health 2011;23(5):782–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saag KG, Doebbeling BN, Rohrer JE, et al. Arthritis health service utilization among the elderly: the role of urban‐rural residence and other utilization factors. Arthritis Care Res 1998;11(3):177–185. [DOI] [PubMed] [Google Scholar]
- 3. Cohen SA, Cook SK, Sando TA, et al. What aspects of rural life contribute to rural‐urban health disparities in older adults? Evidence from a national survey. J Rural Health 2018;34(3):293–303. [DOI] [PubMed] [Google Scholar]
- 4. Moreno‐Llamas A, García‐Mayor J, De la Cruz‐Sánchez E. Urban‐rural differences in perceived environmental opportunities for physical activity: a 2002‐2017 time‐trend analysis in Europe. Health Promot Int 2023;38(4):daad087. [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Li D, Gao J. Health disparities between the rural and urban elderly in China: a cross‐sectional study. Int J Environ Res Public Health 2021;18(15):8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jordan JM, Linder GF, Renner JB, et al. The impact of arthritis in rural populations. Arthritis Care Res 1995;8(4):242–250. [DOI] [PubMed] [Google Scholar]
- 7. Boring MA, Hootman JM, Liu Y, et al. Prevalence of arthritis and arthritis‐attributable activity limitation by urban‐rural county classification ‐ United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66(20):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campos L, Costa D, Donato H, et al. Implementation of digital health in rural populations with chronic musculoskeletal conditions: a scoping review protocol. PLoS One 2023;18(12):e0291638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 2005;33(4):195–200. [DOI] [PubMed] [Google Scholar]
- 12. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;(360):1–8. [PubMed] [Google Scholar]
- 13. Cohen SA, Cook SK, Kelley L, et al. A closer look at rural‐urban health disparities: associations between obesity and rurality vary by geospatial and sociodemographic factors. J Rural Health 2017;33(2):167–179. [DOI] [PubMed] [Google Scholar]
- 14. Messier SP, Callahan LF, Golightly YM, et al. OARSI clinical trials recommendations: design and conduct of clinical trials of lifestyle diet and exercise interventions for osteoarthritis. Osteoarthritis Cartilage 2015;23(5):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50(5):1501–1510. [DOI] [PubMed] [Google Scholar]
- 16. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310(12):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aaboe J, Bliddal H, Messier SP, et al. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage 2011;19(7):822–828. [DOI] [PubMed] [Google Scholar]
- 18. Mench E, West D, Krukowski R, et al. Weight loss success of participants residing in rural and urban areas. J Rural Health 2018;34(4):396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Befort CA, Klemp JR, Sullivan DK, et al. Weight loss maintenance strategies among rural breast cancer survivors: the rural women connecting for better health trial. Obesity (Silver Spring) 2016;24(10):2070–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Befort CA, VanWormer JJ, Desouza C, et al. Effect of behavioral therapy with in‐clinic or telephone group visits vs in‐clinic individual visits on weight loss among patients with obesity in rural clinical practice: a randomized clinical trial. JAMA 2021;325(4):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Symens Smith A, Trevelyan E. In some states, more than half of older residents live in rural areas. United States Census Bureau. Published October 22, 2019. Accessed March 21, 2024. https://www.census.gov/library/stories/2019/10/older-population-in-rural-america.html [Google Scholar]
- 22. Messier SP, Callahan LF, Beavers DP, et al. Weight‐loss and exercise for communities with arthritis in North Carolina (we‐can): design and rationale of a pragmatic, assessor‐blinded, randomized controlled trial. BMC Musculoskelet Disord 2017;18(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messier SP, Beavers DP, Queen K, et al. Effect of diet and exercise on knee pain in patients with osteoarthritis and overweight or obesity: a randomized clinical trial. JAMA 2022;328(22):2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altman R, Asch E, Bloch D, et al; Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 1986;29(8):1039–1049. [DOI] [PubMed] [Google Scholar]
- 25. USDA Economic Research Service . What is rural? Updated March 26, 2024. Accessed March 16, 2022. https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/what-is-rural/ [Google Scholar]
- 26. Bellamy N. Outcome measurement in osteoarthritis clinical trials. J Rheumatol Suppl 1995;43:49–51. [PubMed] [Google Scholar]
- 27. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 28. Griffiths G, Bellamy N, Kean WF, et al. A study of the time frame dependency of responses to the WOMAC osteoarthritis index. Inflammopharmacology 1993;2(1):85–87. [Google Scholar]
- 29. Dougados M, Leclaire P, van der Heijde D, et al. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the Osteoarthritis Research Society International Standing Committee for Clinical Trials response criteria initiative. Osteoarthritis Cartilage 2000;8(6):395–403. [DOI] [PubMed] [Google Scholar]
- 30. Kapstad H, Hanestad BR, Langeland N, et al. Cutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgery. BMC Musculoskelet Disord 2008;9(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siviero P, Limongi F, Gesmundo A, et al; EPOSA Research Group. Minimal clinically important decline in physical function over one year: EPOSA study. BMC Musculoskelet Disord 2019;20(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 33. Clement ND, Bardgett M, Weir D, et al. What is the minimum clinically important difference for the WOMAC index after TKA? Clin Orthop Relat Res 2018;476(10):2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6‐minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23(2):377–381. [DOI] [PubMed] [Google Scholar]
- 35. Ware JE Jr, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–483. [PubMed] [Google Scholar]
- 36. Escobar A, Quintana JM, Bilbao A, et al. Responsiveness and clinically important differences for the WOMAC and SF‐36 after total knee replacement. Osteoarthritis Cartilage 2007;15(3):273–280. [DOI] [PubMed] [Google Scholar]
- 37. Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES‐D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994;10(2):77–84. [PubMed] [Google Scholar]
- 38. Zhang W, O'Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES‐D) among HIV‐positive people in British Columbia, Canada. PLoS One 2012;7(7):e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Owen OE, Kavle E, Owen RS, et al. A reappraisal of caloric requirements in healthy women. Am J Clin Nutr 1986;44(1):1–19. [DOI] [PubMed] [Google Scholar]
- 40. Bennell KL, Nelligan R, Dobson F, et al. Effectiveness of an internet‐delivered exercise and pain‐coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med 2017;166(7):453–462. [DOI] [PubMed] [Google Scholar]
- 41. Bennell KL, Schwartz S, Teo PL, et al. Effectiveness of an unsupervised online yoga program on pain and function in people with knee osteoarthritis: a randomized clinical trial. Ann Intern Med 2022;175(10):1345–1355. [DOI] [PubMed] [Google Scholar]
- 42. Hinman RS, Campbell PK, Kimp AJ, et al. Telerehabilitation consultations with a physiotherapist for chronic knee pain versus in‐person consultations in Australia: the PEAK non‐inferiority randomised controlled trial. Lancet 2024;403(10433):1267–1278. [DOI] [PubMed] [Google Scholar]
- 43. Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ 2018;360:k1274. [DOI] [PubMed] [Google Scholar]
- 44. Hollick RJ, Macfarlane GJ. Association of rural setting with poorer disease outcomes for patients with rheumatic diseases: results from a systematic review of the literature. Arthritis Care Res (Hoboken) 2021;73(5):666–670. [DOI] [PubMed] [Google Scholar]
- 45. Fecht D, Jones A, Hill T, et al. Inequalities in rural communities: adapting national deprivation indices for rural settings. J Public Health (Oxf) 2018;40(2):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form:


