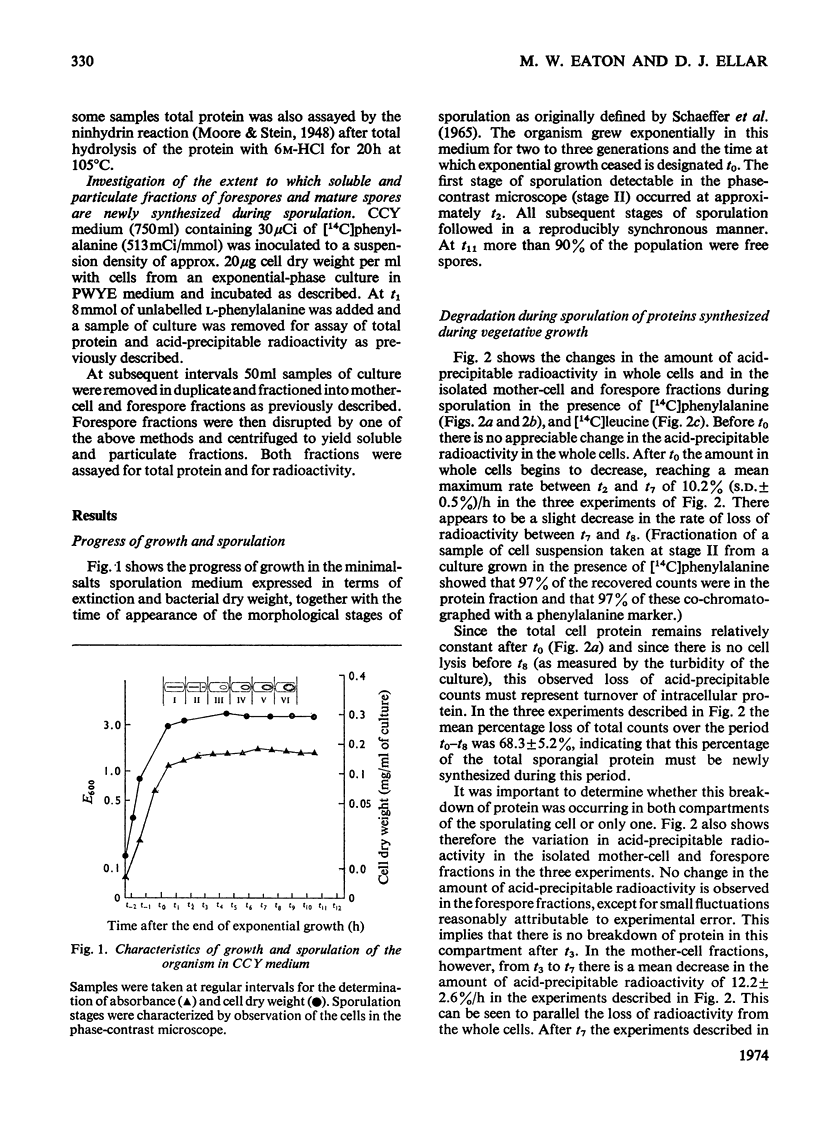
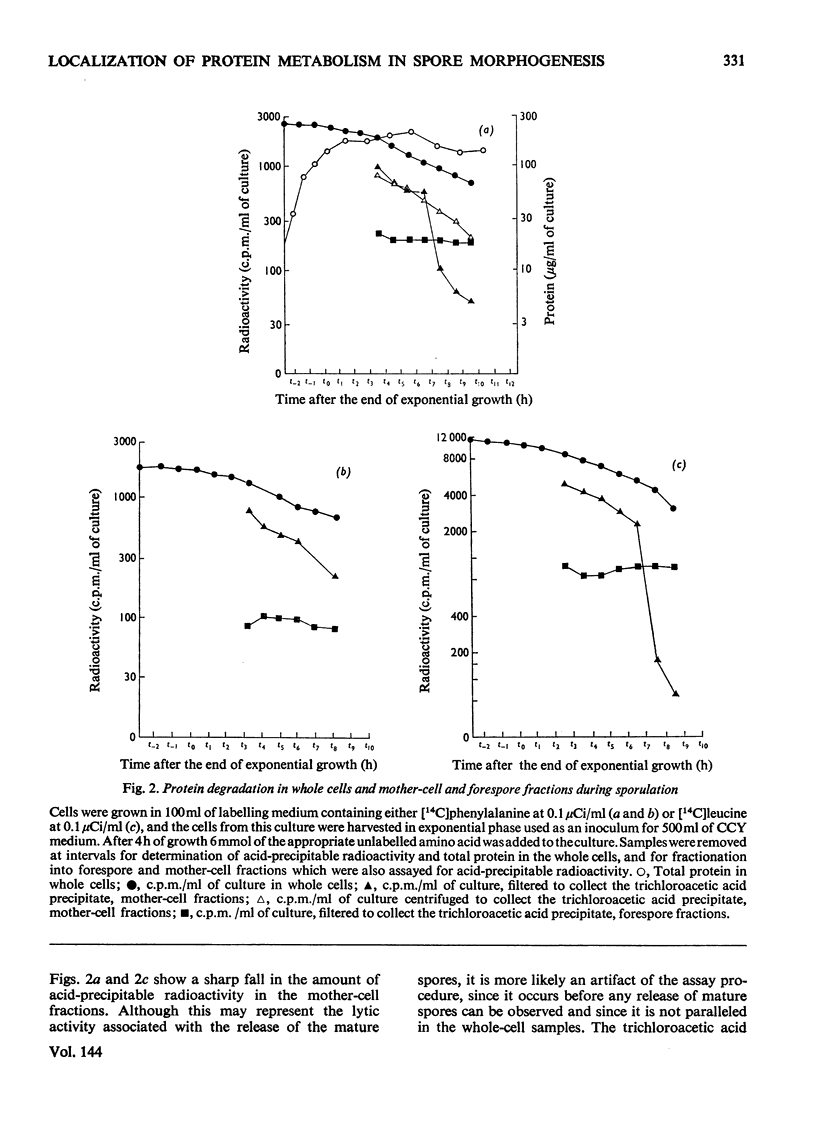
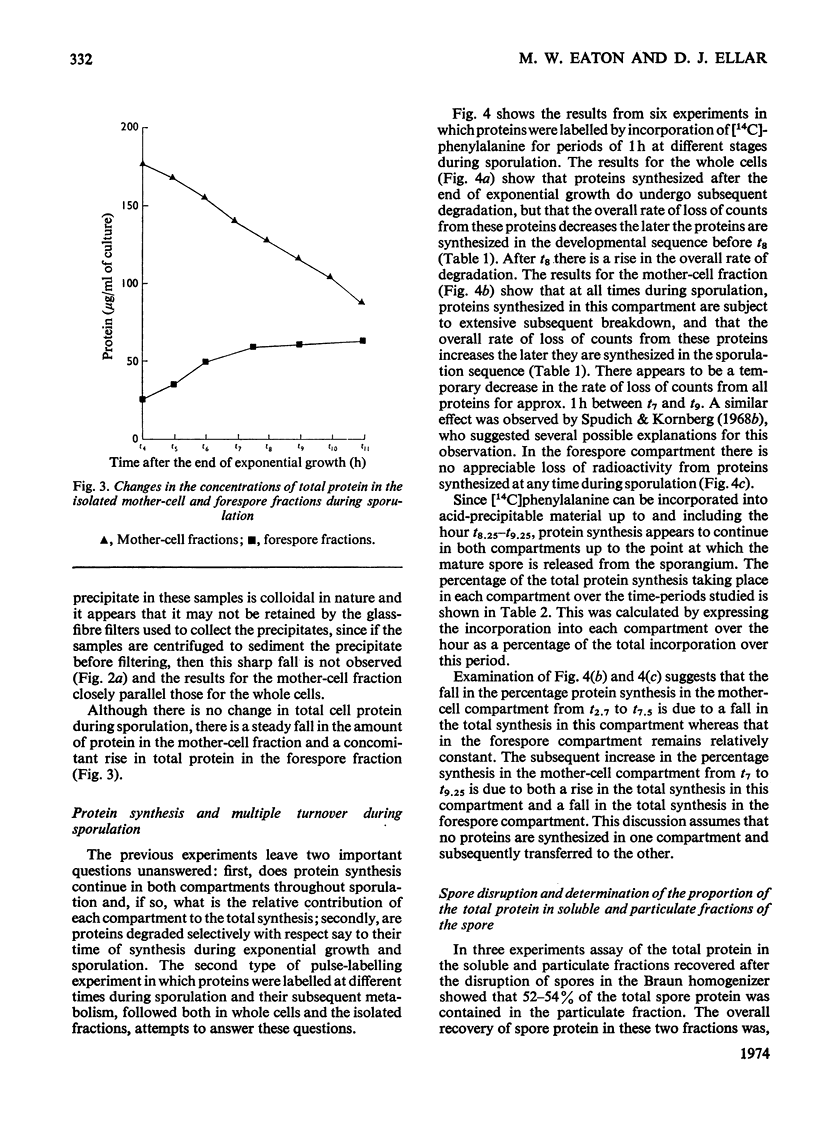
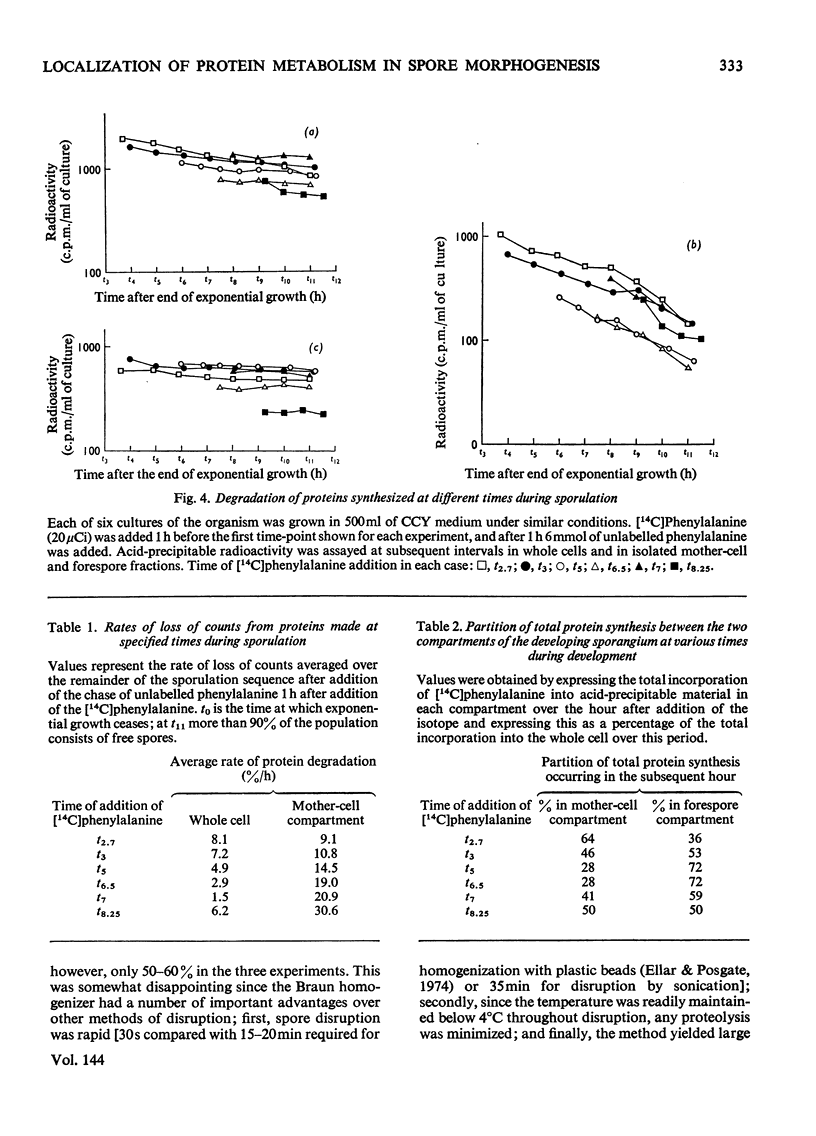
Abstract
Recently developed techniques for isolating forespores from bacilli at all stages of spore morphogenesis have been exploited to investigate the contribution of each of the two compartments of the sporulating cell to the overall pattern of protein synthesis and degradation during sporulation in Bacillus megaterium. These studies have shown: (1) that protein synthesis continues in both compartments throughout spore morphogenesis; (2) that the degradation of proteins made at all times during vegetative growth and sporulation is confined to the mother-cell compartment; (3) that proteins synthesized in the mother-cell compartment during sporulation are subsequently degraded more rapidly than proteins synthesized during vegetative growth. This rate of degradation increases the later the proteins are synthesized in the sporulation sequence. Mature spores were disrupted, and the percentage of the total protein in soluble and particulate fractions was determined. Pulse-labelling experiments were performed to investigate the extent to which the proteins of these two fractions are newly synthesized during sporulation. These data were used to calculate the extent of capture of vegetative cell protein at the time of formation of the forespore septum. The value obtained is consistent with evidence from electron micrographs and supports a model for the origin of spore protein in which there is no protein turnover in the developing forespore.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli A. J., Suehiro S., Sakiyama D., Takemoto J., Vivanco E., Lara J. C., Klute M. C. Release and recovery of forespores from Bacillus cereus. J Bacteriol. 1973 Sep;115(3):1159–1166. doi: 10.1128/jb.115.3.1159-1166.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W., PERRY J. J. Intracellular events occurring during endotrophic sporulation in Bacillus mycoides. J Bacteriol. 1954 Mar;67(3):295–302. doi: 10.1128/jb.67.3.295-302.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki C., Kondo M., Teshima K. [Biological nature of forespores of Bacillus subtilis]. Nihon Saikingaku Zasshi. 1967 Aug;22(8):468–471. doi: 10.3412/jsb.22.468. [DOI] [PubMed] [Google Scholar]
- Kay D., Warren S. C. Sporulation in Bacillus subtilis. Morphological changes. Biochem J. 1968 Oct;109(5):819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONRO R. E. Protein turnover and the formation of protein inclusions during sporulation of Bacillus thuringiensis. Biochem J. 1961 Nov;81:225–232. doi: 10.1042/bj0810225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelstam J. Recurring patterns during development in primitive organisms. Symp Soc Exp Biol. 1971;25:1–26. [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germination. VI. Origin of spore core and coat proteins. J Biol Chem. 1968 Sep 10;243(17):4588–4599. [PubMed] [Google Scholar]


