Abstract
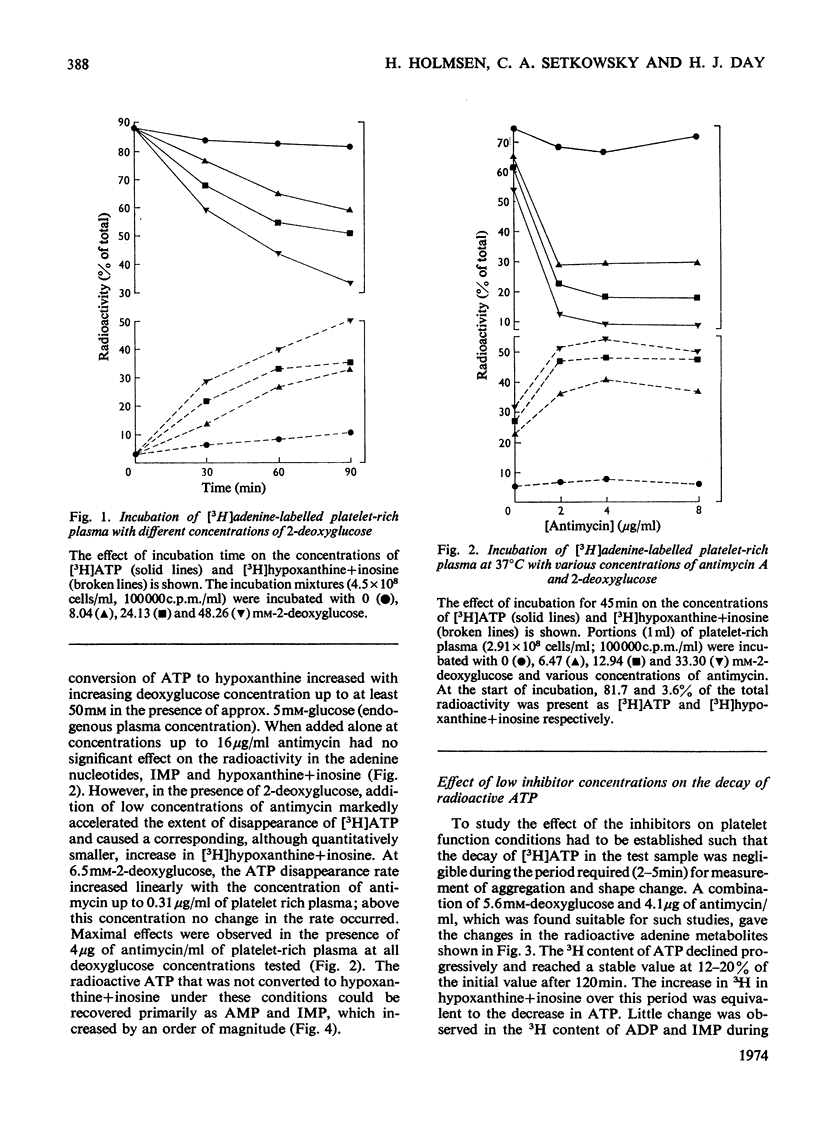
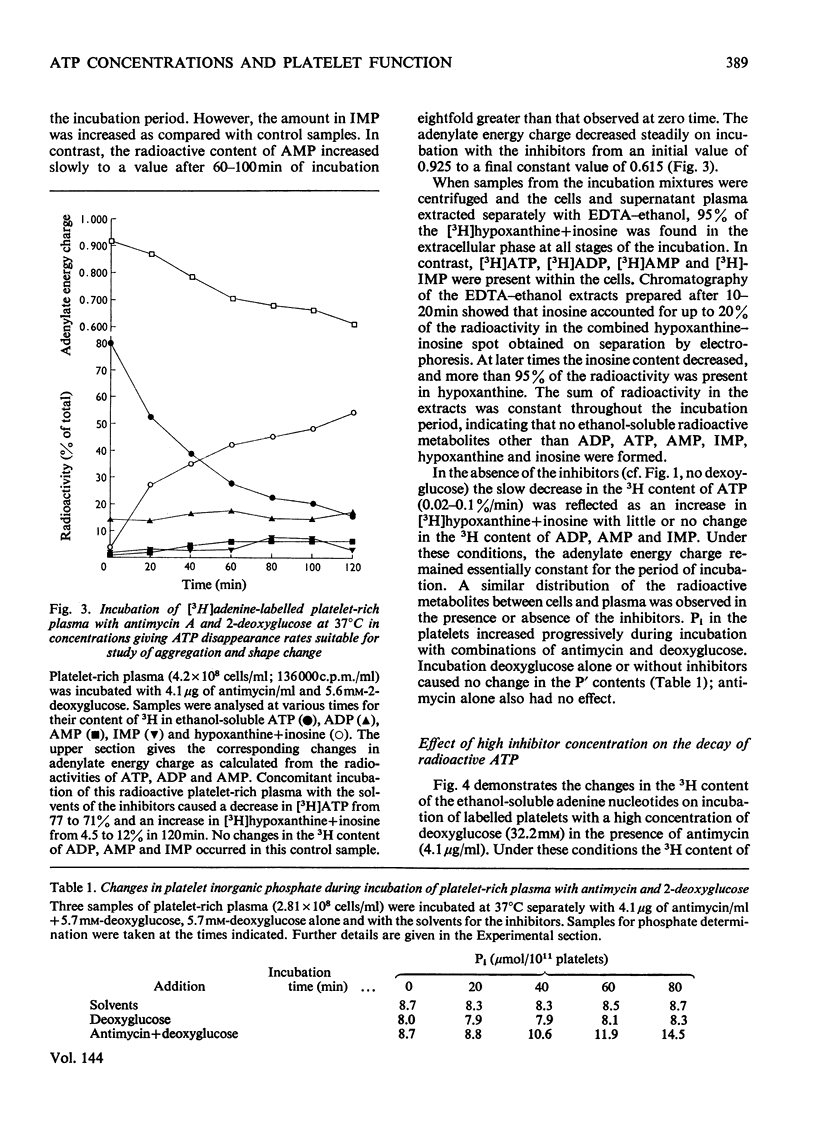
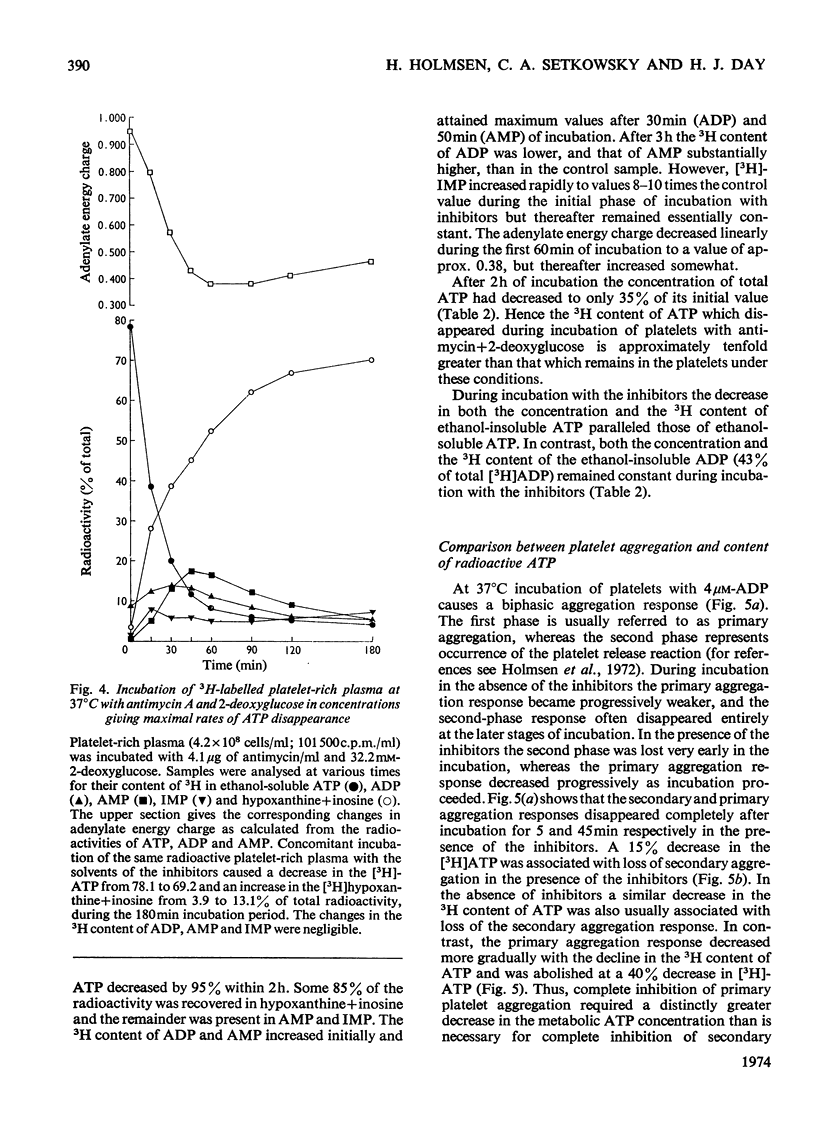
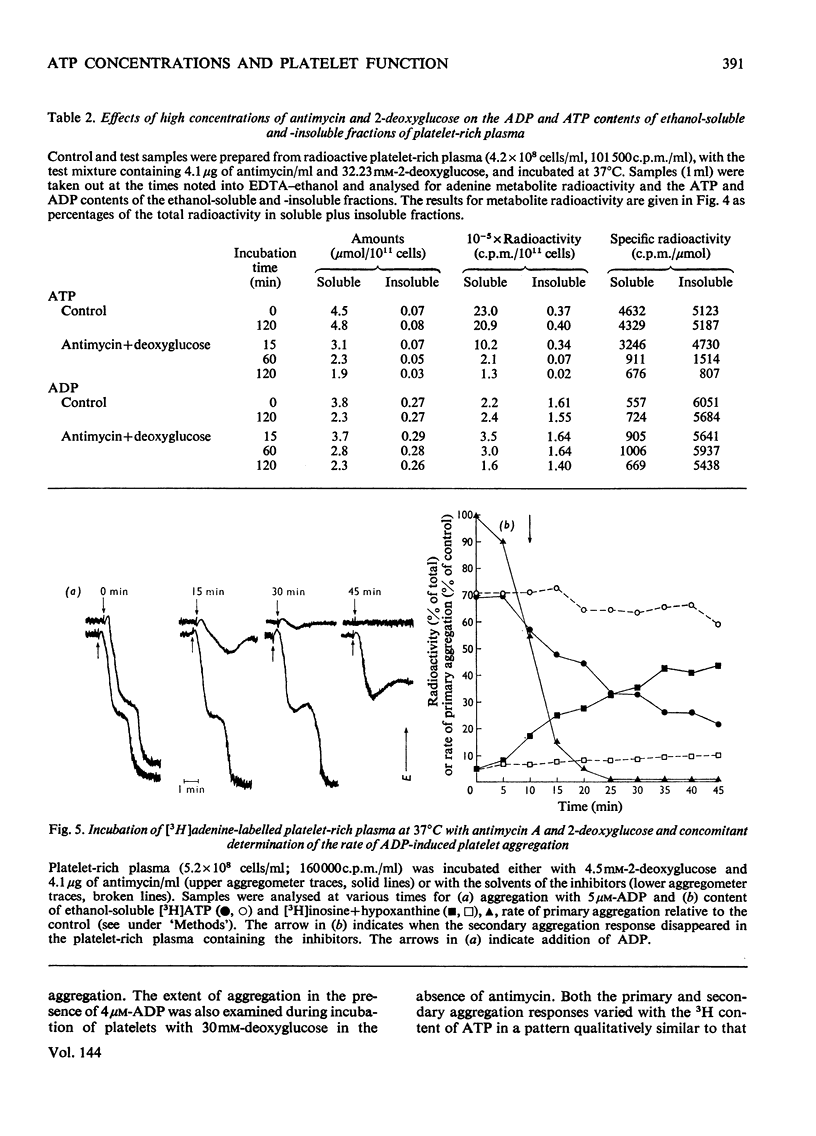
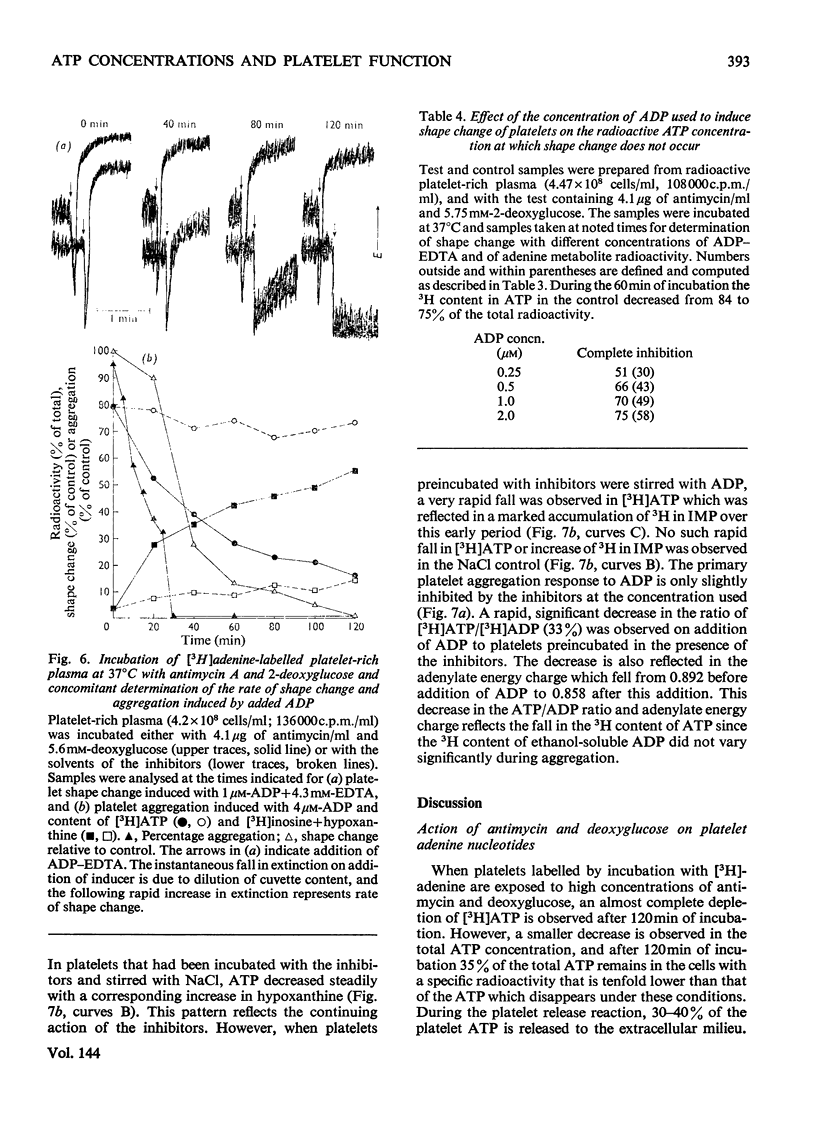
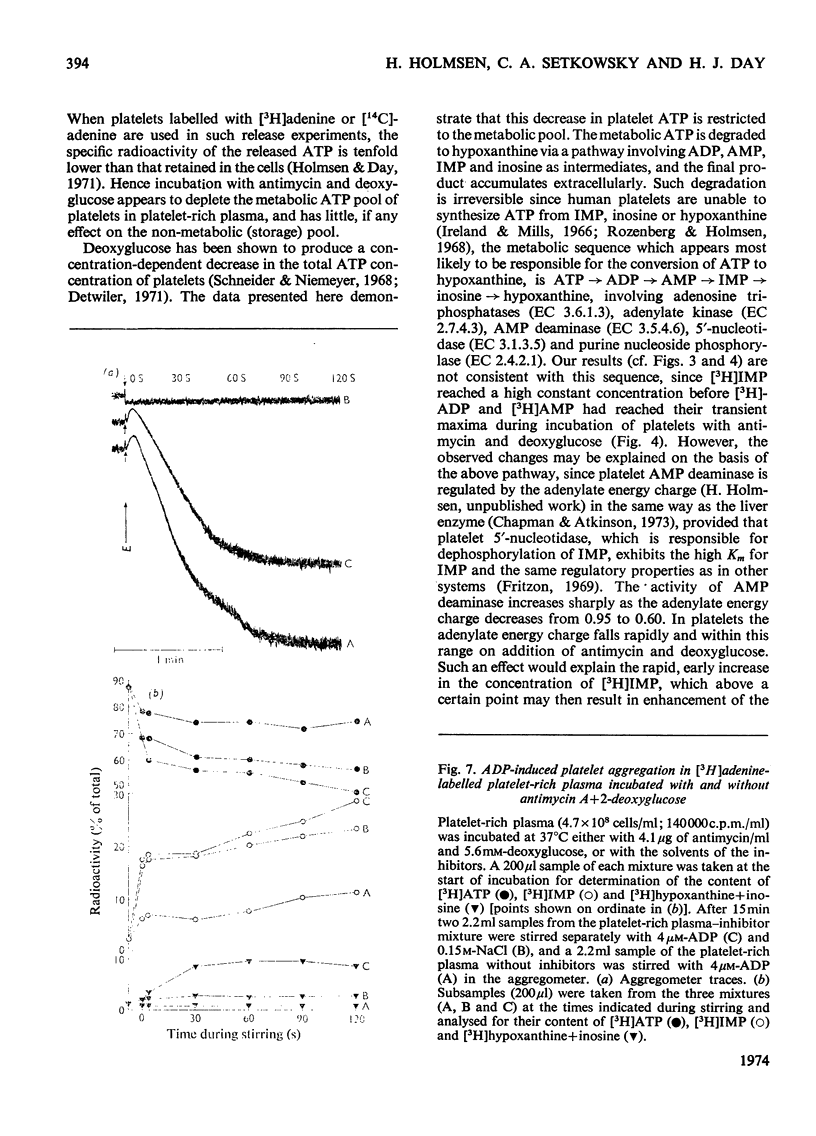
1. Human platelet-rich plasma prelabelled with [3H]adenine was incubated at 37°C with antimycin A and 2-deoxy-d-glucose. Variations in the amounts of ATP, ADP and Pi, and in the radioactivity of ATP, ADP, AMP, IMP, hypoxanthine+inosine and adenine were determined during incubation. Adrenaline- and ADP-induced platelet aggregation and the ADP-induced shape change of the platelets were determined concurrently. 2. 2-Deoxyglucose caused conversion of [3H]ATP to [3H]hypoxanthine+inosine. The rate of this conversion increased with increasing 2-deoxyglucose concentration and was markedly stimulated by addition of antimycin, which had no effect alone. At maximal ATP–hypoxanthine conversion rates, the IMP radioactivity remained at values tenfold higher than control, whereas [3H]ADP and [3H]AMP radioactivity gave variations typical for product/substrates in consecutive reactions. The specific radioactivityof ethanol-soluble platelet ATP decreased during incubation to less than one-tenth of its original value. The amounts and radioactivity of ethanol-insoluble ADP did not vary during incubation with the metabolic inhibitors. 3. The rate of ADP- and adrenaline-induced primary aggregation decreased as the amount of radioactive ATP declined, and complete inhibition of aggregation was obtained at a certain ATP concentration (metabolic ATP threshold). This threshold decreased with increasing concentration of inducer ADP. 4. Secondary platelet aggregation (release reaction) had a metabolic ATP threshold markedly higher than that of primary aggregation. 5. Shape change was gradually inhibited as the ATP radioactivity decreased, and had a metabolic ATP threshold distinctly lower than that of primary aggregation, and which decreased with increasing concentration of ADP. 6. A small but distinct fraction of [3H]ATP disappeared rapidly during the combined shape change–aggregation process induced by ADP in platelets incubated with metabolic inhibitors, whereas no ATP disappearance occurred during aggregation in their absence.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Ball G., Fulwood M., Ireland D. M., Yates P. Effect of some inhibitors of platelet aggregation on platelet nucleotides. Biochem J. 1969 Sep;114(3):669–671. doi: 10.1042/bj1140669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Day H. J., Holmsen H. Concepts of the blood platelet release reaction. Ser Haematol. 1971;4(1):3–27. [PubMed] [Google Scholar]
- Detwiler T. C. Effects of deoxyglucose on platelet metabolism. Biochim Biophys Acta. 1971 Aug 19;244(2):303–310. doi: 10.1016/0304-4165(71)90230-3. [DOI] [PubMed] [Google Scholar]
- EXER B. Uber die Wirkung von Phenylbutazon auf den Tricarbonsäurezyklus und andere Fermentsysteme. Experientia. 1956 Aug 15;12(8):294–296. doi: 10.1007/BF02159616. [DOI] [PubMed] [Google Scholar]
- Fritzson P. Nucleotidase activities in the soluble fraction of rat liver homogenate. Partial purification and properties of a 5'-nucleotidase with pH optimum 6.3. Biochim Biophys Acta. 1969 May 27;178(3):534–541. doi: 10.1016/0005-2744(69)90222-8. [DOI] [PubMed] [Google Scholar]
- HELLEM A. J., ODEGAARD A. E. INVESTIGATIONS ON ADENOSINE DIPHOSPHATE (ADP) INDUCED PLATELET ADHESIVNESS IN VITRO. II. STUDIES ON THE MECHANISM. Thromb Diath Haemorrh. 1964 Jul 31;11:305–326. [PubMed] [Google Scholar]
- Harrison M. J., Emmons P. R., Mitchell J. R. The effect of sulphydryl and enzyme inhibitors on platelet aggregation in vitro. Thromb Diath Haemorrh. 1966 Jul 31;16(1):122–133. [PubMed] [Google Scholar]
- Holmsen H., Day H. J. Adenine nucleotides and platelet function. Ser Haematol. 1971;4(1):28–58. [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Setkowsky C. A. Secretory mechanisms. Behaviour of adenine nucleotides during the platelet release reaction induced by adenosine diphosphate and adrenaline. Biochem J. 1972 Aug;129(1):67–82. doi: 10.1042/bj1290067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Storm E., Day H. J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972 Apr;46(2):489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Hereditary defect in the platelet release reaction caused by a deficiency in the storage pool of platelet adenine nucleotides. Br J Haematol. 1970 Nov;19(5):643–649. doi: 10.1111/j.1365-2141.1970.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Homsen H. Ethanol-insoluble adenine nucleotides in platelets and their possible role in platelet function. Ann N Y Acad Sci. 1972 Oct 27;201:109–121. doi: 10.1111/j.1749-6632.1972.tb16292.x. [DOI] [PubMed] [Google Scholar]
- Ireland D. M., Mills D. C. Detection and determination of adenosine diphosphate and related substances in plasma. Biochem J. 1966 May;99(2):283–296. doi: 10.1042/bj0990283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattlove H. E. Platelet ATP in ADP-induced aggregation. Am J Physiol. 1974 Feb;226(2):325–329. doi: 10.1152/ajplegacy.1974.226.2.325. [DOI] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Packham M. A., Mustard J. F. The effect of glucose on adenosine diphosphate-induced platelet aggregation. J Lab Clin Med. 1970 May;75(5):780–787. [PubMed] [Google Scholar]
- Lee Y. P., Wang M. H. Studies of the nature of the inhibitory action of inorganic phosphate, fluoride, and detergents on 5'-adenylic acid deaminase activity and on the activation by adenosine triphosphate. J Biol Chem. 1968 May 10;243(9):2260–2265. [PubMed] [Google Scholar]
- Mills D. C. Changes in the adenylate energy charge in human blood platelets induced by adenosine diphosphate. Nat New Biol. 1973 Jun 13;243(128):220–222. doi: 10.1038/newbio243220a0. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Thomas D. P. Blood platelet nucleotides in man and other species. Nature. 1969 Jun 7;222(5197):991–992. doi: 10.1038/222991a0. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. A comparative study of the action of release inducers upon platelet release and phosphorus metabolism. Biochim Biophys Acta. 1969 Oct 7;192(1):138–140. doi: 10.1016/0304-4165(69)90020-8. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Clot retraction and energy metabolism of platelets. Effect and mechanism of inhibitors. Biochim Biophys Acta. 1969 Feb 25;172(2):266–276. doi: 10.1016/0005-2728(69)90069-3. [DOI] [PubMed] [Google Scholar]
- Mürer E. H., Hellem A. J., Rozenberg M. C. Energy metabolism and platelet function. Scand J Clin Lab Invest. 1967;19(3):280–282. doi: 10.3109/00365516709090638. [DOI] [PubMed] [Google Scholar]
- Mürer E. H. Release reaction and energy metabolism in blood platelets with special reference to the burst in oxygen uptake. Biochim Biophys Acta. 1968 Oct 1;162(3):320–326. doi: 10.1016/0005-2728(68)90118-7. [DOI] [PubMed] [Google Scholar]
- Overgaard-Hansen K. Metabolic regulation of the adenine nucleotide pool. I. Studies on the transient exhaustion of the adenine nucleotides by glucose in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1965 Jul 8;104(2):330–347. [PubMed] [Google Scholar]
- ROBINSON C. W., Jr, MASON R. G., WAGNER R. H. EFFECT OF SULFHYDRYL INHIBITORS ON PLATELET AGGLUTINABILITY. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:857–861. doi: 10.3181/00379727-113-28512. [DOI] [PubMed] [Google Scholar]
- Rozenberg M. C., Holmsen H. Adenine nucleotide metabolism of blood platelets. II. Uptake of adenosine and inhibition of ADP-induced platelet aggregation. Biochim Biophys Acta. 1968 Feb 26;155(2):342–352. [PubMed] [Google Scholar]
- Salganicoff L., Fukami M. H. Energy metabolism of blood platelets. I. Isolation and properties of platelet mitochondria. Arch Biochem Biophys. 1972 Dec;153(2):726–735. doi: 10.1016/0003-9861(72)90391-8. [DOI] [PubMed] [Google Scholar]
- Sixma J. J., Holmsen H., Trieschnigg A. C. Adenine nucleotide metabolism of blood platelets. 8. Transport of adenine into human platelets. Biochim Biophys Acta. 1973 Mar 16;298(2):460–468. doi: 10.1016/0005-2736(73)90373-8. [DOI] [PubMed] [Google Scholar]
- Slater E. C. The mechanism of action of the respiratory inhibitor, antimycin. Biochim Biophys Acta. 1973 Dec 7;301(2):129–154. doi: 10.1016/0304-4173(73)90002-5. [DOI] [PubMed] [Google Scholar]
- Yushok W. D. Control mechanisms of adenine nucleotide metabolism of ascites tumor cells. J Biol Chem. 1971 Mar 25;246(6):1607–1617. [PubMed] [Google Scholar]


