Abstract
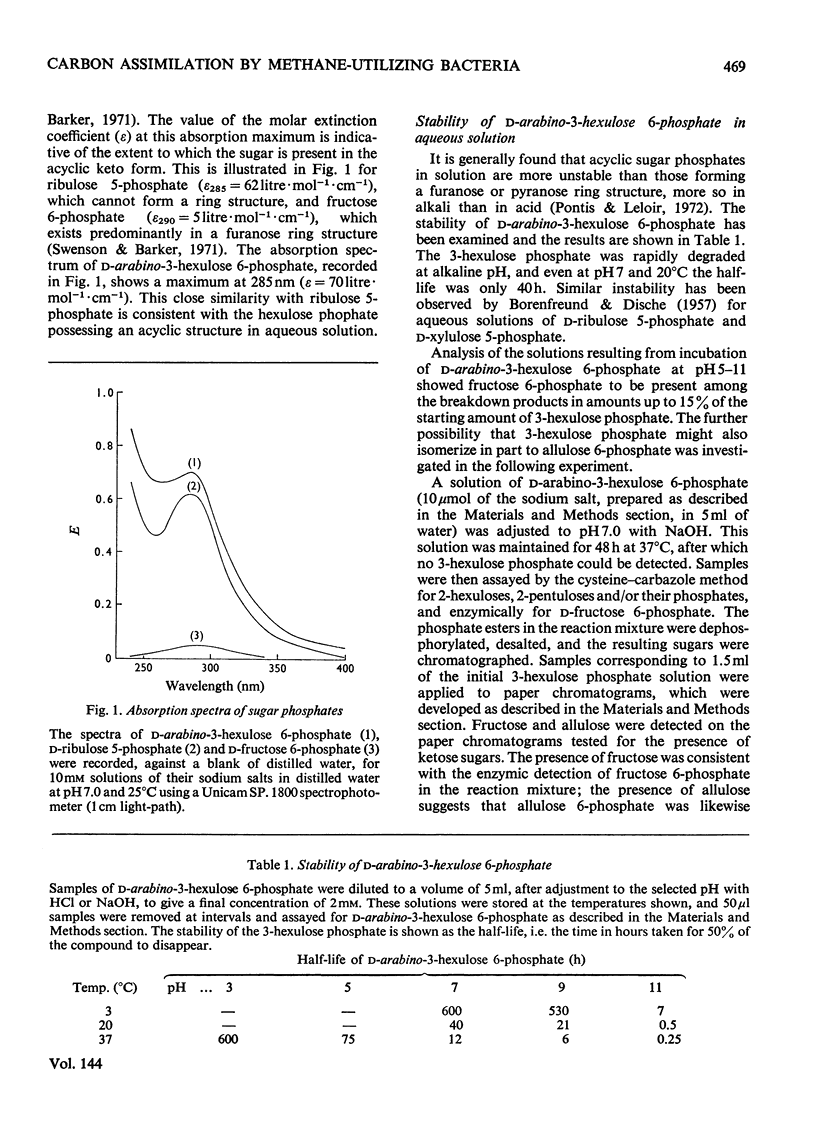
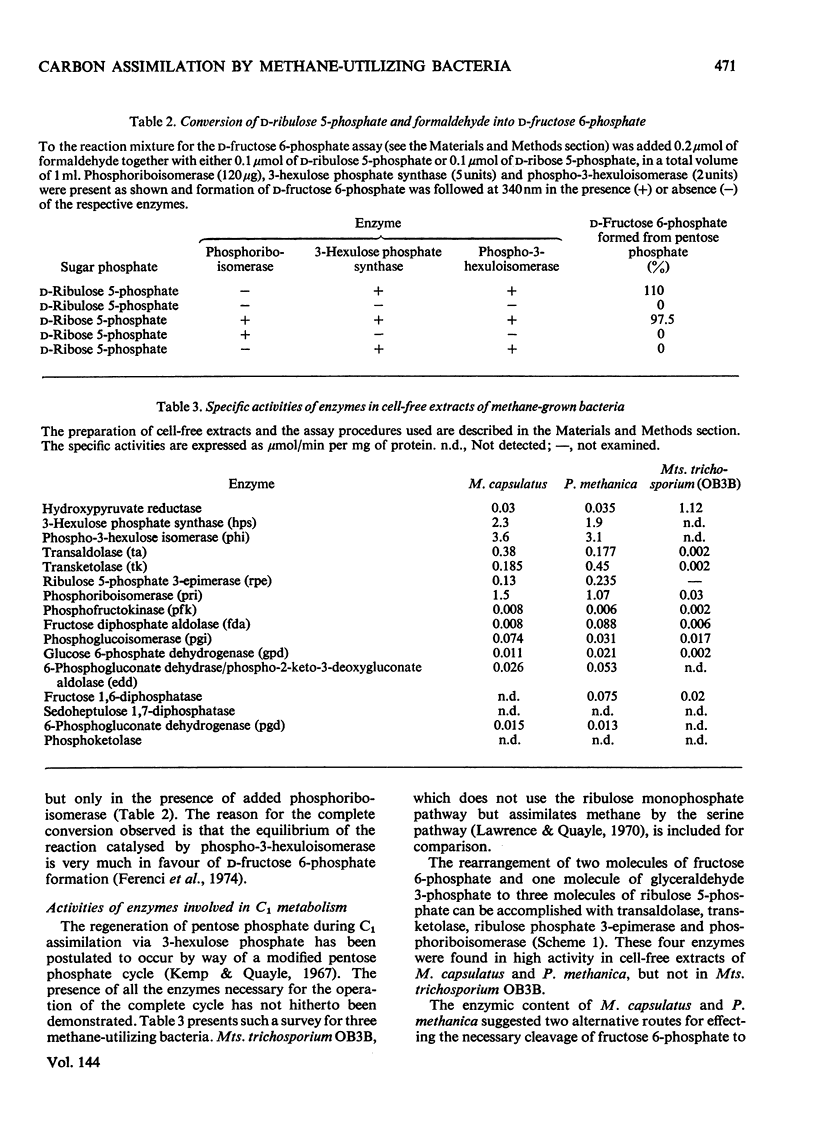
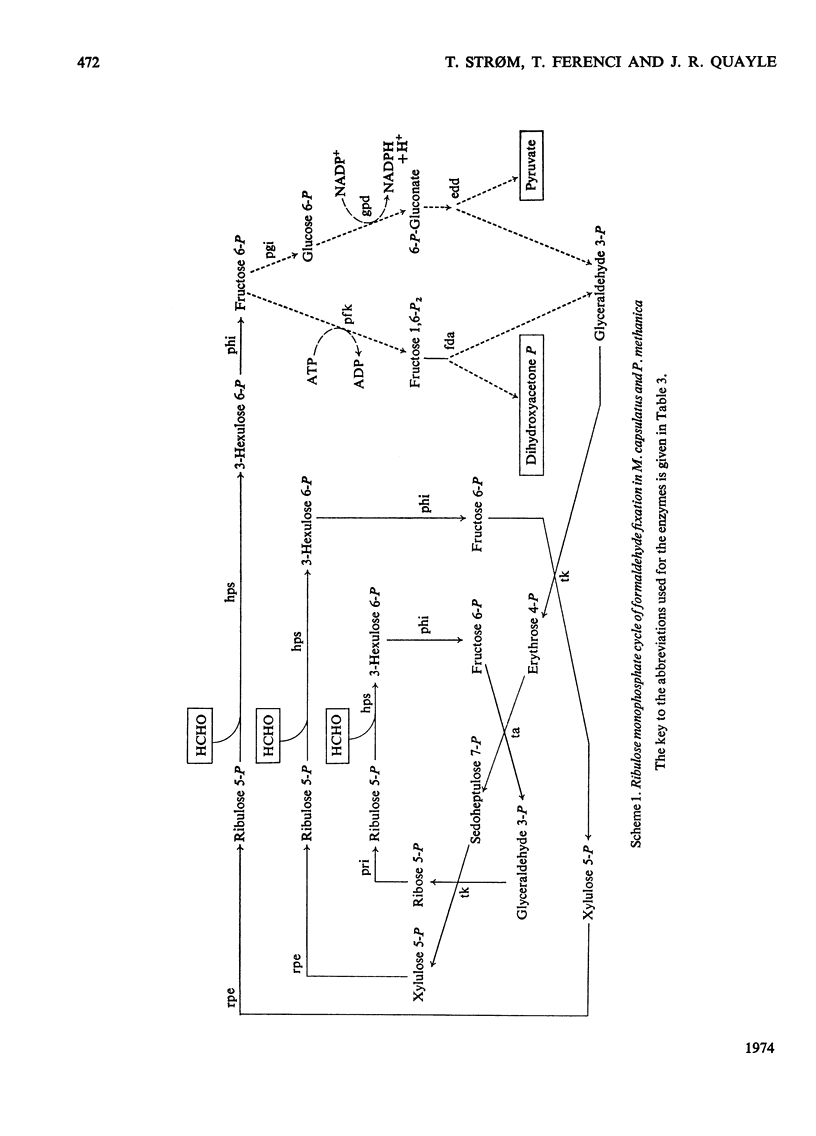
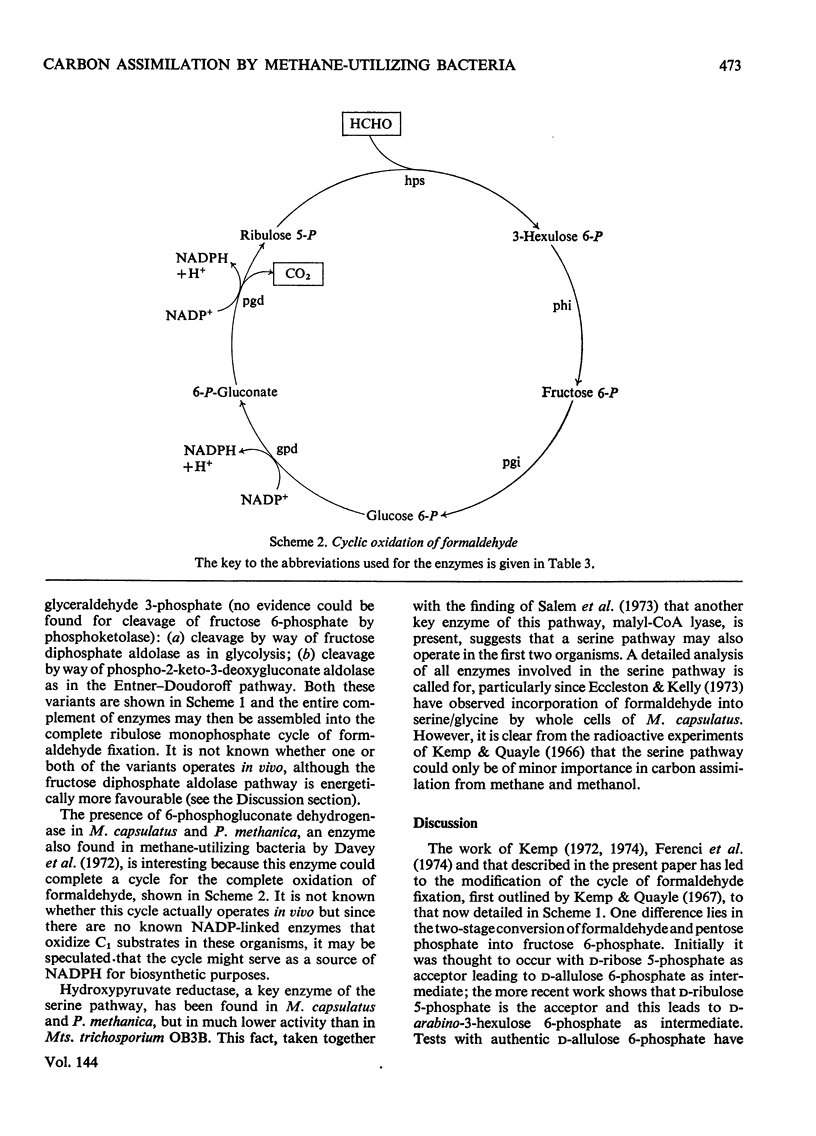
d-arabino-3-Hexulose 6-phosphate was prepared by condensation of formaldehyde with ribulose 5-phosphate in the presence of 3-hexulose phosphate synthase from methane-grown Methylococcus capsulatus. The 3-hexulose phosphate was unstable in solutions of pH greater than 3, giving a mixture of products in which, after dephosphorylation, allulose and fructose were detected. A complete conversion of d-ribulose 5-phosphate and formaldehyde into d-fructose 6-phosphate was demonstrated in the presence of 3-hexulose phosphate synthase and phospho-3-hexuloisomerase (prepared from methane-grown M. capsulatus). d-Allulose 6-phosphate was prepared from d-allose by way of d-allose 6-phosphate. No evidence was found for its metabolism by extracts of M. capsulatus, thus eliminating it as an intermediate in the carbon assimilation process of this organism. A survey was made of the enzymes involved in the regeneration of pentose phosphate during C1 assimilation via a modified pentose phosphate cycle. On the basis of the presence of the necessary enzymes, two alternative routes for cleavage of fructose 6-phosphate are suggested, one route involves fructose diphosphate aldolase and the other 6-phospho-2-keto-3-deoxygluconate aldolase. A detailed formulation of the complete ribulose monophosphate cycle of formaldehyde fixation is presented. The energy requirements for carbon assimilation by this cycle are compared with those for the serine pathway and the ribulose diphosphate cycle of carbon dioxide fixation. A cyclic scheme for oxidation of formaldehyde via 6-phosphogluconate is suggested.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWELL G., HICKMAN J. Enzymatic formation of xylulose 5-phosphate from ribose 5-phosphate in spleen. J Biol Chem. 1957 May;226(1):65–76. [PubMed] [Google Scholar]
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- BORENFREUND E., DISCHE Z. Instability of ketopentose-5-phosphates in buffer solutions of varying pH. Biochim Biophys Acta. 1957 Jul;25(1):215–216. doi: 10.1016/0006-3002(57)90454-7. [DOI] [PubMed] [Google Scholar]
- Baker D. C., Horton D., Tindall C. G., Jr Large-scale preparation of D-allose: observations on the stereoselectivity of the reduction of 1,2:5,6-di-O-isopropylidene- -D-ribo-hexofuranos-3-ulose hydrate. Carbohydr Res. 1972 Sep;24(1):192–197. doi: 10.1016/s0008-6215(00)82279-x. [DOI] [PubMed] [Google Scholar]
- Bassham J. A. The control of photosynthetic carbon metabolism. Science. 1971 May 7;172(3983):526–534. doi: 10.1126/science.172.3983.526. [DOI] [PubMed] [Google Scholar]
- Bellion E., Hersh L. B. Methylamine metabolism in a pseudomonas species. Arch Biochem Biophys. 1972 Nov;153(1):368–374. doi: 10.1016/0003-9861(72)90457-2. [DOI] [PubMed] [Google Scholar]
- Colby J., Zatman L. J. Hexose phosphate synthese and tricarboxylic acid-cycle enzymes in bacterium 4B6, an obligate methylotroph. Biochem J. 1972 Aug;128(5):1373–1376. doi: 10.1042/bj1281373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Studies on Pseudomonas methanica (Söhngen) nov. comb. J Bacteriol. 1956 Nov;72(5):646–659. doi: 10.1128/jb.72.5.646-659.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. S., Mehta R. J., Hoare D. S. New obligate methylotroph. J Bacteriol. 1972 Feb;109(2):916–921. doi: 10.1128/jb.109.2.916-921.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. F., Whittenbury R., Wilkinson J. F. The distribution in the methylobacteria of some key enzymes concerned with intermediary metabolism. Arch Mikrobiol. 1972;87(4):359–366. doi: 10.1007/BF00409135. [DOI] [PubMed] [Google Scholar]
- Diel F., Held W., Schlanderer G., Dellweg H. Comparative investigations on the metabolism of formaldehyde in the presence of ribose-5-phosphate in cell-free extracts of yeasts grown on methanol. FEBS Lett. 1974 Jan 15;38(3):274–276. doi: 10.1016/0014-5793(74)80071-2. [DOI] [PubMed] [Google Scholar]
- Eccleston M., Kelly D. P. Assimilation and toxicity of some exogenous C1 compounds, alcohols, sugars and acetate in the methane-oxidizing bacterium Methylococcus capsulatus. J Gen Microbiol. 1973 Mar;75(1):211–221. doi: 10.1099/00221287-75-1-211. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Purification and properties of 3-hexulose phosphate synthase and phospho-3-hexuloisomerase from Methylococcus capsulatus. Biochem J. 1974 Dec;144(3):477–486. doi: 10.1042/bj1440477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Davis R. H. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol. 1966 May;91(5):1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., KORNBERG H. L., BURTON K. A survey of the energy transformations in living matter. Ergeb Physiol. 1957;49:212–298. [PubMed] [Google Scholar]
- Kemp M. B. Hexose phosphate synthase from Methylcoccus capsulatus makes D-arabino-3-hexulose phosphate. Biochem J. 1974 Apr;139(1):129–134. doi: 10.1042/bj1390129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Quayle J. R. Microbial growth on C1 compounds. Uptake of [14C]formaldehyde and [14C]formate by methane-grown Pseudomonas methanica and determination of the hexose labelling pattern after brief incubation with [14C]methanol. Biochem J. 1967 Jan;102(1):94–102. doi: 10.1042/bj1020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEADBETTER E. R., FOSTER J. W. Studies on some methane-utilizing bacteria. Arch Mikrobiol. 1958;30(1):91–118. doi: 10.1007/BF00509229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Kemp M. B., Quayle J. R. Synthesis of cell constituents by methane-grown Methylococcus capsulatus and Methanomonas methanooxidans. Biochem J. 1970 Feb;116(4):631–639. doi: 10.1042/bj1160631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A. J., Quayle J. R. Alternative carbon assimilation pathways in methane-utilizing bacteria. J Gen Microbiol. 1970 Nov;63(3):371–374. doi: 10.1099/00221287-63-3-371. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Simpson F. J. The purification and properties of D-allosephosphate isomerase of Aerobacter aerogenes. Can J Microbiol. 1966 Apr;12(2):313–321. doi: 10.1139/m66-042. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLS A., DE LA FUENTE G., VILLARPALASI C., ASENSIO C. Substrate specificity and some other properties of baker's yeast hexokinase. Biochim Biophys Acta. 1958 Oct;30(1):92–101. doi: 10.1016/0006-3002(58)90245-2. [DOI] [PubMed] [Google Scholar]
- Sahm H., Wagner F. Mikrobielle Verwertung von Methanol. Einbau von Formaldehyd in Fructose- und Glucose-phosphat im zellfreien Extrakt von Candida boidinii. Arch Microbiol. 1974 Apr 19;97(2):163–168. doi: 10.1007/BF00403055. [DOI] [PubMed] [Google Scholar]
- Salem A. R., Hacking A. J., Quayle J. R. Cleavage of malyl-Coenzyme A into acetyl-Coenzyme A and glyoxylate by Pseudomonas AM1 and other C1-unit-utilizing bacteria. Biochem J. 1973 Sep;136(1):89–96. doi: 10.1042/bj1360089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz B., Mateles R. I. Methanol metabolism in pseudomonad C. J Bacteriol. 1973 Apr;114(1):390–398. doi: 10.1128/jb.114.1.390-398.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson C. A., Barker R. Proportion of keto and aldehydo forms in solutions of sugars and sugar phosphates. Biochemistry. 1971 Aug 3;10(16):3151–3154. doi: 10.1021/bi00792a026. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]


