Abstract
Environmental light exposure plays a role in the entrainment of the infant circadian rhythm, which is crucial for growth and development. This scoping review aims to evaluate existing literature linking the role of light exposure in the development of the infant circadian rhythm. This scoping review is conducted in accordance with the PRISMA-ScR guidelines. The search strategy was conducted in a total of six databases (PubMed, Cochrane Database of Systematic Reviews, Science Direct, Google Scholar, Taylor and Francis, and Wiley) as of August 2024. Reviews, narrative studies, observational studies, and experimental studies published from 2012 to 2024 were extracted. These studies discussed the role of light exposure on the development of infant circadian rhythm. A total of 25 studies were retrieved (3 observational studies, 6 experimental studies, and 16 reviews). Evidence showed that cycled lighting is beneficial for the entrainment of the infant circadian rhythm according to the 24-h light–dark cycle. Cycled lighting improved nighttime sleep and daytime wakefulness, promoting optimum growth and development. Limited experimental studies were conducted due to the ethical considerations of infants as study participants.
Conclusions: Given the benefits of cycled lighting in the development of the circadian rhythm development, it should be implemented in both healthcare and home settings to promote optimum growth and development of the infant.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-024-05951-3.
Keywords: Circadian rhythm, Neonatal, Environmental light, Infant growth, Scoping review
Introduction
The circadian clock dictates our body’s physiological activities by regulating a 24-h rhythm with respect to the environment variations. Behavioral factors that regulate circadian rhythm include light exposure, food habits, shift work, and seasonal variation [1]. Changes in the rhythmicity of the circadian system occur when there is a misalignment of the circadian clock with environmental cues, which alters the sleep–wake cycle. Circadian disruption can lead to negative health effects such as impaired glucose tolerance, cardiovascular disease, diabetes, and psychiatric disorders [2, 3] and possible link to dysregulation of ocular growth [4, 5].
Light acts as a zeitgeber which entrains the circadian rhythm to regulate rest-wake activity. Sunlight has always played an important role in the determination of melatonin rhythm onset and sleep time [6]. However, the recent modern lifestyle with the availability of artificial light has changed the lighting environment, especially during the evening and at night [7]. This could lead to the misalignment of the endogenous circadian rhythm with the external light–dark cycle, leading to disruption of the circadian rhythm.
Circadian rhythm has been shown to be essential for optimal health and well-being. Past literature has shown that circadian rhythm development starts during the fetal phase by the mother’s hormonal signalling via the placenta [1]. However, the infant’s circadian rhythm can be influenced by environmental cues during the postnatal period from as early as 7 days after birth by changing the lighting conditions and feeding patterns [2, 3]. In fact, a study conducted by Benavente et al. reported observation of a prominent P2 wave, which indicates the development of visual functions, present in the flash visually evoked potentials in full-term infants at 12 h to 5 days postnatal [4]. The P2 wave is linked to the complex interaction of the intrinsic photosensitive retinal ganglion cells (ipRGC) and cone-mediated visual processing [5, 6]. The ipRGCs are light-sensitive at birth, and their axons form the retinohypothalamic tract (RHT) which projects to suprachiasmatic nuclei and finally the pineal gland, a regulatory body responsible for rhythmic production of melatonin hormone entrains to light [7]. The development of infant circadian rhythm is important for growth, sleep–wake rhythm development, cognitive development, and long-term health outcomes [8, 9]. Hence, the periods of infant circadian rhythm development should be explored to determine the critical period for lighting interventions.
The importance of understanding the role of light exposure on the development of infant circadian rhythm warrants the research to investigate this relationship. However, the current literature was mainly focused on nutritional status and feeding practices of infants in determining growth and developmental outcome [10]. The complexity of infant development involves multiple factors, and the exclusive emphasis on infant nutrition can only explain a partial understanding of its role on infant development. Moreover, the existing literatures have been focusing on light exposure in neonatal intensive care units (NICU) which do not reflect the normal infant light exposure at home settings [11–13]. Infants spend a significant portion of time at home, and the lighting environment in home settings and NICU settings is disparate which are significant contributors to infant growth and development. Studies have shown that higher quality home environment promotes cognitive development and is associated with non-screen-based habits [14, 15].
The current scoping review is necessary to assess the existing research evidence to identify potential gaps in the role of light exposure with infant circadian rhythm development. Specifically, this scoping review focused on infants below 1 year old and without any congenital diseases while infants in NICU settings were discussed separately. Hence, the aim of this scoping review is to discuss the existing literature on the role of light exposure on infant circadian rhythm development.
Methodology
This scoping review adheres to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist, and, as such, there was no protocol registered.
Eligibility criteria
The current scoping review included reviews, narrative study, observational study, and experimental study that meets our research objective, which is to assess literature presenting the association of infant light exposure with circadian rhythm development (e.g., rest-wake activity, cortisol rhythm, melatonin rhythm). The study population for this scoping review are infants below 1 year old. Studies published from 2012 to 2024 in the English language were included. For this review, studies that reported infants with congenital diseases, physical disabilities, or developed jaundice and receiving phototherapy were excluded.
Search strategy
Six databases (PubMed, Google Scholar, Science Direct, Cochrane Database of Systematic Reviews, Taylor and Francis, and Wiley) were searched using predefined keywords as shown in Table 1. A preliminary search was done to identify articles on the topic and finalize the keywords. A manual search of publications which meets the eligibility criteria and grey literature was also conducted to ensure that no significant articles were missed. Whenever possible, Boolean operators such as OR or AND, as well as phrasal-level search, were used. The search process was conducted between 12 April 2022 and 29 August 2024.
Table 1.
Predefined keywords included in search strategy for article identification
| Concept 1: Light | Concept 2: Infant | Concept 3: Circadian Rhythm | Concept 4: Sleep |
|---|---|---|---|
|
Light Lighting Sunlight Artificial light Natural light Fluorescent light Incandescent light Light exposure Illumination Daylight Day light Blue light Light at night Environmental integrative lighting Circadian lighting Illuminance |
Infant Newborn Neonate Neonatal Early childhood Infancy Baby Pediatrics Fetal Early life Birth Premature |
Circadian Circadian rhythm Biological clock Periodicity Activity cycles Sleep wake disorder Body clock Internal clock Circadian system Circadian clock Sleep wake pattern Sleep wake disorder Circadian preference Circadian entrainment Chronological alignment Circadian misalignment Circadian disruption Circadian phase Circadian shift Circadian rhythmicity Circadian timing Rest activity cycle Rest activity pattern ipRGCs |
Sleep Slumber Sleep quality Sleep duration Sleep disorder Sleep time Sleep onset Wake time Polysomnography Sleep disturbance Sleep hygiene Sleep habit Sleep phase Wakefulness |
Study selection
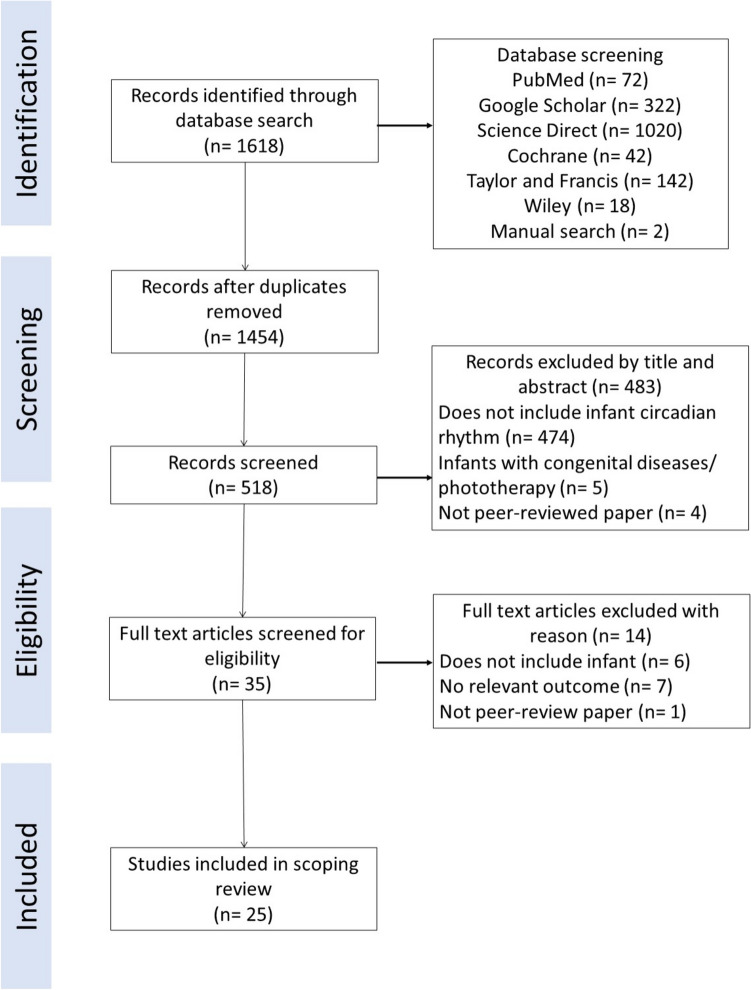
Duplicates and non-peer reviewed papers were removed. The remaining articles were selected based on title by EYK, JEFT, and SK, while abstract screening was done by EYK, JEFT, and NHMS based on the predetermined eligibility criteria. Full texts were evaluated by EYK, JEFT, SK, MT, and NAR according to the similar criteria. Any discrepancies for article selection were discussed among all authors until a consensus reached. The flow diagram of article selection was depicted in Fig. 1.
Fig. 1.
Flow diagram of article identification and selection in scoping review
Data extraction
A data extraction tool was created as a joint effort and reviewed continually by the research team. Data from the article were retrieved and compiled in a systematic manner using the adapted data collection instrument from JBI System for the Unified Management, Assessment and Review of Information (JBI SUMARI; JBI, Adelaide, Australia) relevant to the review’s objectives. The data included standard article information (author, year of publication, country), study design, settings, aims and objectives, study population, sample size, methodology, and key findings of study. EYK, SK, MT, NAR, and JEFT extracted the data independently and charted the data based on thematic analysis. Thematic analysis is an analysis technique used to identify, analyze, organize, describe, and reporting themes recognized within the extracted data [16]. Four themes were developed based on similarities in key findings or methodology. The main themes were evaluated among the reviewers, and any disagreements were resolved with agreement of all authors.
Results
A total of 1618 articles were identified through the search strategy as depicted in Fig. 1. After removing the duplicates and screened for title and abstracts, 35 full text articles were screened for eligibility. The excluded articles did not meet the eligibility criteria, such as did not meet the study objectives, study participants of infants with congenital diseases or jaundice, or the paper was not peer-reviewed. The final number of articles selected for this scoping review was 25.
Among the 25 articles included, there were 16 review articles, consisting of 12 narrative reviews, one scoping review, and three systematic reviews. There were six experimental studies retrieved, of which one was a nested intervention study, and four randomized controlled trials. Additionally, there were three observational studies retrieved, with two cross-sectional studies and one with longitudinal design. The studies were conducted in different regions, namely Europe (n = 11), North America (n = 7), Asia (n = 6), and South America (n = 1).
Observational studies
In terms of observational studies as seen in Table 2, a cross-sectional study with 22 infants was conducted to determine the association of light exposure and circadian rest-wake activity [17]. Light exposure and rest-wake activity were determined using a light-activity actigraphic monitor and a sleep-activity record. Another cohort study involved 72 healthy 8-month-old infants which assessed the sleep architecture during different seasons of birth (spring, summer, autumn, and winter) [18]. Infants were divided into four subgroups representing the four birth seasons with different levels of light exposure and underwent ambulatory overnight polysomnography. Another recent cross-sectional study was conducted among 43 infants to determine the role of light exposure and co-sleeping with sleep consolidation into nighttime [19]. Infant sleep parameters were recorded using actigraphy for four consecutive days, while light stimulation and co-sleeping behavior data was obtained from a sleep events diary and questionnaire respectively.
Table 2.
Observational studies (n = 3)
| Author | Study design | Participants and sample size | Location of study conducted | Methodology/approach | Key findings |
|---|---|---|---|---|---|
| Yoshida et al. (2023) | Cross-sectional study | 43 infants aged 3 to 5 months | Home | Sleep parameters and light exposure of the infant was recorded via actigraphy for four consecutive days, a sleep events diary, and a questionnaire completed by the mother | Light exposure during daytime promoted efficient sleep at night and daytime waking. Co-sleeping consolidated nighttime sleep among infants. |
| Karki et al. (2020) | Cohort study | 72 healthy 8-month old infants | Home | Sleep architecture was recorded among infants at 8 months via ambulatory overnight polysomnography (PSG). Infants were categorized into four subgroups based on the amount of light at their birth seasons and at home. A 1-night PSG was conducted at home | No significant impact observed for sleep architecture of infants based on season of birth. However, less N3 sleep and more N2 sleep was detected among infant’s PSG in spring than in autumn. Infants’ sleep is affected by the season or light environment which is the same as adults. |
| Tsai et al. (2012) | Cross-sectional study | 22 infants aged 1 to 2 months | Home | Infant light exposure and activity were monitored using an actigraphy device (Actiwatch-L) for seven continuous days. A sleep activity record was completed by the parent to record infant sleep behavior at 15-min interval | Increased duration of daily exposure to > 100 lx of illumination and increased amplitude of circadian rhythm of light were associated with stronger circadian patterns of infant activity. |
Experimental studies
Experimental studies were focused among preterm infants in hospital environment, whereby the common aim was to determine the outcomes of preterm infants in different light environments [Table 3]. Guyer et al. (2015) had an experimental group with 34 preterm infants, while 14 term infants were selected as control [12]. Another randomized interventional study compared 38 preterm infants in NICU grouped into light–dark cycle group and continuous light group [20]. Intervention groups from both studies were exposed to cycled lighting by allowing light exposure from 7 a.m. to 7 p.m. and off during other timing. Lighting to imitate “dark” hours were done by installation of bed curtains, usage of quilts on incubators, or using a specially designed acrylic helmet. Assessment of circadian rhythm was found to be done using actigraphy recording or salivary melatonin rhythm. On the other hand, a randomized controlled trial had a group of 42 preterm infants randomly assigned to either experimental group with red LED light, or control group with white LED light exposure at night, both groups having approximately the same level of daytime lighting exposure [13]. Actigraphy data was obtained to assess the activity during daytime and nighttime. The study conducted by Watanabe et al. reported infants in the experimental group were placed under a light filter which cuts the wavelength that could be detected by melanopsin and rhodopsin in the eye, while the control group infants were placed in normal clear covers. The light filter mimics an artificial night in a continuous bright light environment in the NICU, which provides a light–dark cycle environment to the infant [21]. Similarly, using an incubator cover, Varvara and colleagues reported that there was increased duration of deep sleep as the intensity of light at night mimics near darkness to consolidate sleeping patterns among preterm infants [22]. On the other hand, Valizadeh et al. conducted a randomized controlled trial which both groups were placed into incubators with thick cover, while the intervention group had face covers to reduce light exposure [23]. It was observed that sleep duration was significantly longer among the intervention group, while the control group had decreased sleep periods instead.
Table 3.
Experimental/intervention studies (n = 6)
| Author | Study design | Participants and sample size | Location of study conducted | Methodology/approach | Key findings |
|---|---|---|---|---|---|
| Guyer et al. (2015) | Prospective cohort with nested intervention trial | 34 preterm (< 32 weeks of gestational age) + 14 term infants | NICU | Preterm infants were divided into two experimental groups: cycled lighting and dim light condition, while term infants served as control group. Cycled lighting was achieved by limiting light exposure through taking away the curtains and turning on overhead room lights during daytime (7:00 a.m. to 7:00 p.m.) Dim light condition was the standard condition in ward, with bed curtains opened only for few minutes every 3 to 4 h during feeding time. Actigraphy data was obtain for ten consecutive days complemented with a sleep diary completed by the parent | Nighttime sleep and longest consolidated sleep period between 12 a.m. and 6 a.m. was longer among preterm infants compared to term infants. Among the experimental group, infants in cycled light had the longest nighttime sleep duration, while dim light group was the least active. |
| Kaneshi et al. (2016) | Randomized controlled trial | 42 preterm infants (< 36 weeks of gestational age) | NICU | Preterm infants were randomly assigned to periodic exposure (according to light–dark cycle, 15 h light – 06:00 a.m. to 9:00 p.m. and 9 h dark – 9:00 p.m. to 06:00 a.m.) of either white or red LED lighting | No significant differences observed between the two groups. Light exposure for less than 15 min at 3- to 4-h interval does not interfere with the circadian rhythm development if they are under regular light–dark cycles. |
| Vasquez Ruiz et al. (2014) | Randomized control trial | 38 preterm infants (32 weeks gestational age) | NICU | Preterm infants were divided into two groups: light dark environment (LD) or traditional continuous light (LL) | Infants in light dark environment had promoted rapid weight gain and a decrease in hospitalization period, suggesting beneficial implications for the infant to be incorporated into home environment and reducing nosocomial infections from the hospital. |
| Watanabe et al. (2013) | Randomized control trial | 39 preterm infants (35 weeks gestational age) | NICU | The experimental group (n = 19) were placed under a light filter (red filter) (> 30 lx, between 5:30 a.m. and 8:00 p.m.; < 30 lx, between 8:00 p.m. and 5:30 a.m.) which cuts the wavelength that could be detected by melanopsin and rhodopsin in the eye, while the control group (n = 20) were placed in normal clear covers (> 30 lx). Actigraphy data, illuminance in the incubator, and weight gain were recorded | The experimental group had increased activity during daytime, and increased weight gain compared to those without the filter. This difference is more apparent as the infant age due to the maturation of the eye. |
| Varvara et al. (2016) | Randomized controlled trial | 32 neonates with postmenstrual age of at least 31 weeks | NICU | Amplitude integrated electroencephalogram (aEEG) was used to record sleep for two consecutive meal intervals between 8:00 a.m. and 12:00 a.m., for three successive days. Sound intensity was measured with a sound level meter, while light intensity was assessed using a lux meter device. The baseline light intensity was 204 ± 29 lx; after installing incubator covers on the third day, light intensity was recorded at 1.45 ± 0.35 lx | Deep sleep (NREM) was increased as the intensity of sound and light was reduced. Light intensity reduction is significantly beneficial for the neonatal central nervous system development identified by the increase of quiet sleep duration. |
| Valizadeh et al. (2017) | Randomized controlled trial | 60 preterm infants (28 – 32 weeks gestational age) | NICU | Preterm infants were randomly categorized into intervention and observation group. Incubators from both groups were covered with a thick cover, while only the infants from intervention groups had their faces covered with a face cover to reduce light exposure mimicking near darkness conditions | Sleep periods were reported to be significantly longer among the intervention groups with 59 min of increase from first to sixth day, compared to control group which sleep periods decreased by 67 min. |
Review studies
The findings of the review articles from Table 4 were explored in a few themes, such as the periods of infant circadian rhythm development, consequences of infant circadian rhythm disruption, cycled lighting vs continuous lighting, and other recommended lighting conditions.
Table 4.
Review studies (n = 16)
| Author | Study design | Aim/objectives | Key findings |
|---|---|---|---|
| Brooks et al. (2013) | Narrative review | To discuss the timing of the circadian system at postnatal period and current existing literature on the long-term effects of light environment | Circadian rhythm starts to develop during the fetal period. Immediately after birth, it is entrained by the maternal circadian system instead of directly via light exposure. The environmental influence will be in favor at 10 days after birth via entrainment through the suprachiasmatic nucleus (SCN). |
| Correia and Lourenco (2020) | Scoping review (9 articles) | To review the existing literature addressing strategies to promote newborns’ sleep in the NICU | Cycled lighting, which simulates the daytime and nighttime environment, supports the development of the circadian rhythm of newborns in NICU environment. |
| Engwall et al. (2014) | Systematic review (15 articles) | To assess literature reporting the outcomes and measurements of cycled light in ICU | Cycled lighting shown positive effect for preterm infants in terms of their weight gain and development. It was reported that preterm infants in NICU with cycled lighting significantly shorter time on ventilator and hospital stay, indicating the beneficial role of cycled lighting for preterm infants’ physiological development |
| Escobar et al. (2021) | Narrative review | To explore the endogenous functionality of the circadian system in the early stages of development |
Fetal SCN starts to function at the last trimester of pregnancy. Rhythmicity is not apparent in preterm infants until 6 weeks after birth. Preterm infants exposed to light–dark (LD) cycle environment for more than 2 weeks before hospital discharge promoted sleep–wake patterns aligned with the LD cycle, which promotes adaptive responses in the baby. Continuous light exposure constantly stimulates the SCN, resulting in loss of circadian rhythm. |
| Fonken and Nelson (2016) | Narrative review | To discuss the development of the mammalian circadian system and implications of disruptive light exposure during early critical periods | Early lighting environment can affect sleep and biological rhythms, ultimately affecting physiological and psychological development. Disturbances in sleep and circadian rhythm implicate the etiology of mood disorders including schizophrenia. |
| Hazelhoff et al. (2021) | Narrative review |
To review the effects of light–dark cycles on clinical outcomes of preterm neonates in the NICU and its alignment with the development of the circadian system |
Light–dark cycles in the NICU have beneficial effects on preterm infants such as weight gain and hospitalization time than infants exposed to constant light or constant near-darkness. |
| Logan and McClung (2019) | Narrative review | To identify research gaps between circadian disruption and disorders of the central nervous system | Circadian rhythms can be entrained by light exposure after birth, which is beneficial for premature infants in terms of shortening hospitalization, maturation of sleep–wake activity, and improved temperament. Disrupted circadian rhythms have been associated with neurodevelopmental disorders such as ADHD, autism, and Parkinson’s disease. |
| McKenna et al. (2018) | Narrative review | To review evidence to support a chronobiological approach to neonatal care |
The neonatal circadian rhythms entrain via the light–dark cycle gradually, starting with an increase in amplitude, which progressively becomes more stable upon 3 months after birth The influence of birth seasons with their sleep–wake rhythm shows that more “morning types” were born in the shorter photoperiods of autumn and winter, while “night owls” are more likely to be born in spring and summer, with greater variations at higher latitudes. Repeated exposures to bright light can be solved by infant goggles or helmets and incubator light filters. |
| Morag et al. (2016) | Narrative review | To review effectiveness of cycled lighting and irregularly dimmed light among preterm infants | Infants exposed to cycled light (CL) reported improved growth at 3 months compared with those exposed to continuous bright light. Length of hospital stay was shortened with cycled light in the nursery compared with near darkness or with continuous bright light. CL contributed to improved birth weight, total movements focused during daytime, and total night sleep hours. Cycled light could be defined as less than 20 lx during the nighttime for 12 h and greater than 200 lx during the daytime with 12 h; “Near darkness” is defined as less than 20 lx for 24 h. |
| Ramachandran et al. (2013) | Narrative review | To review the Developmental Care Interventions (DCI) for preterm very low birth weight infants | The infant visual pathway matures around 39 to 40 weeks. Hence, protective measures such as rhythmic low level ambient lights for entrainment of circadian rhythm, prevention of eyes from direct light exposure, and facilitation of sleep cycles should be implemented. Infants exposed to cycled lighting in NICU had improved weight gain, earlier transition to oral feeding, and shorter hospital stay. |
| Rodriguez et al. (2016) | Narrative review | To discuss practical recommendations for an appropriate lighting environment in NICU | A cycled lighting schedule should be implemented to achieve optimum growth and development of preterm infants. Daytime light should be between 100 and 200 lx, with some exposure to natural light. At night, artificial light should be lower than 50 lx. |
| Santos et al. (2015) | Narrative review | To summarize existing literature of exposures in NICU environment and its role in neurodevelopmental outcomes of preterm infants | The illuminance recommendation for NICU environment is cycled lighting between 10 and 600 lx. However, standard levels of hospital lighting are continuous bright light of 400–1000 lx, which disrupts the circadian rhythm. Cycled lighting has been found to improve motor skills development, improved stability, and growth. Light exposure can be controlled in NICU environment using accessories such as light-reducing goggles and acrylic helmets, which mimics the light–dark cycle and at the same time, does not affect lighting required for healthcare routine. |
| van den Hoogen et al. (2017) | Systematic review (14 studies) | To systematically review the evidence determining NICU-based interventions promoting neonatal sleep | Interventions in optimizing NICU environment such as cycled lighting did not show significant improvement in sleep duration. Polysomnography is recommended for future studies to assess sleep quality and the effectiveness of light interventions. |
| Wong et al. (2022) | Narrative review | To assess the early stages of circadian rhythm development and common factors resulting in circadian misalignment | Prenatal circadian rhythm is entrained by maternal signals environmental exposures dictates the infant circadian rhythm during postnatal period, complemented by maternal signal synchronization via breastmilk. During pregnancy, a woman’s physiological determines the onset of circadian clock for the fetus. Exposure to light at night disrupts the circadian system, leading to circadian entrainment via external cues, and adaptation to environmental stimuli. |
| Zores‐Koenig et al. (2020) | Narrative review | To develop a practical recommendations of optimal light environment for neonatal intensive care units | Light protection should be used for infants of < 32 weeks of postmenstrual age and but must be individualized to each infant. Infants should not be exposed to continuous high light levels regardless of their term and postnatal age. Cycled light before discharge seemed to be safe and beneficial. For medical caregivers’ well-being, higher light levels and access to natural light are recommended. Special attention should be given to protecting neonatal patients from high light levels that may be necessary when performing specific care procedures. |
| Lewis et al. (2024) | Systematic review (56 studies) | To review existing evidence related to chronobiological factors and human neonates in NICUs and nursery wards | The benefits of cycled light had weak supporting evidence due to limitations in study design and the nature of population which are in the NICU, which findings cannot be generalized, and long-term effects are not determined. However, cycled lighting was still the best option, and there were no detrimental effects reported for neonatal health. |
The phases of infant circadian rhythm development
The infant circadian rhythm development involving light exposure occurs in four phases. Firstly, the main oscillator of the circadian rhythm, the SCN, starts developing during the fetal phase; however, it is only coordinated during the postnatal period [8]. The entrainment of infant circadian rhythm is guided via environmental cues and breastmilk consumption, which was influenced by content of melatonin and cortisol during the postnatal period [24]. Next, the infant cortisol rhythm responsible for wakefulness develops as early as 8 weeks, followed by melatonin rhythm establishment at approximately 9 weeks for sleep onset [2]. The maturation of visual pathways is the last phase which occurs around 39 to 40 weeks after birth with proper exposure of rhythmic ambient lights to entrain the circadian rhythm [25]. During the neonatal period, the VIP-expressing neurons of the SCN continues to develop and reaches full maturation by the first 2 years of life [24]. Hence, the entrainment of infant circadian rhythm occurs in separate stages before the full maturation of the SCN, indicating the need for optimum environmental cues such as avoiding artificial light at night and maternal breastfeeding, to ensure the development of SCN pathway.
On the other hand, preterm infants had different phases in circadian rhythm development. From Escobar et al. (2021), the circadian rhythm of preterm infants is not apparent until 6 weeks after birth [9]. Preterm infants were also found to have prematurely lost the circadian connection from the pregnant mother, and their circadian rhythm was replaced with environmental cues following the light–dark cycle. This can be noted from the distinct sleep–wake rhythm development of preterm infants when placed under cycled light compared to term infants who were placed under the same conditions, indicating the role of external time cues may mask the influence of the maternal rhythm [24].
Difference in seasonal effects will also determine the circadian rhythm development due to the different photoperiods. Shorter photoperiods during autumn and winter were related to more “early-morning” types born, and more “night owls” born during spring and summer with longer photoperiods, and this effect is most prominent among population from higher latitudes where the variation of photoperiod is greatest [26]. Seasonal variation of birth time had an impact on infant’s sleep at 8 months, notably infants born in spring had less slow wave sleep (N3) and longer stage two non-rapid eye movement (N2) sleep compared to those born in autumn [18]. This finding was consistent with another large cohort study with 1302 pairs of mother-infant dyads, reporting that although infant sleep patterns are similar to their mothers, their nighttime sleep duration was longer during spring compared to autumn [27].
Consequences of infant circadian rhythm disruption
The infant’s lighting environment can disrupt the circadian rhythm development by affecting their sleep–wake cycle. The disruption of circadian rhythm during early life implicates the etiology of mood disorders such as schizophrenia [28]. Circadian dysfunction at the developmental stage of life was also associated with brain disorders such as autism, depression, and Parkinson’s disease [29].
Cycled lighting vs continuous lighting
Cycled lighting was deployed to simulate the daytime and nighttime environment, and was reported to influence hormonal production which is essential in the healing processes [30]. It has been a favorable method to entrain the infant circadian rhythm, as observed from reduced fussiness and improved sleep [11]. A regulated behavioral state after birth can be assessed by reduced daytime crying patterns and consolidated sleep [31]. From the study, the cycled light group showed a day and night period of 23.99 ± 0.04 h, while the dim light group was longer than the 24-h period with 24.77 ± 0.3 h as measured using an actigraphy watch. This shows that cycled lighting can entrain the sleep–wake rhythm to adapt to the 24-h light dark cycle. Studies also found that infant circadian rhythm was entrained with a low intensity (200 lx) cycled light environment rather than constant dim light [24].
Among preterm infants, sleep–wake cycle synchronized with the 24-h light dark cycle was found to shorten the period of hospitalization and promoted the adaptive responses such as heart rate, food responsiveness, and body weight when exposed to more than 2 weeks of cycled lighting [24, 26, 32, 33]. Light reduction was also shown to improve infant stability, including their respiratory rate and heart rate [34]. It was also reported that cycled-lighting was beneficial and showed no detrimental effects compared to continuous lighting or darkness [35]. Regardless, the lighting conditions should be personalized according to the infants condition, healthcare providers’ visibility to conduct nursing care, and tools used for lighting protection [36].
Observational studies have shown that cycled lighting plays an important role in establishing the infant circadian rhythm, particularly in aligning the sleep–wake schedule according to the 24-h light dark cycle. Cycled lighting was found to contribute to longer nighttime sleep duration between midnight and 6 a.m. among preterm infants compared to term infants, while dim light exposure resulted in less active preterm infants [12]. It also promoted rapid weight gain and significantly decreased the duration of hospital stay for preterm infants, as well as reducing nosocomial infection [20]. When investigating the role of different colors of LED light on preterm infants’ rest-wake activity patterns, there were no significant differences between control (white LED light) and experimental groups (red LED light) [13]. Preterm infants were not able to detect the red LED light with a wavelength of > 675 nm as the retinal photoreceptors were premature. On the other hand, the white LED light consists of a wider range of wavelengths which the preterm infant was able to receive. However, the brief white light exposure was overwhelmed by the effects of cycled lighting. Hence, the development of infant circadian rhythm mainly depends on the light exposure according to the 24-h light dark cycle instead of the color of light source. A summary of cycled lighting timing, intensity, and its outcomes from the reported studies was depicted in Fig. 2.
Fig. 2.
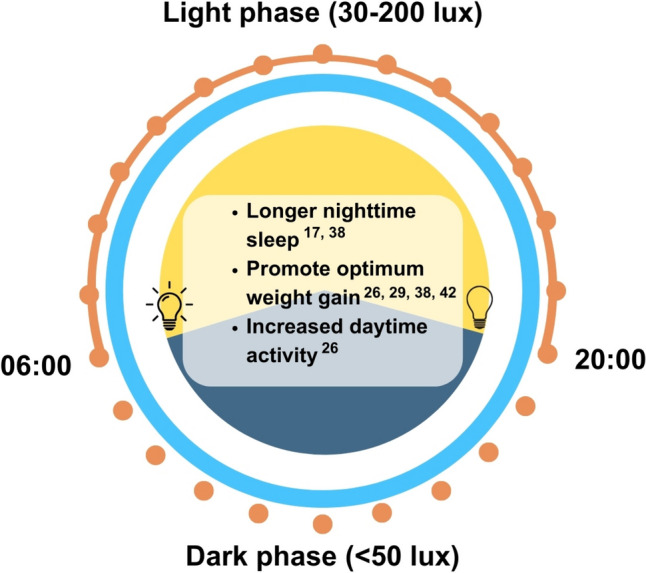
Timing, intensity, and outcomes of cycled lighting exposure. This figure is based on the findings of this review which excludes natural light exposure
Other recommended lighting conditions
When the lux levels remained constant at 249 ± 11 lx, melatonin level was not stimulated and remained low, while cycled lighting of 249 ± 11 lx during the day and 27 ± 0.8 lx at night exhibited a daily rhythmicity of salivary melatonin among preterm infants [9], while Morag et al. suggested that cycled lighting conditions can be defined as less than 20 lx for 12 h at night, greater than 200 lx for 12 h during the day [33]. Rodriguez et al. stated that cycled light with 100 to 200 lx during the day and artificial light lower than 50 lx should be implemented in NICU settings [34]. There were also studies reported that standard levels of hospital lighting was 400 to 1000 lx, while the recommended levels should be 10 to 600 lx [26, 37].
There is a need to balance the preservation of nighttime darkness with lighting conditions to perform medical procedures safely in the NICU as even a short period of 5 min of bright light exposure can delay the melatonin phase by 3 h [26]. Hence, protective accessories to avoid bright light exposure such as infant goggles or helmets, and installing a light filter in incubators could be implemented to retain the dark condition [21, 26]. Other strategies such as health promotion programs to increase awareness of the importance of sleep and circadian health should be executed, as well as providing sufficient human resource and technical support [38]. From observational studies, results showed that duration of light exposure with intensity of more than 500 lx was a significant predictor of infant circadian rhythm development measured by greater amplitudes in acrophases of rest-wake activity (β = 0.50, p = 0.01), indicating stronger circadian activity [17]. Recent studies looking into lighting environments in home settings have reported that more daytime light exposure was associated with improve daytime wakefulness of infants and improves sleep efficiency at night, indicating less nighttime waking and prolonged night sleep for the infant [39].
Discussion
To the best of our knowledge, this is the first scoping review that attempted to distinguish the role of light exposure on infant circadian rhythm development including preterm and term infants from NICU and natural environments. To date, many studies have focused on determining the role of light exposure on sleep rhythm development among preterm infants rather than infants born full term. This is because preterm infants were considered a highly vulnerable population to neurodevelopmental issues due to environmental exposures in the NICU [37]. Traditional lighting—continuous bright light in the NICU has posed negative impacts in the preterm infant development in terms of emotional and physical [40]. Cycled lighting was also found to have increased body weight and reduction in length of hospital stay [41]. Hence, the emergence of experimental studies had resulted in formal recommendations for NICU lighting conditions from reducing light intensity, to suggesting cycled lighting to improve preterm infant health outcomes [34]. These findings are not only significant within the healthcare community, but also warrant for collaborative work from other industries such as architecture and engineering to build a healthy and optimal environment for the infant.
Although findings from the current literature search suggest that infant circadian rhythm was established from as early as 8 weeks postnatal for term infants, and 6 weeks postnatal for preterm infants, studies should also explore the role of in-utero circadian rhythm development. As mentioned previously, circadian rhythms are entrained via environmental cues, especially among preterm infants as they are separated from the maternal environmental prematurely, reducing the opportunity for in-utero circadian entrainment [8]. In the NICU, there are many factors disrupting the circadian rhythm such as continuous light exposure or darkness, noise exposure, erratic feeding schedules, and stressful clinical procedures [42]. Studies have shown that the in-utero circadian rhythm could influence the postnatal cortisol rhythm, with higher infant cortisol levels reported during the evening at 8 weeks after birth which is unsynchronized with the 24-h light dark cycle [43]. As circadian rhythm is affected by seasonal variation due to the amount of sunlight exposure and changes in melatonin rhythms, we also observed seasonal variations in circadian rhythm development of the infants [44]. The production of melatonin among infants born in summer was reported to be the highest, while the difference was unobserved by 16 weeks of age [45]. This states that the maternal influence on the melatonin rhythm during the prenatal period may play a role in determining the postnatal rhythm as well. Maternal melatonin reprogramming has been found to have significant effects to entrain the infant circadian rhythm during the postnatal period via breastmilk feeding [46]. Hence, the factors influencing infant circadian rhythm development should also be noted if the pregnancy period occurs during different seasons of the year.
The establishment of infant circadian rhythm involves the role of light environment according to the light–dark cycle. Generally, it was found that cycled lighting played a significant role in determining the preterm infants’ circadian rhythms compared to those with continuous lighting. One of the studies included in this review revealed that the melatonin rhythmicity was established among preterm infants in NICU settings when exposed to cycled lighting [9]. The authors identified the positive results were due to temporal signalling of light exposure, and the experimental design was not specified in detail. Nevertheless, existing literature concluded that postnatal development of melatonin rhythmicity starts around 8–12 weeks among full-term infants, and even later among preterm infants [47]. Hence, more experimental studies with vigorous study design should be conducted to determine the role of light exposure in circadian rhythm development of preterm infants. Not only that, but the comparison with full-term infants cannot also be concluded as previous literatures did not explore the effects of light exposure in home settings on infant development. The lack of experimental studies conducted among full-term infants may be due to ethical considerations as full-term infants are usually raised in regular light–dark cycles [48]. However, it is worth noting that external factors such as erratic family schedules (e.g., shift workers) or artificial lighting conditions at home (e.g., darkened room during daytime naps) may influence the light–dark cycle of the infant at home as well [24, 49]. Therefore, rather than altering the lighting conditions of full-term infants, it would be more meaningful to report the real-time light exposure of infants in home settings and its effects on circadian rhythm development from now on.
Light intensity measured in terms of lux levels was reported to be controlled in cycled lighting conditions for NICU settings. Lux levels ranging from 100 to 250 lx for daytime, and 20–50 lx to imitate nighttime lighting in the hospital [24, 33, 34]. Interestingly, the difference in the range of light intensity to imitate day and night for the controlled environment is comparable to infants staying at their own homes with natural lighting. Lux levels of indoor natural light was 200 lx, while artificial lighting ranges from 20 to 100 lx [50], which is in agreement with the cycled lighting in controlled environment. However, the notable difference would be on the darkness lux levels with 0.2 lx only in home settings, which cannot be implemented in hospital settings due to safety and medical procedure needs [51]. The lighting environment between home settings and a controlled environment in healthcare facilities is remarkably different, and the study outcomes could not be compared within the same context due to complexities of infant growth in terms of gaps in milestones for preterm and full-term birth infants. Furthermore, the duration and timing of cycled light should be in accordance with the 24-h light dark cycle for circadian rhythm development. From our current findings, cycled lighting was set at various durations of 12 h, 14.5 h, and 15 h, with time of light exposure starting at 05:30 h, 06:00 h, and 07:00 h respectively. The difference in 24-h light dark cycled should be addressed according to time zones of respective regions. Hence, the difference between circadian rhythm establishment of infants in different settings has yet to be determined, and it should be done to further validate the recommended light intensity for the optimum growth of the infant. Nevertheless, these findings have demonstrated the beneficial outcomes of cycled lighting for both preterm and term infants in terms of their circadian rhythm development, sleep architecture, and growth.
While previous studies have revealed the role of light exposure in infant sleep, it has been presented that disrupted sleep–wake cycle during the developmental stage is associated with mood and developmental disorders [28, 29]. By 6 months, infant sleep gradually transitions from active sleep into REM (rapid eye movement) sleep, while quiet sleep transitions into NREM (non-rapid eye movement) sleep [52]. NREM sleep aids in the consolidation of declarative memories, while REM sleep is involved in processing nondeclarative and procedural memories, as well as mood regulation [53, 54]. However, infants with intense bursts of REM sleep (REM sleep storms) after 6 months were found to have poorer development at 1 year old [55]. The increase in REM sleep can be attributed to the exposure of dim artificial light at night during sleep, which was observed in a study conducted among healthy adults [56]. Hence, dim artificial light exposure during sleep can disrupt the proportion of sleep states and lead to adverse developmental outcomes. However, the effect of dim artificial lighting was only studied among the adult population, which bodily functions have fully matured. More studies on the role of artificial light and infant sleep states should be done to confirm this finding. It has been evident that disrupted sleep–wake cycle may lead to poor cognitive and mood function, hence interventions focusing on improving infant sleep environment such as controlling light exposure and temperature to ensure sleep quality should be proposed.
From this scoping review, it can be observed that most of the studies reporting on the influence of light exposure on the circadian rhythm development of infants are from narrative reviews. The limited number of clinical trials for humans, especially infants, have been challenging yet most warranted. As infant circadian rhythm had been determined using actigraphy, which is a non-invasive method and promotes compliance as it does not interrupt daily activities of the infant [17–19], future studies could incorporate the light exposure component by using a dosimeter, which is a device that is worn at the wrist or ankle to detect ultraviolet light [57]. Besides, only one paper reporting on the seasonal effects of infant circadian rhythm development was included, which warrants more studies to replicate the study design in other regions to strengthen this finding.
Despite the efforts made to enhance the search strategy for a comprehensive extraction of studies, there were a few limitations from this scoping review. Firstly, only published full articles were selected from the stated six databases only; hence, there might be publication bias. Besides that, only peer-reviewed articles published in English were selected; hence, there may be findings published in other languages that were not discussed in this scoping review.
Conclusion
This scoping review has shown that the field of infant circadian rhythm research is a highly warranted field to be explored. The current review suggests that infant circadian rhythm can be entrained by cycled lighting of ~ 20 to ~ 250 lx to simulate night and daytime respectively, while usage of protective measures such as goggles and light filter can be recommended in NICU settings. Light exposure of infants plays a role from as early as 6 weeks after birth, and seasonal variation may influence the circadian rhythm development due to difference in photoperiods, which should be further studied due to the limited number of studies conducted among infants. Hence, future studies should consider longitudinal designs to investigate the role of seasonal variations and photoperiods, home lighting environment, and usage of objective measurements to assess infant circadian rhythm development. As many more reviews related to this topic have been published with strong evidence-based recommendations established, more clinical trials and cohort studies should be conducted to further concrete the findings for health policies to be implemented in home settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
All authors contributed to the study conception and design. EYK performed the literature search. EYK, SK, and JEFT conducted the title screening. EYK, NAR, and JEFT performed the abstract screening. EYK and NMHS conducted the full text screening. All authors were involved in the data extraction of the final articles selected. EYK drafted the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was funded by UCSI University Research Excellence and Innovation Grant (REIG-FAS-2023/004).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bates K, Herzog ED (2020) Maternal-fetal circadian communication during pregnancy. Front Endocrinol (Lausanne) 11:. 10.3389/fendo.2020.00198 [DOI] [PMC free article] [PubMed]
- 2.Yates J (2018) Perspective: the long-term effects of light exposure on establishment of newborn circadian rhythm. J Clin Sleep Med 14:1829–1830. 10.5664/jcsm.7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGraw K, Hoffmann R, Harker C, Herman JH (1999) The development of circadian rhythms in a human infant. Sleep 22:303–310. 10.1093/sleep/22.3.303 [DOI] [PubMed] [Google Scholar]
- 4.Benavente I, Tamargo P, Tajada N et al (2005) Flash visually evoked potentials in the newborn and their maturation during the first six months of life. Doc Ophthalmol 110:255–263. 10.1007/s10633-005-0818-0 [DOI] [PubMed] [Google Scholar]
- 5.Adhikari P, Uprety S, Feigl B, Zele AJ (2024) Melanopsin-mediated amplification of cone signals in the human visual cortex. Proc R Soc B Biol Sci 291:20232708. 10.1098/rspb.2023.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zele AJ, Adhikari P, Cao D, Feigl B (2019) Melanopsin driven enhancement of cone-mediated visual processing. Vision Res 160:72–81. 10.1016/j.visres.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 7.Polese D, Riccio ML, Fagioli M et al (2022) The newborn’s reaction to light as the determinant of the brain’s activation at human birth. Front Integr Neurosci 16:. 10.3389/fnint.2022.933426 [DOI] [PMC free article] [PubMed]
- 8.Wong SD, Wright KP, Spencer RL et al (2022) Development of the circadian system in early life: maternal and environmental factors. J Physiol Anthropol 41:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escobar C, Rojas-Granados A, Angeles-Castellanos M (2021) Development of the circadian system and relevance of periodic signals for neonatal development. Handb Clin Neurol 179:249–258 [DOI] [PubMed] [Google Scholar]
- 10.Cheikh Ismail L, Al Dhaheri AS, Ibrahim S et al (2022) Nutritional status and adequacy of feeding practices in infants and toddlers 0–23.9 months living in the United Arab Emirates (UAE): findings from the Feeding Infants and Toddlers Study (FITS) 2020. BMC Public Health 22:319. 10.1186/s12889-022-12616-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engwall M, Fridh I, Bergbom I, Lindahl B (2014) Let there be light and darkness: findings from a prestudy concerning cycled light in the intensive care unit environment. Crit Care Nurs Q 37:273–298 [DOI] [PubMed] [Google Scholar]
- 12.Guyer C, Huber R, Fontijn J et al (2015) Very preterm infants show earlier emergence of 24-hour sleep–wake rhythms compared to term infants. Early Hum Dev 91:37–42. 10.1016/j.earlhumdev.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Kaneshi Y, Ohta H, Morioka K et al (2016) Influence of light exposure at nighttime on sleep development and body growth of preterm infants. Sci Rep 6(CC-N):21680. 10.1038/srep21680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kracht CL, Redman LM, Casey PH et al (2021) Association between home environment in infancy and child movement behaviors. Child Obes 17:100–109. 10.1089/chi.2020.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nurliyana AR, Mohd Shariff Z, Mohd Taib MN et al (2020) Early growth and home environment are associated with cognitive development in the first year of life of Malaysian infants. Early Hum Dev 140:104890. 10.1016/j.earlhumdev.2019.104890 [DOI] [PubMed] [Google Scholar]
- 16.Braun V, Clarke V (2006) Using thematic analysis in psychology. Qual Res Psychol 3:77–101. 10.1191/1478088706qp063oa [Google Scholar]
- 17.Tsai S-Y, Thomas KA, Lentz MJ, Barnard KE (2012) Light is beneficial for infant circadian entrainment: an actigraphic study. J Adv Nurs 68:1738–1747. 10.1111/j.1365-2648.2011.05857.x [DOI] [PubMed] [Google Scholar]
- 18.Kärki A, Paavonen EJ, Satomaa A-L et al (2020) Sleep architecture is related to the season of PSG recording in 8-month-old infants. Chronobiol Int 37:921–934. 10.1080/07420528.2020.1754845 [DOI] [PubMed] [Google Scholar]
- 19.Yoshida M, Ikeda A, Adachi H (2023) Contributions of the light environment and co-sleeping to sleep consolidation into nighttime in early infants: a pilot study. Early Hum Dev 189:105923. 10.1016/j.earlhumdev.2023.105923 [DOI] [PubMed] [Google Scholar]
- 20.Vasquez Ruiz G, Castellanos M, Escobar Briones C (2012) Preterm infants have improved growth in cycled light/dark compared with continuous light. Pediatr Res 72:110 [Google Scholar]
- 21.Watanabe S, Akiyama S, Hanita T et al (2013) Designing artificial environments for preterm infants based on circadian studies on pregnant uterus. Front Endocrinol (Lausanne) 4:113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varvara B, Effrossine T, Despoina K et al (2016) Effects of neonatal intensive care unit nursing conditions in neonatal NREM sleep. J Neonatal Nurs 22:115–123. 10.1016/j.jnn.2015.11.004 [Google Scholar]
- 23.Valizadeh S, Bagher Hosseini M, Jafarabadi MA et al (2017) Comparison of 2 methods of light reduction on preterm infants ’ sleep pattern in NICU : a randomized controlled trial. Crescent J Med Biol Sci 4:211–216
- 24.Wong SD, Wright KP, Spencer RL et al (2021) Development of the circadian system in early life: maternal and environmental factors. Handb Clin Neurol 41:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran S, Dutta S (2013) Early developmental care interventions of preterm very low birth weight infants. Indian Pediatr 50:765–770 [DOI] [PubMed] [Google Scholar]
- 26.McKenna H, Reiss IKM (2018) The case for a chronobiological approach to neonatal care. Early Hum Dev 126:1–5 [DOI] [PubMed] [Google Scholar]
- 27.Iwata S, Fujita F, Kinoshita M et al (2017) Dependence of nighttime sleep duration in one-month-old infants on alterations in natural and artificial photoperiod. Sci Rep 7:44749. 10.1038/srep44749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonken LK, Nelson RJ (2016) Effects of light exposure at night during development. Curr Opin Behav Sci 7:33–39 [Google Scholar]
- 29.Logan RW, McClung CA (2019) Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci 20:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correia A, Lourenço M (2020) Sleep promotion in neonatal intensive care units: scoping review. Enfermería Glob 19:561–575 [Google Scholar]
- 31.Guyer C, Huber R, Fontijn J et al (2012) Cycled light exposure reduces fussing and crying in very preterm infants. Pediatrics 130:e145–e151. 10.1542/peds.2011-2671 [DOI] [PubMed] [Google Scholar]
- 32.Hazelhoff EM, Dudink J, Meijer JH, Kervezee L (2021) Beginning to see the light: lessons learned from the development of the circadian system for optimizing light conditions in the neonatal intensive care unit. Front Neurosci 15:634034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morag I, Ohlsson A (2016) Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev 2016(8):CD006982. 10.1002/14651858.CD006982.pub4 [DOI] [PMC free article] [PubMed]
- 34.Rodríguez RG, Pattini AE, Roberto GR, Andrea EP (2016) Neonatal intensive care unit lighting: update and recommendations. Arch Argent Pediatr 114:361–367 [DOI] [PubMed] [Google Scholar]
- 35.Lewis P, Wild U, Pillow JJ et al (2024) A systematic review of chronobiology for neonatal care units: what we know and what we should consider. Sleep Med Rev 73:101872. 10.1016/j.smrv.2023.101872 [DOI] [PubMed] [Google Scholar]
- 36.Zores-Koenig C, Kuhn P, Caeymaex L (2020) Recommendations on neonatal light environment from the French Neonatal Society. Acta Paediatr 109:1292–1301. 10.1111/apa.15173 [DOI] [PubMed] [Google Scholar]
- 37.Santos J, Pearce SE, Stroustrup A (2015) Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr 27:254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Hoogen A, Teunis CJ, Shellhaas RA et al (2017) How to improve sleep in a neonatal intensive care unit: a systematic review. Early Hum Dev 113:78–86 [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Ikeda A, Adachi H (2024) Contributions of the light environment and co-sleeping to sleep consolidation into nighttime in early infants: a pilot study. Early Hum Dev 189:105923. 10.1016/j.earlhumdev.2023.105923 [DOI] [PubMed] [Google Scholar]
- 40.Westwood E, Smith S, Mann D et al (2023) The effects of light in children: a systematic review. J Environ Psychol 89:102062. 10.1016/j.jenvp.2023.102062 [Google Scholar]
- 41.Sánchez-Sánchez M, García TL, Heredia D et al (2022) Effect of a light-darkness cycle on the body weight gain of preterm infants admitted to the neonatal intensive care unit. Sci Rep 12:17569. 10.1038/s41598-022-22533-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lammertink F, Vinkers CH, Tataranno ML, Benders MJNL (2020) Premature birth and developmental programming: mechanisms of resilience and vulnerability. Front Psychiatry 11:531571. 10.3389/fpsyt.2020.531571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwata O, Okamura H, Saitsu H et al (2013) Diurnal cortisol changes in newborn infants suggesting entrainment of peripheral circadian clock in utero and at birth. J Clin Endocrinol Metab 98:E25–E32. 10.1210/jc.2012-2750 [DOI] [PubMed] [Google Scholar]
- 44.Honma K, Honma S, Kohsaka M, Fukuda N (1992) Seasonal variation in the human circadian rhythm: dissociation between sleep and temperature rhythm. Am J Physiol 262:R885–R891. 10.1152/ajpregu.1992.262.5.R885 [DOI] [PubMed] [Google Scholar]
- 45.Sivan Y, Laudon M, Tauman R, Zisapel N (2001) Melatonin production in healthy infants: evidence for seasonal variations. Pediatr Res 49:63–68. 10.1203/00006450-200101000-00015 [DOI] [PubMed] [Google Scholar]
- 46.Häusler S, Lanzinger E, Sams E et al (2024) Melatonin in human breast milk and its potential role in circadian entrainment: a nod towards chrononutrition? Nutrients 16:. 10.3390/nu16101422 [DOI] [PMC free article] [PubMed]
- 47.Paditz E (2024) Postnatal development of the circadian rhythmicity of human pineal melatonin synthesis and secretion (systematic review). Children 11:. 10.3390/children11101197 [DOI] [PMC free article] [PubMed]
- 48.Tsai S-Y, Barnard KE, Lentz MJ, Thomas KA (2010) Mother-infant activity synchrony as a correlate of the emergence of circadian rhythm. Biol Res Nurs 13:80–88. 10.1177/1099800410378889 [DOI] [PubMed] [Google Scholar]
- 49.Petrov ME, Whisner CM, McCormick D et al (2021) Sleep-wake patterns in newborns are associated with infant rapid weight gain and incident adiposity in toddlerhood. Pediatr Obes 16:e12726. 10.1111/ijpo.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajaj A, Rosner B, Lockley SW, Schernhammer E (2011) Validation of a light questionnaire with real-life photopic illuminance measurements: the Harvard Light Exposure Assessment Questionnaire. Cancer Epidemiol Biomarkers Prev 20:1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engwall M, Fridh I, Johansson L et al (2015) Lighting, sleep and circadian rhythm: an intervention study in the intensive care unit. Intensive Crit Care Nurs 31:325–335. 10.1016/j.iccn.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 52.Yasova Barbeau D, Weiss MD (2017) Sleep disturbances in newborns. Children 4:90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang F (2019) Sleep and early brain development. Ann Nutr Metab 75:44–54 [DOI] [PubMed] [Google Scholar]
- 54.Kumar D, Yanagisawa M, Funato H (2024) Sleep-dependent memory consolidation in young and aged brains. Aging Brain 6:100124. 10.1016/j.nbas.2024.100124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenehan SM, Fogarty L, O’Connor C et al (2023) The architecture of early childhood sleep over the first two years. Matern Child Health J 27:226–250. 10.1007/s10995-022-03545-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho C-H, Lee H-J, Yoon H-K et al (2016) Exposure to dim artificial light at night increases REM sleep and awakenings in humans. Chronobiol Int 33:117–123. 10.3109/07420528.2015.1108980 [DOI] [PubMed] [Google Scholar]
- 57.Maslin D, Veitch D, Williams HC (2020) Direct infant ultraviolet light exposure is associated with eczema and immune development: a critical appraisal. Br J Dermatol 182:300–303. 10.1111/bjd.18087 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.