Correction to: Cancer Immunology, Immunotherapy (2022) 72:475–491 10.1007/s00262-022-03253-x
In the original version of this article, there is a minor typo in the third sentence of the introduction section where "USA" should be "EU".
The third sentence of the introduction section, which previously read
"Cemiplimab, another PD-1 inhibitor, was the first drug ever to demonstrate a statistically significant and clinically meaningful OS benefit in pretreated patients with persistent/recurrent/metastatic CC, for which it gained regulatory approval in the USA".
Should have read
"Cemiplimab, another PD-1 inhibitor, was the first drug ever to demonstrate a statistically significant and clinically meaningful OS benefit in pretreated patients with persistent/recurrent/metastatic CC, for which it gained regulatory approval in the EU".
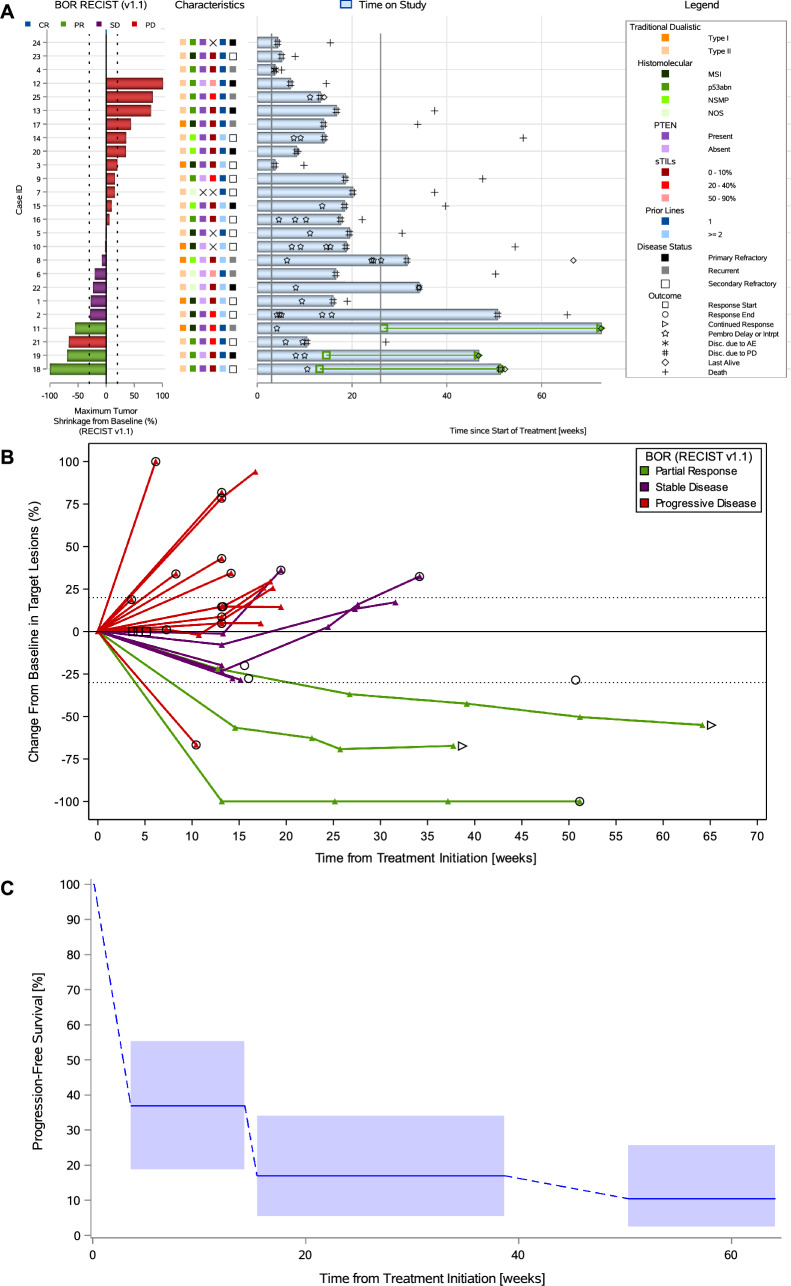
The vertical dotted lines in Fig. 3A (the waterfall plot) were incorrectly depicted as a solid line. The Fig. 3 should have appeared as shown below.
Fig. 3.
Antitumor activity to study treatment in the endometrial cohort. A Combined waterfall and swimmer plot. B Spider plot. C Interval-censored progression-free survival per immune-related response criteria. AE adverse event, BOR best overall response, CR complete response, MSI microsatellite instability, NOS not otherwise specified, NSMP no specific molecular profile, PD progressive disease, PR partial response, PTEN phosphatase and tensin homolog, RECIST v1.1 Response Evaluation Criteria in Solid Tumors version 1.1, SD stable disease, sTILs stromal tumor-infiltrating lymphocytes
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.