Abstract
Background
Colorectal cancer (CRC) is the most common digestive cancer in the world. Microsatellite stability (MSS) and microsatellite instability (MSI-high) are important molecular subtypes of CRC closely related to tumor occurrence and progression and immunotherapy efficacy. The presence of CD8+ CXCR5+ follicular cytotoxic T (TFC) cells is strongly associated with autoimmune disease and CD8+ effector function. However, the roles of TFC cells in MSI-high CRC and MSS CRC are unclear. Here, we aimed to explore the characteristics of TFC cells in CRC and compare their biological functions between MSI-high and MSS CRC.
Methods
We explored the expression of TFC cell in tumor tissues and peripheral blood in our clinical cohort and public datasets. By combining single-cell RNA sequencing (scRNA-seq) and bulk RNA sequencing, we explored the potential function of TFC cells and developed a prediction model for CRC. We also compared the biological functions of these cells between MSS and MSI-high CRC and used flow cytometry and coculture experiments to explore their potential regulatory functions.
Results
TFC cell markers are downregulated in tumor tissues and patient peripheral blood vs. controls. The prediction model for CRC performed well in the training and validation cohorts (KM plot p < 0.001). MSS CRC patients exhibit enrichment of genes related to the cell cycle (MKI67) and T cell activation (CD38 and HLA-DR) and decreased enrichment of immune checkpoint markers (PD1, TIM3, and LAG3). The expression of TFC cell-related genes is positively correlated with that of CD8+IFN-γ+-related genes and closely related to that of TLS-related genes in MSS CRC. The proportion of TFC cells is positively correlated with that of CD19+CD38+ B cells in MSS CRC.
Conclusions
The prognostic prediction model has good predictive value. In MSS CRC, TFC cells function mostly in T cell activation and the cell cycle and have low expression of immune checkpoint molecules, which may influence the effectiveness of ICB therapy. TFC cells may regulate antitumor function by regulating CD19+ CD38+ B cells and TLSs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-024-03887-z.
Keywords: TFC, Colorectal cancer, scRNA-seq, Machine learning model
Introduction
Colorectal cancer (CRC) is a common digestive tract tumor worldwide and one of the main causes of cancer-related death [1, 2]. The antitumor function of the immune system plays a necessary role in regulating cancer progression [3]. Many studies have shown that immune cell infiltration and costimulatory cytokines in colorectal tumors can have prognostic value [4].
As a large family of small-molecular weight cytokines, chemokines can be divided into four subgroups (CC, CXC, XC, and CX3C). Cytokines can act as important biological regulatory factors in cell proliferation, respiration bursts and antimicrobial effects [5]. Cytokines play a necessary role in homing and positioning lymphocytes, including mature T cells, B cells, and plasma cells [6, 7]. CXCR5 is the receptor of CXCL13 and is significantly expressed on B cells, follicular helper T cells and antigen-carrying dendritic cells [8]. CXCR5 mainly transmits signals through the CXCL13‒CXCR5 axis, which mediates the immune response of B and T lymphocytes [9]. The CXCL13-CXCR5 axis plays a dual role in the occurrence and development of tumors. The CXCL13-CXCR5 axis can induce tumor cell growth and invasion by activating phosphatidylinositol 3-kinase (PI3K) in the Raf/MEK/ERK pathway and promoting the recruitment of MDSCs and Treg cells [10, 11]. The CXCL13-CXCR5 axis can also promote the formation of tumor tertiary lymphoid organs (TLOs) by recruiting B cells and follicular helper T (TFH) cells [12].
CXCR5 expression on CD4+ T cells is the marker of the TFH cell subset and is crucial in the B cell-mediated immune response [13]. CXCR5-producing CD8+ T cells were previously named follicular cytotoxic T (TFC) cells [14]. Compared with CXCR5-CD4 + T cells, TFC cells can express granzyme B (GZMB), interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), which can enhance the antitumor immune response in different kinds of tumors [14, 15]. High expression of CXCR5 and low expression of CCR7 subgroups can regulate the expression of the molecules CD62L and CXCR3, which influence cell trafficking and migration [16]. Tpex cells constitute a self-renewing cell population that highly expresses TCF1 and CXCR5 [17]. They can migrate to tumors and undergo terminal differentiation, thereby replenishing the depleted T cell pool within the tumor. Tpex cells are enriched in the tertiary lymphoid structures (TLSs) or T cell APC regions of some human solid tumors, and the establishment of this immune niche is associated with improved clinical prognosis [18]. Microsatellite instability (MSI) is caused by mismatch repair (MMR) gene defects and is closely associated with the occurrence of CRC. CRC can be divided into two main subtypes: microsatellite-stable (MSS) CRC and microsatellite instability (MSI)-high CRC. CRC can be divided into two main subtypes: microsatellite-stable (MSS) CRC and microsatellite instability (MSI)-high CRC. Compared with MSS CRC, MSI-high CRC is more responsive to immune checkpoint blockade (ICB)-based treatments [19]. Previous studies have shown that TFC cells play important roles in the ICB response, but relatively few in-depth studies have investigated this relationship in CRC. The difference between the proportions of TFC cells between MSS and MSI-high CRC is also unclear.
Therefore, in our studies, we explored TFC expression in tumor tissue and peripheral blood and developed a model to predict the OS of patients with CRC. We also explored the different interactions and functions between MSI-high CRC and MSS CRC. Our results revealed that a high proportion of TFC cells is associated with a better prognosis and is related to the expression of TLS-related genes and positively correlated with the proportion of CD8+IFN-γ+ cells. B cells can regulate TFC cell function, and TFC cells are closely related to CD19+CD38+ B cells. These results indicate that intratumoral TFC cells play roles in the cell cycle and T cell activation. TFC cells have potential prognostic value and may be associated with the response to ICB. The different biological functions between MSS and MSI-high CRC indicate that TFC cells can regulate the antitumor response by regulating CD19+CD38+ B cells.
Methods
Data download and preprocessing
We downloaded single-cell RNA sequencing (scRNA-seq) and sequencing datasets for 1021 CRC patient tumor tissues samples, 12 adjacent tissue samples and 5 normal controls from The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/) and the Gene Expression Omnibus (GEO) (https://ncbi.nlm.nih.gov/geo). The 10X scRNA sequencing datasets (GSE188711 [20], GSE221575 [21] and GSE132465 [22]) included information on 31 CRC patients. The TCGA-CRC datasets and clinical information were combined for the TCGA-COAD and TCGA-READ cohorts. The GEO dataset GSE39582 [23], which includes information on 562 CRC patients, was downloaded and used to construct the prognostic model.
scRNA-seq data and RNA-seq analysis
The scRNA-seq data were 10x-seq data. We first used the Seurat and dplyr R packages to read the raw data and applied the following exclusion criteria: the number of cells was lower than 0, and the min feature was lower than 200. We selected the cells with at least 200 genes and no more than 7000 genes and cells for which the percentage of mitochondrial genes was less than 20%. We then used the R package DoubletFinder to identify platelets. After processing single-cell data for each patient individually, we used the Harmony R package to eliminate interbatch differences and normalize the scRNA-seq data. We also used FindNeighbors cluster data and used TSNE and UMAP for nonlinear dimensional reduction for our data. The TCGA-CRC database and clinical information were combined for TCGA-COAD and TCGA-READ. The raw data were converted to transcripts per kilobase million (TPM) format, and the lncRNAs and mRNAs were separated. The GEO data used in the prognostic model (GSE17537 [24], GSE38582) were annotated via the Affymetrix GPL570 platform for symbol genes. For the scRNA-seq analysis, we utilized the “Seurat” R package. First, we used “harmony” R packages to normalize the data and used “FindClusters” to divide cells into clusters and then used biomarkers annotate cells as different subpopulations: T cells (CD3D, CD3E), B cells (CD19, MS4A1), plasma cells (SDC1, MZB1), epithelial cells (EPCAM, CDH1), endothelial cells (PECAM1, CDH5), mast cells (TPSAB1, KIT), fibroblasts (FAP, COL3A1), myeloid cells (CD14, FCGR3A) and glial cells (S100B) [25].
TFC cell marker expression in the peripheral blood
We utilized TIMER (http://timer.comp-genomics.org/timer/) and public scRNA-seq data to explore TFC cell marker expression in tissues. We collected tumor tissue from patients who were diagnosed with CRC in our hospital. The tissue was stored in tissue preservation solution (MACS Tissue Storage Solution, 130-100-008), and the tissue was transferred to single-cell solution (MACS, Tumor Dissociation Kit, human, 130-095-929) following the instructions in the kit manual. We collected peripheral blood from CRC patients and healthy people in EDTA anticoagulant tubes. Antibodies, including those against CXCR5, PD1, CD39, CD4, CD3, Foxp3, CD45, and CD8, were applied using a Foxp3/transcription factor staining buffer kit following the manufacturer’s instructions; this approach was used for analysis of both tissue samples and peripheral blood mononuclear cells (PBMCs).
Prognostic genes identified via the machine learning prognostic model
We utilized the “FindMarkers” function to explore the unique genes of CXCR5 + CD8 + T cells. We utilized the NMF clustering method to divide the data into 2 parts and identify prognostic genes. The enriched biological functions were explored via “clusterProfiler” and “org.Hs.eg.db”.
Next, we explored the prognostic hub genes via least absolute shrinkage and selection operator (LASSO) regression and multivariate Cox regression. To develop a high-accuracy and stable prognostic prediction model, we used 8 kinds of deep machine learning models, including LASSO regression. We used Kaplan–Meier (KM) curves for the training cohort and validation cohorts to evaluate the models’ performance.
Cell communication and pseudotime analysis
To explore the patterns of crosstalk among TFC cells and other cells, we utilized the “CellChatDB.human” database of the “CellChat” R package in different patient groups (CRC patients and healthy controls). We utilized identifyOverExpressedGenes and identifyOverExpressed Interactions to identify overexpressed genes and filtered cell‒cell communication data via “computeCommunProb” and “computeCommunProbPathway”. On the basis of the T clusters we identified, we continued to explore the differentially expressed genes with p < 1E−02. On the basis of the identified genes (that is, those specific to this cell type), we utilized the “mono” R package and the “setOrderingFilter” and “orderCells” functions to explore the developmental trajectory of the TFC cells.
Identification of TFC cell function
We compare the proportions of TFC cells in different tissues according to the expression of marker genes. The genes related to the cell cycle, immunoregulation, naïve memory, cytotoxicity, resident memory T cells, endoplasmic reticulum stress, angiogenesis, TLSs and the stem-like program-related genes were previously studied [26–28]. A heatmap was generated via the R package “ComplexHeatmap”. The total UMI counts were calculated for each cell, and the expression levels are shown in the heatmap.
Analysis of CRC tissue and PBMC single-cell suspensions and coculture experiments
We collected the tumor tissues and peripheral blood from the same patients. We separated PBMCs using lymphocyte isolation solution (human lymphocyte separation medium, 7111012, DAKEWE) according to the reagent instructions. At baseline, we assessed the expression of the following T cell subtype markers: CXCR5 (FITC anti-human CD185 antibody, 356,914, Biolegend), PD1 (PE anti-human CD279 antibody, 329,906, Biolegend), CD39 (PE/Dazzle™ 594 anti-human CD39 antibody, Biolegend), CD4 (PE/CY7, Raisecare), CD3 (Percp, Raisecare), Foxp3 (Monoclonal Antibody, PCH101, Thermo Fisher), CD45 (redFluor™ 710 anti-human, HI30, CYTEK), and CD8 (APC/CY7, Raisecare) using targeted antibodies in a Foxp3/transcription factor staining buffer kit (TNB-0607-KIT) following the manufacturer’s instructions.
We treated PBMCs with LPS and anti-IgM for 6 days to explore the immunoregulatory relationship between TFC cells and CD8+IFN-γ+ T cells. We also cocultured PBMCs and tumor cells with LPS and anti-IgM after treatment with a cell stimulation cocktail (000-4975-93, Thermo Fisher) for 6 days. After being stimulated for 5 h, the cells were harvested and stained with CD3, CD8, IFN-γ (APC, anti-human IFN-γ, 1997143), and CD45. All the cells were cultured in 10% FBS, 1% antibiotics (penicillin streptomycin, 2585616), or 1640 medium (BioLegend).
Statistical analysis
FlowJo v10.8.1 was used to analyze the flow cytometry fcs files. Statistical analysis of the data was performed with R software (4.0.3). Nonnegative matrix factorization (NMF) clustering analysis was performed via the “survival”, “caret”, “glmnet”, and “surviminer” R packages. Multivariate Cox regression and LASSO regression analyses were performed via the R packages “glmnet” and “survival”. The machine learning models were applied with the following packages: “ggplot2”, “ggsci”, “survival”, “randomForestSRC”, “glmnet”, “plsRcox”, “superpc”, “gbm”, “CoxBoost”, “survivalsvm”, “dplyr”, “tibble”, “BART” and “ggbreak”. p < 0.05 was considered to indicate statistical significance.
Result
Identification of major CRC cell types
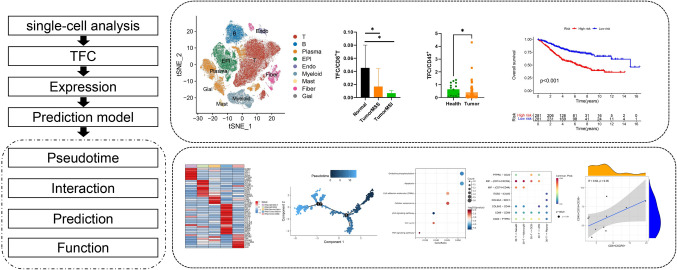
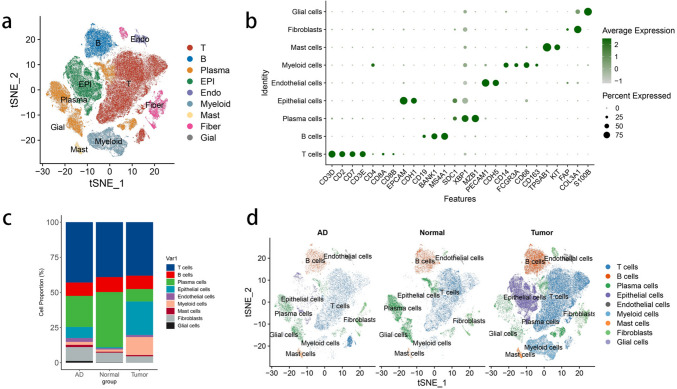
The workflow is shown in Fig. 1, we explored the TFC differential expression in tumor tissue and peripheral blood, based on the genes of TFC we developed a model to predict the OS of patients with CRC. We included 5 normal tissues, 9 MSS adjacent tumor tissues, 27 MSS tumor tissues, 3 MSI-H adjacent tumor tissues and 4 MSI-H tumor tissues. As shown in Fig. 2A, we used the normalized data to divide cells into 9 types: T cells, B cells, plasma cells, epithelial cells, endothelial cells, mast cells, fibroblasts, myeloid cells and glial cells (Fig. 2). We then analyzed the T cells and divided them into 32 clusters. CD8+ T-30 cells expressed relatively high levels of CD8 and CXCR5, and we renamed these cells as TFC cells (Supplementary Fig. 1).
Fig. 1.
Workflow of this study
Fig. 2.
Single-cell atlas samples of normal, adjacent tumor and tumor tissues. A: TSNE map of normal, adjacent tumor and tumor tissues. B: Dot plots showing the classical markers in the cell clusters. C: Bar plot of different proportions of cell clusters in normal tissues, adjacent tissues and tumor tissues. D: TSNE of the indicated cell clusters in different tissues
Expression of TFC in CRC tissue and peripheral blood
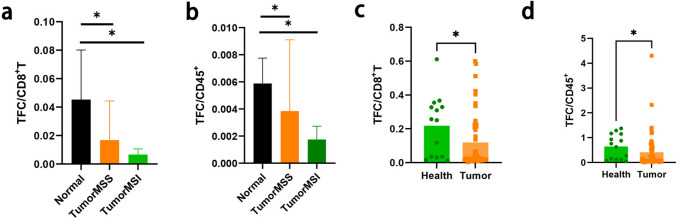
We assessed the expression of the identified TFC cell in normal, adjacent tumor and tumor tissues. As shown in Supplementary Fig. 2, in public datasets, CXCR5 expression in CRC tumor tissue was lower than that in normal tissues. The same results are shown in Fig. 3A: the proportion of TFC/CD8+ T cells in normal tissue was greater than that in tumor tissue, and the same trend was also observed for the TFC/CD45+ cells (Fig. 3B). To further explore the predictive value of the proportion of TFC cells in peripheral blood, we also assessed the expression of TFC in a clinical cohort, including 51 CRC patients and 14 healthy controls. As shown in Fig. 3C, D, TFC expression was lower than that in the healthy cohorts.
Fig. 3.
Expression of TFC cell markers in tissues and peripheral blood. A: Proportion of TFC cells among CD8+ T cells from different tissue types in public datasets. B: Proportion of TFC cells among CD45 + cells from different tissue types in public datasets. The black bar represents normal tissues, the orange bar represents MSI-high CRC tissues, and the green bar represents MSS CRC tissues. c: Proportion of TFC cells among CD8 + T cells between CRC patients and healthy people. D: Proportion of TFC cells among CD45.+ cells between CRC patients and healthy people. Green bar (healthy people), orange bar (CRC patients)
Development and validation of a prognostic model based on TFC cells in CRC
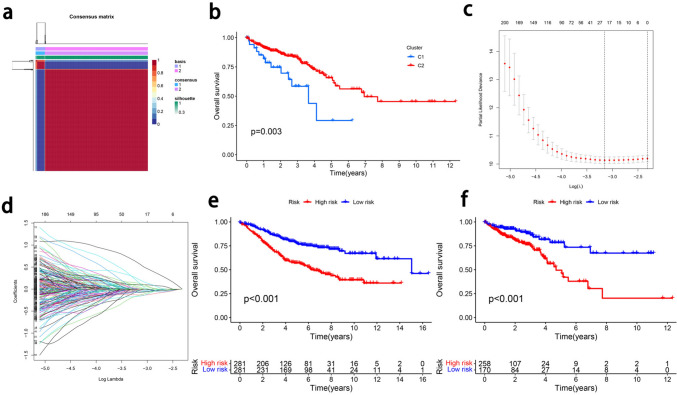
To explore the prognostic value of TFC cells, we identified TFC markers with the following screening criteria: p < 0.05 and |logFc|≥ 1. We first used univariate Cox regression analysis to identify prognosis-related genes, and 521 genes were found to have potential prognostic value. We subsequently identified a cluster of 323 genes via NMF analysis, and we then performed LASSO regression to identify hub genes related to prognosis. Volcano plots and KM survival curves for patients grouped according to some of the prognostic genes are shown in Supplementary Fig. 3. Twenty-four prognostic hub genes were included in the prognostic model (Fig. 4). On the basis of the hub genes, we developed a prognostic model via deep learning machine models to calculate the prognostic risk score. The following formula was used: Risk score = IDS*(0.092) + FBLN7*(− 0.026) + MRPL4*(− 0.073) + PTRHD1*(− 0.0005) + AMOTL1*(0.096) + TMX2*(− 0.046) + GMNN(− 0.134) + RANBP1(− 0.138) + FDFT1*(− 0.028) + NOA1*(− 0.019) + NIN*(0.573) + NUDCD2*(− 0.005) + THG1L*(− 0.004) + TNFSF13B*(− 0.031) + PRCD*(0.043) + LARS2*(− 0.001) + HNRNPM*(0.128) + DNAH11*(0.138) + TNFRSF1B(− 0.161) + HTR3A*(0.001) + HTR3A*(0.0009) + SNRNP25*(− 0.044) + NPR2*(0.016) + KIF15*(− 0.067) + CR1*(0.213). The hub prognostic genes were enriched in antitumor functions and the cell cycle. As shown in Fig. 4e, f, patients with low risk scores had better prognoses than those with high risk scores did. The KM plot for patients grouped according to the risk score revealed that the model had good prognostic value (training group: p < 0.001; validation group: p < 0.001).
Fig. 4.
Construction of a prognostic model for CRC. A: Clustering of genes related to TFC cells and prognosis. B: KM plots of NMF model clusters. C: Determining the optimal value for genes related to TFC cells parameter λ in the Lasso regression model using the cross-validation method. D: LASSO regression trajectory plot of genes related to TFC cells and prognosis. e: KM plot of the prognostic model training cohort. F: KM plot of the prognostic model of the validation cohort
Pseudotime analysis and potential interactions
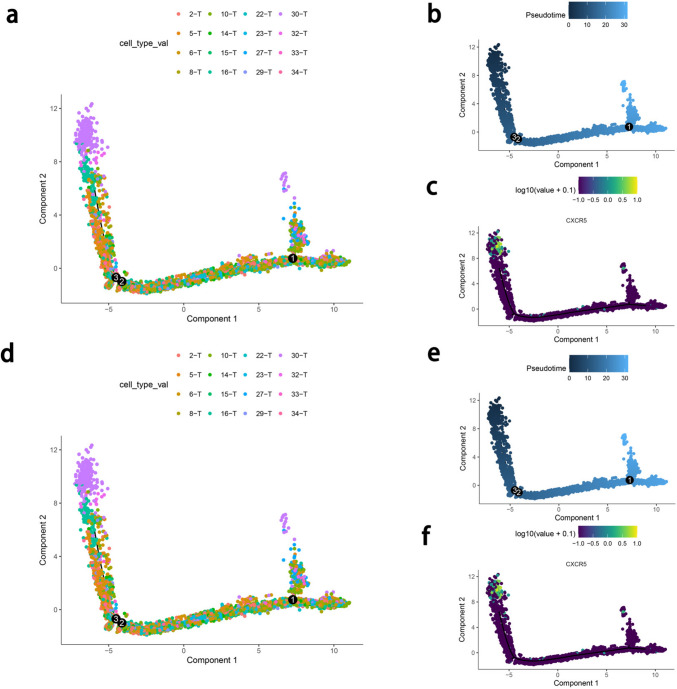
To investigate the effect of TFC cells as depleted precursor cells on the differentiation of other CD8+ T cells, we conducted a pseudotime analysis of CD8+ T cells. As shown in Fig. 5, for MSS CRC, TFC cells are produced at the beginning of the T cell trajectory and at the end of the T cell trajectory and are the primary regulated T cell population. TFC cells appear in the early and late stages of CD8+ T cell differentiation in both MSS and MSI CRC. After the appearance of the TFC cells, cytotoxic cells appeared (GZMA, GZMB, and GZMK highly expressed), indicating that these cells may induce the production of these cells and affect the antitumor immune response of patients with CRC. Among the T cell subsets, TFC cells also highly expressed the stem-like progenitor markers TCF1 and CXCR5, which may be characteristics of exhausted progenitor T (Tpex) cells; notably, the induced T cells may have stronger cytotoxic effects after immunotherapy.
Fig. 5.
Pseudotime analysis of T cells in MSI-high and MSS CRC. a: Pseudotime trajectory of sub-T clusters in MSS CRC. B: Pseudotime of sub-T cells in MSS CRC. C: CXCR5 expression over time in MSS CRC. d: Pseudotime trajectory of sub-T clusters in MSI-high CRC. e: Pseudotime of sub-T cells in MSI-high CRC. f: CXCR5 expression over time in MSI-high CRC
Different biological functions between MSS and MSI-high CRC
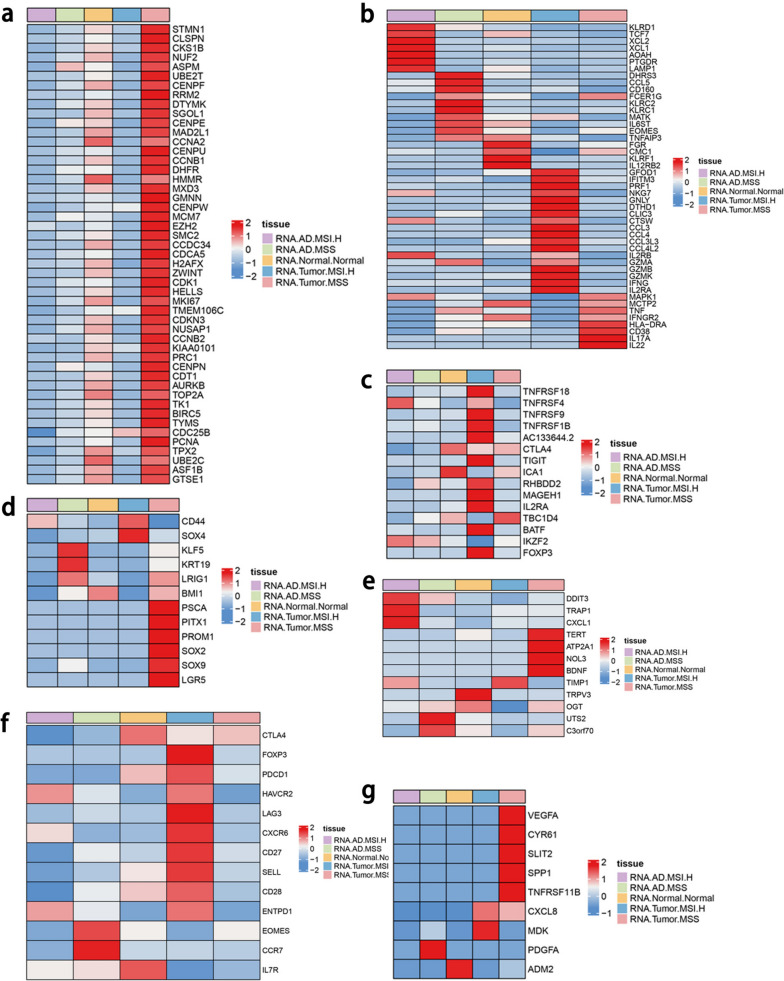
Owing to the different responses of MSS and MSI CRC to immunotherapy, our results indicate that the difference in immune infiltration levels between the two is not significant, but MSS CRC is significantly less sensitive to immunotherapy than MSI. Therefore, it is speculated that there are differences in the functions of TFC cells between MSS and MSI-high CRC and that further analysis of functional changes in MSS CRC is important (Fig. 6). As shown in Fig. 6a, MSS CRC tumors expressed more cell cycle-related genes than MSI tumors did, indicating that TFC cells proliferate in MSS tissue. In terms of cytokine expression, MSI tumors highly express MHC-I molecules and inflammation-related genes, such as HLA-C, HLA-B, HLA-A, IL-1B, and IL-2RA; MSS tumors highly express MHC-II molecules, genes related to antitumor function and inflammation-related genes, such as IL-1A, IL-17A, IL-1B, IL-17RA, TNF, and HLA-DR.
Fig. 6.
Heatmap of biological function genes between different kinds of tissues. A: Expression of cell type-related genes in different tissues. B: Expression of cytotoxicity-related genes in different tissues. C: Expression of immunoregulatory genes in different tissues. D: Expression of stem-like genes in different tissues. E: Expression of endoplasmic reticulum stress-related genes in different tissues. F: Expression of T cell exhaustion-related genes in different tissues. G: Expression of angiogenesis-related genes in different tissues
In terms of cytotoxic marker expression, MSI-high tumors highly express the function-related genes GZMA, GZMB, GZMK and IFNG, but MSS tumors highly express antitumor and T cell activation-related genes, such as TNF, IFNGR2, HLA-DRA1, CD38, IL17A and IL22 (Fig. 6b). In terms of immunoregulation-related markers, MSI tumors highly express B cell-activating factors and immune tolerance factors, such as TNFRSF18, TNFRSF4, TNFRSF9, TNFRSF1B, IL2RA and FOXP3; MSS tumors highly express TBC1D4, which is related to glucose metabolism (Fig. 6c). MSI-high CRC tumors highly express the stem-like-related genes sCD44 and SOX4; MSS tumors highly express PSCA, PITX1, PROM1, SOX2, SOX9 and LGR5 (markers of TFC cells), and these tumors have a higher degree of lineage plasticity (Fig. 6d). In the context of endoplasmic reticulum stress, MSI-high tumors express high levels of TIMP1, whereas MSS tumors highly express TERT, ATP2A1, NOL3 and BDNF (Fig. 6e). In terms of exhaustion-related markers, MSI-high tumors highly express the immune checkpoints PDCD1, TIM3, and LAG3, but MSS tumors highly express CTLA4, which indicates that TFC cells may be related to the poor response to PD-1 ICB therapy (Fig. 6f). Both MSS and MSI-high tumors highly express angiogenesis-related genes; MSI-high tumors highly express MDK, and MSS-high tumors highly express VEGFA and TNFRSF11B (Fig. 6g). In terms of naïve-related markers, MSI-high tumors highly express SELL, LEF1, SOX4, and TOB1 (Supplementary Fig. 4a). Among the resident memory-related genes, sSYTL3, GPR171 and PDE4D are highly expressed in MSI tumors, whereas IL18R1 is highly expressed in MSS tumors (Supplementary Fig. 4b). These results indicate that TFC cells may be associated with cell cycle regulation and T cell activation.
Because biological function-related genes were differentially expressed between MSS and MSI-high CRC, we next analyzed the TFC -specific genes that were differently expressed between MSS and MSI-high CRC. The genes highly expressed in MSS tumor tissues were involved in inflammation-related functions. As shown in Supplementary Fig. 5, genes highly expressed in MSS CRC were enriched in the TNF signaling pathway, cell adhesion molecules, oxidative phosphorylation, antigen processing and presentation and the B cell receptor signaling pathway; genes highly expressed in MSI-high CRC were enriched in transcriptional regulation in cancer, the phosphatidylinositol signaling system and Fc-γ R-mediated phagocytosis.
Potential interaction between TFC cells and immune cells
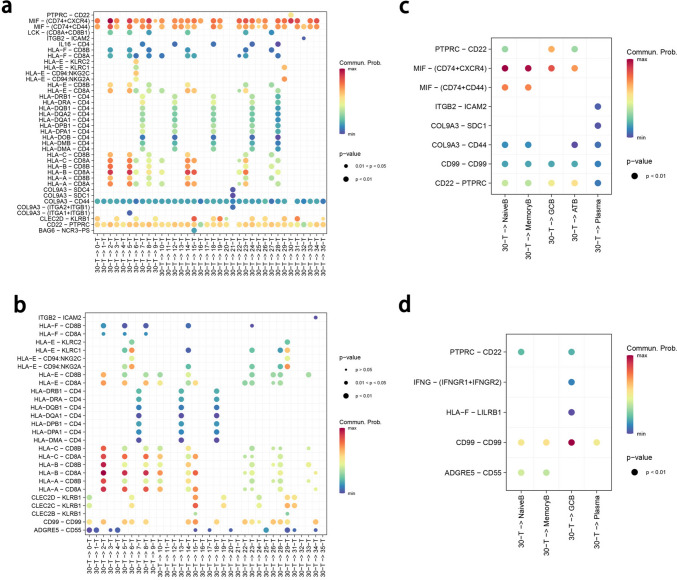
The expression of CXCR5 is associated with immune infiltration in CRC (Supplementary Fig. 6). To further explore the interaction of TFC cells, we continued to explore the cell‒cell interaction signals via CellChat. As shown in Fig. 7, both MSS and MSI tumor TFC cells were related to the HLA-CD8 signal. MSS tumor TFC cells interact with T cells through the MIF (CD74-CXCR4) and MIF (CD74-CD44) signaling pathways, indicating that TFC cells can regulate CD8 + T cells through inflammation and cell proliferation signal transduction.
Fig. 7.
CellChat analysis of MSS and MSI-high CRC. A: Dot plot of CellChat interactions between TFC cells and T cells from MSS CRC patients. b: Dot plot of CellChat interactions between TFC cells and T cells from MSI-high CRC patients. c: Dot plot of CellChat interactions between TFC cells and B cells from MSS CRC patients. A: Dot plot of CellChat interactions between TFC cells and B cells of MSI-high CRC
In MSI-high tumors, TFC cells interact with T cells through CD99‒CD99 interactions, indicating that TFC cells can regulate T cells through cell adhesion. For B cells and plasma cells, MSS tumor TFC cells can communicate with naïve B cells, memory B cells and germinal center (GC)-like B cells (GCB); MSI tumor TFC cells interact with GCB cells, suggesting that TFC cells may affect humoral immune responses by influencing the differentiation of B cells through GCB cells. The other immune cells are shown in Supplementary Figs. 7 and 8.
Relationship between TFC cells and CD19+CD38+ B cells
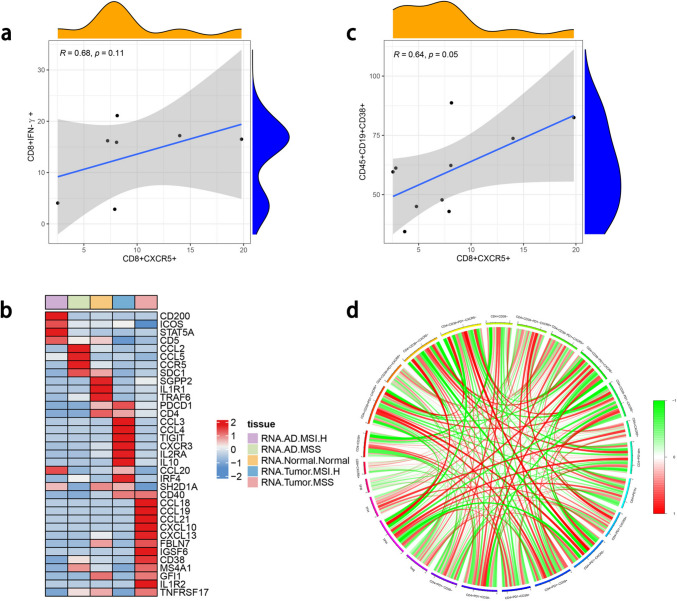
Because of the different interactions between B cells, we further explored the relationships between TFC cells and B cells in patients. In the baseline tumor tissue, the proportion of TFC cells was positively correlated with the proportion of CD19+CD38+ B cells and negatively correlated with those of CD19+CD38+IgG+ B cells, CD19+CD138+ B cells, TPH cells, TFH cells and Tregs. We continued to explore the interaction between TFC cells and the cytotoxic function of CD8. After PBMCs and tumor cells were cocultured, the expression of TFC cell was positively correlated with that of IFN-γ-producing CD8 + T cells (Fig. 8a).
Fig. 8.
Antitumor function and relationship between B cells. A: Relationship between TFC and CD8+IFN-γ. b: Relationships between TFC cell- and TLS-related genes. C: Heatmap of the relationships between TFC cell- and TLS-related genes. D: Relationships between TFC cells and T cells
Because the presence of TLSs may be a prognostic and predictive factor, to further confirm their antitumor function, we assessed TLS-related gene expression in TFC cells [25]. As shown in Fig. 8b, TFC cells were enriched in TLS-related genes and highly enriched in genes related to B cell regulation and cytotoxic function. With respect to B cells, the proportion of TFC cells was positively correlated with the proportion of CD19+CD38+ B cells (Fig. 8c) but weakly related to the proportions of other T cell types (Fig. 8d).
Conclusion
TFC cells in CRC have potential prognostic value, and our prognostic model exhibited good predictive value in both the training and validation cohorts. In MSS CRC, TFC cells function more strongly in terms of T cell activation and the cell cycle and has lower expression of immune checkpoint molecules, which may influence the effectiveness of ICB therapy. TFC cells may regulate antitumor function by regulating CD19+CD38+ B cells and TLSs.
Discussion
Colorectal cancer (CRC) is one of the most common digestive tract tumors in the world and the second leading cause of cancer-related death [29]. Under tumor conditions, the immune cells, fibroblasts, and microbiota in the intestine undergo changes, leading to a decrease in their ability to kill tumors, and these changes can even promote tumor development [30]. Follicular T cells are a special subset of T cells that express the chemokine receptor CXCR5 and play important roles in antibody-mediated immune responses [30, 31]. They primarily support germinal center (GC) responses, generating high-affinity and long-term humoral immunity in GC responses. At present, research on follicular T cells has focused mostly on CD4+ T lymphocytes, but relatively few studies have focused on CD8+CXCR5+ follicular cells (follicular cytotoxic T cells (TFC cells) in CRC, which are associated with multiple types of diseases [32, 33]. Single-cell transcriptomics, an important approach, can be used to divide CRC cells into different cell types and subgroups and identify the unique gene expression patterns of cell clusters [25]. Deep learning based on computational data might be useful for systematically predicting patient prognosis [29, 34]. In our studies, via scRNA-seq, we identified TFC cells and explored their different functions between MSS and MSI-high CRC.
We assessed the expression of TFC cell in tissues, and the results revealed that lower expression in patients with tumors indicated the inhibition of TFC cell infiltration in the tumor state. These results show that TFC cell can be used as predictive biomarkers. Owing to the noninvasive nature of peripheral blood biomarkers, we continued to explore the expression of TFC cell in the peripheral blood of CRC patients and healthy individuals. The expression of TFC cell was also lower in the blood of tumor patients than in that of normal controls, indicating that TFC cell can be a potential biomarkers for predicting CRC prognosis with blood samples. Because of its relatively low expression, we also built a prognostic model for predicting CRC patient survival. The prediction model also showed good predictive value for CRC.
The depleted T cell pool is maintained by a type of stem-like progenitor cell that can self-renew but also produces terminally differentiated T cells during long-term exposure to antigens. Studies have been performed to better understand the developmental relationship between exhausted T cell subsets; these studies revealed that transcriptional regulator T cell factor 1 (TCF1) and C-X-C chemokine receptor type 5 (CXCR5) are markers of exhausted T cell subsets [18, 35]. CXCR5 is expressed mainly in mature B cells and follicular helper T cells. Abnormal activation of the CXCL13/CXCR5 signaling pathway affects tumor cell metastasis and infiltration and is also associated with autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis [36]. In our studies, TFC cells were identified as stem-like progenitor cells that express TCF1; notably, TFC cells appeared in the initial and final stages of CD8+ T cell differentiation. As development progressed, cytotoxic CD8+ T cells gradually emerged. These results indicate that this TFC cells may be have the characteristics of exhausted progenitor T cells (Tpex) [37], which are crucial for maintaining sustained T cell responses over the long term and can induce the production of cytotoxic CD8 T cells associated with the therapeutic efficacy of ICBs [38]. Naïve CD4+ and CD8+ T cells proliferate into different functional subsets through the T cell receptor (TCR) and peptide-major histocompatibility complex (pMHC), which are costimulatory and cytokine receptors [39]. In our studies, the cytotoxic molecules and effector cytokines involved in the different cytotoxic programs differed between MSS and MSI-high CRC. We performed experiments to further explore the characteristics of TFC cells in different kinds of tissues. We also explored exhausted T cell-related biomarkers in MSS CRC and explore the expression of CTLA and classical exhausted markers, such as PD1, PDL1, LAG3 and Tim3, in MSI-H CRC. These findings also indicate that MSS CRC may have different response pathways for immunotherapy and antibody class switching [40]. Previous studies have shown that CXCR5+CD8+ T cells play roles in antitumor immunity [41], and we obtained the same results.
The homeostasis of the endoplasmic reticulum (ER) is an important factor for cell growth, differentiation, and apoptosis. Studies have shown that endoplasmic reticulum stress (ERS) is one of the prognostic factors affecting CRC [28]. In our study, there were different ERS-related genes between MSI-high CRC and MSS CRC, and ERS-related genes enriched in the MSS subtype of CRC were related to calcium ion metabolism. Compared with those in MSI-high CRC, cell cycle-related genes were more enriched in MSS CRC, indicating that TFC cells are associated with T cell development in MSS CRC. To further investigate the antitumor function of TFC cells, we explored the correlation between the proportion of TFC cells and that of CD8 + IFN-γ + cells. The results revealed a positive correlation between the two variables, but due to the large number of cases, the difference was not significant. Therefore, we continued to explore the correlations between TLS-related genes and TFC cell. These results indicate that MSS and MSI-high CRC are associated with genes related to tertiary lymphoid structures. Previous studies have reported that the presence of TLSs is a prognostic factor [26]; therefore, the presence of TFC cells in patients with CRC is associated with a good prognosis.
The differentially expressed genes between MSI-high CRC and MSS CRC are associated with different regulatory pathways. Genes enriched in MSS CRC TFC cells are associated with immune-related pathways, whereas those enriched in MSI-high CRC TFC cells are associated with metabolic-related pathways. The same results were also shown for different interactions among fibroblasts, endothelial cells and monocytes. The expression of CXCR5 can drive CXCR5+CD8+ T cells to migrate away from the T cell region toward B cell follicles [42]. In our studies, GCB cells were found to play a key role in the interactions between normal, MSS and MSI-high CRC via different pathways. In MSS CRC, more interactions involved naïve B and memory B cells, and our experiments revealed a close relationship between these cell types and CD19+CD38+ B cells. B cells play a crucial role in TLSs [43], and our results indicate the TFC cells can influence CD19+CD38+ B cells to regulate TLS function. However, our work has several limitations. In the future, we will further verify the findings in TFC cells, validate our model in a large sample cohort and further explore the regulatory mechanism among TFC cells, B cells and immunotherapy.
In summary, TFC cell-specific genes can serve as prognostic biomarkers for CRC, and TFC cells have antitumor immune functions. We also constructed a risk score model for predicting the OS of CRC patients. Our study provides a new direction for immunotherapy and CRC prognosis prediction.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The study was supported and funded by CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-012 and Key Project of National Key R & D Plan “Research on Prevention and Control of Major Chronic Non-Communicable Diseases”(No. 2019YFC1315705). In addition, the authors wish to thank all teachers at the Departments of Clinical Laboratory and Colorectal Surgery in National Cancer Center/Cancer Hospital.
Abbreviations
- TFC cell
CD8+ CXCR5+ follicular cytotoxic T cell
- CRC
Colorectal cancer
- ER
Endoplasmic reticulum
- ERS
Endoplasmic reticulum stress
- TFC cells
Follicular cytotoxic
- TFH
Follicular helper T cells
- GC
Germinal center
- GZMB
Granzyme B
- ICB
Immune checkpoint blockade
- IFN-γ
Interferon-γ
- MSS
Microsatellite-stable
- MSI
Microsatellite instability
- PI3K
Phosphatidylinositol 3-kinase
- scRNA-seq
Single-cell RNA sequencing
- TLS
Tertiary lymphoid structure
- TLO
Tumor tertiary lymphoid organs
- TNF-α
Tumor necrosis factor-α
Author contributions
Wei FZ and Xu XT designed the research; Wei FZ, Xu XT, JW, Mei SH, Zhao FQ and Liu ZR organized the data; Huang F, Xiao TX, Wei BJ, Huang SK, Wang GJ and Li YY analyzed and visualized the data; Xu XT and Wei FZ drafted the article; Cui W revised the paper.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-012 and Key Project of National Key R & D Plan “Research on Prevention and Control of Major Chronic Non-Communicable Diseases” (No. 2019YFC1315705).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethical approval
This study was approved by the ethics committee of the Cancer Hospital.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fangze Wei and Xiaotian Xu contributed equally to this work and are regarded as co-first authors.
References
- 1.Farhad I, Emily C M, Blake T, et al. (2019) Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. 2024, (0) [DOI] [PubMed]
- 2.Murthy SS, Trapani D, Cao B et al (2024) Premature mortality trends in 183 countries by cancer type, sex, WHO region, and World Bank income level in 2000–19: a retrospective, cross-sectional, population-based study. Lancet Oncol 25(8):969–978. 10.1016/S1470-2045(24)00274-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christopher JMW, Richard G, Robert KH et al (2024) Evaluation of CD3 and CD8 T-Cell immunohistochemistry for prognostication and prediction of benefit from adjuvant chemotherapy in early-stage colorectal cancer within the QUASAR trial. J Clin Oncol 42:3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maria P, Stephanie LG, Frank JL et al (2024) Adoptive transfer of personalized neoantigen-reactive TCR-transduced T cells in metastatic colorectal cancer: phase 2 trial interim results. Nat Med 30:2586 [DOI] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O (2000) Chemokines. Immunity 12(2):121–127. 10.1016/S1074-7613(00)80165-X [DOI] [PubMed] [Google Scholar]
- 6.Binhan W, Manni W, Danyi A et al (2022) CXCL13-CXCR5 axis: Regulation in inflammatory diseases and cancer. Biochim et Biophys Acta Rev Cancer 1877(5):188799 [DOI] [PubMed] [Google Scholar]
- 7.Matthew TT, James DB, Claire D et al (2019) Preferential small intestine homing and persistence of CD8 T cells in rhesus macaques achieved by molecularly engineered expression of ccr9 and reduced ex vivo manipulation. J Virol. 10.1128/JVI.00896-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster R, Emrich T, Kremmer E, Lipp M (1994) Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 84(3):830–840. 10.1182/blood.V84.3.830.bloodjournal843830 [PubMed] [Google Scholar]
- 9.Förster R, Mattis AE, Kremmer E et al (1996) A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87(6):1037–1047. 10.1016/S0092-8674(00)81798-5 [DOI] [PubMed] [Google Scholar]
- 10.El-Haibi C, Singh R, Gupta P et al (2012) Antibody microarray analysis of signaling networks regulated by Cxcl13 and Cxcr5 in prostate cancer. J Proteom Bioinform 5(8):177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Takemoto Y, Deng H et al (2017) Histidine decarboxylase (HDC)-expressing granulocytic myeloid cells induce and recruit Foxp3 regulatory T cells in murine colon cancer. OncoImmunology 6(3):e1290034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimenta E, Barnes BJC (2014) Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancer 6(2):969–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hideki UJJCI (2016) Human circulating T follicular helper cell subsets in health and disease. J Clin Immunol 36:34 [DOI] [PubMed] [Google Scholar]
- 14.Quigley M, Gonzalez V, Granath A et al (2007) CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol 37(12):3352–3362 [DOI] [PubMed] [Google Scholar]
- 15.Brummelman J, Mazza E, Alvisi G et al (2018) High-dimensional single cell analysis identifies stem-like cytotoxic CD8 T cells infiltrating human tumors. J Ex Med 215(10):2520–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Im SJ, Hashimoto M, Gerner MY et al (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537(7620):417–421. 10.1038/nature19330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolanda B, Emilia MCM, Giorgia A et al (2018) High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med 215(10):2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan X, Zebley CC, Youngblood B (2023) Cellular and molecular waypoints along the path of T cell exhaustion. Sci Immunol. 10.1126/sciimmunol.adg3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Wang D, Li Y et al (2024) Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell 42(7):1268–85.e7 [DOI] [PubMed] [Google Scholar]
- 20.Wei G, Cuiyu Z, Xia W et al (2021) Resolving the difference between left-sided and right-sided colorectal cancer by single-cell sequencing. JCI Insight. 10.1172/jci.insight.152616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linlin J, Gongbo F, Mengxi H et al (2024) scRNA-seq of colorectal cancer shows regional immune atlas with the function of CD20(+) B cells. Cancer Lett 584:216664 [DOI] [PubMed] [Google Scholar]
- 22.Hae-Ock L, Yourae H, Hakki Emre E et al (2020) Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat Genet 52(6):594 [DOI] [PubMed] [Google Scholar]
- 23.Laetitia M, Aurélien DR, Alex D et al (2013) Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 10(5):e1001453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshua J, Natasha GD, Fei W, Nipun BM, Bing Z, Aixiang J et al (2009) Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology 138(3):958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jingjing Q, Hongxiang S, Yao Z et al (2022) Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun 13(1):1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catherine S-F, Florent P, Julien C et al (2019) Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 19(6):307 [DOI] [PubMed] [Google Scholar]
- 27.Mathieu U, Jerome CM, Luka M et al (2022) Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med 28(4):766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng J, Guo Y, Xie M et al (2023) Characteristics of endoplasmic reticulum stress in colorectal cancer for predicting prognosis and developing treatment options. Cancer Med 12(10):12000–12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng C, Yoshino T, Ruíz-García E et al (2024) Colorectal cancer. Lancet 404(10449):294–310. 10.1016/S0140-6736(24)00360-X [DOI] [PubMed] [Google Scholar]
- 30.Yonekura S, Terrisse S, Silva CAC et al (2021) Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov 12(4):1128–1151. 10.1158/2159-8290.CD-21-0999 [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez-Melo N, Baumjohann D (2023) T follicular helper cells in cancer. Trends Cancer 9(4):309–325. 10.1016/j.trecan.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Fan Z, Tao B et al (2024) Density of tertiary lymphoid structures predicts clinical outcome in breast cancer brain metastasis. J ImmunoTherapy Cancer 12(7):e009232. 10.1136/jitc-2024-009232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceylan A, Artac M, Kocak M et al (2024) Epidermal growth factor receptor and programmed cell death-1 expression levels in peripheral T cell subsets of patients with non-small cell lung cancer. Scand J Immunol 100:e13398 [DOI] [PubMed] [Google Scholar]
- 34.Adam JW, Minita S, Amanda F et al (2024) Ultrasensitive plasma-based monitoring of tumor burden using machine-learning-guided signal enrichment. Nat Med 30(6):1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caroline SJ, Nataliya P, Viraj AM et al (2019) An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576(7787):465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazanietz M, Durando M, Cooke MJFIE (2019) CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front Endocrinol 10:471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallies A, Zehn D, Utzschneider DJNRI (2020) Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol 20(2):128–136 [DOI] [PubMed] [Google Scholar]
- 38.Sade-Feldman M, Yizhak K, Bjorgaard S et al (2019) Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 176:404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinfang Z, William EPJB (2008) CD4 T cells: fates, functions, and faults. Blood 112(5):1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristen MV, Dan D, Travis JL et al (2018) CD8 follicular T cells promote B cell antibody class switch in autoimmune disease. J Immunol 201(1):31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Junjie X, Chenxin Z, Xiaohong Y et al (2017) CXCR5(+)CD8(+) T cells infiltrate the colorectal tumors and nearby lymph nodes, and are associated with enhanced IgG response in B cells. Exp Cell Res 356(1):57 [DOI] [PubMed] [Google Scholar]
- 42.Di Y, Lilin YJTI (2018) A portrait of CXCR5(+) follicular cytotoxic CD8(+) T cells. Trends Immunol 39(12):965 [DOI] [PubMed] [Google Scholar]
- 43.Fridman W, Meylan M, Pupier G et al (2023) Tertiary lymphoid structures and B cells: an intratumoral immunity cycle. Immunity 56(10):2254–2269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.