Abstract
Background
Cardiogenic shock is a critical cardiac condition characterized by low cardiac output leading to end-organ hypoperfusion and associated with high in-hospital mortality rates. It can manifest following acute myocardial infarction or acute exacerbation of chronic heart failure. Despite advancements, mortality rates remain elevated, prompting interest in multidisciplinary approaches to improve outcomes. This manuscript presents a review focused on the concept of a cardiogenic shock team and its potential impact on patient management and outcomes.
Methods
A comprehensive search was performed on March 19, 2023, covering PubMed, Web of Science, Scopus, Embase, and Cochrane Library. We included primary studies (prospective and retrospective) only and evaluated their quality using the Newcastle–Ottawa Quality Scale. This review was registered in PROSPERO (CRD42023440354).
Results
Six relevant studies with 2066 cardiogenic shock patients were included, of which 1071 were managed by shock teams and 995 received standard care. Findings from the reviewed studies indicated the favorable outcomes associated with implementing cardiogenic shock teams. Patients managed by these teams exhibited higher 30-day and in-hospital survival rates compared to those without team intervention. The implementation of cardiogenic shock teams was linked to reduced in-hospital and intensive care unit mortality rates. Additionally, shock team involvement was associated with shorter door-to-balloon times.
Conclusion
The findings suggest that cardiogenic shock teams play a crucial role in improving patient outcomes through earlier detection and timely interventions. Despite challenges in team implementation, their potential to reduce mortality and improve efficiency in patient care warrants further research and greater integration of multidisciplinary strategies into clinical practice.
Supplementary Information
The online version contains supplementary material available at 10.1186/s43044-024-00594-z.
Keywords: Cardiogenic shock, Shock team, Mechanical circulatory support, Resuscitation, Multidisciplinary care
Background
Cardiogenic shock (CS) is a hemodynamically complex cardiac disorder associated with low cardiac output that leads to clinical manifestations and biochemical evidence of end-organ hypoperfusion. CS can present with different phenotypes, most commonly as a complication of acute myocardial infarction (AMI-CS). It can also result from different etiologies, like an acute exacerbation of chronic heart failure (non-AMI-CS). Notably, CS is associated with high in-hospital mortality ranging between 30 and 60% [1]. AMI-CS mortality has slightly improved due to early intervention with Percutaneous Coronary Intervention (PCI) to 50.3%, as reported in the SHOCK trial [2].
Subsequent clinical trials have failed to show improvement in mortality with additional mechanical support for revascularization, such as Intra-aortic balloon pump (IABP) [3–5]. However, early application of mechanical support was reported to improve mortality [6]. Tehrani et al. reported that every 1-h delay in mechanical circulatory support (MCS) therapy was associated with a 9.9% increased risk of death [7]. The improvement in outcome with the early application of MCS goes back to the pathophysiology of CS. CS progresses from a treatable hemodynamic problem to a hemo-metabolic problem that does not respond to the MCS. Knowing that the concept of “door to support” time started to gain more attention in treating CS [10].
Early identification of CS can be challenging because patients can present to the ER or the health care facility in different stages and phenotypes. In other critical health conditions like pulmonary embolism and stroke, early identification and intervention by a dedicated rapid response team were associated with a marked decrease in mortality and morbidity [11, 12].
From here, the idea of creating a multidisciplinary team for the early identification and management of CS started to be implemented. Different centers applied this concept and found that managing CS with a rapid-response multidisciplinary team was associated with decreased mortality [7, 13–18]. Despite the promising outcomes from individual centers, there is limited synthesis of evidence on the overall effectiveness of cardiogenic shock teams across different settings. This review aims to address this gap by systematically analyzing the impact of cardiogenic shock teams on patient outcomes. Specifically, we seek to evaluate whether the implementation of such teams leads to improved survival and reduced mortality rates, as well as other key clinical outcomes.
Methods
Search strategy
We searched PubMed, Web of Science, Scopus, Embase, and Cochrane Center till March 19, 2023. We used the following search strategy to find all the studies discussing the management of cardiogenic shock with versus without shock teams (“ECMO team” OR “Multidisciplinary Care Team” OR “Interdisciplinary Health Team” OR “Shock team” OR “Rapid Response Team”) AND (“Cardiac shock” OR “Cardiogenic Shock”) as shown in the appendix. We gathered the search terms from the MeSH database and the literature and then built the strategy as described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 4.4.4) [18].
Our review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [19]. This review was registered in PROSPERO (CRD42023440354).
Selection criteria and screening
We included primary studies (prospective and retrospective) published in peer-reviewed journals comparing critical outcomes of cardiogenic shock in adult patients who were managed with shock teams versus those without shock teams. We excluded animal studies, case reports, letters to the editor, conference abstracts, and secondary studies.
Three authors (A.S.E, A.M.A, M.A) screened the articles by title and abstract, then by reading the full texts using Covidence systematic review software (Available at www.covidence.org). Any disagreement was resolved through consensus.
Data extraction
We extracted data summarizing the included studies’ criteria, demographics, and baseline characteristics of their patients, including CS etiology, cardiac arrest, baseline lab values, and cardiovascular risks.
Additionally, we extracted the studies’ outcome data, including the rates of 30-day survival, in-hospital survival, in-hospital mortality, ICU mortality, time to treatment (door-to-balloon time), rate of MCS utilization, and the use of mechanical ventilation and renal replacement therapy. Two authors (A.S.E., A.M.A.) extracted the data and then all the extracted data were revised by a third author (M.A.).
Quality assessment
Two authors (A.S.E, A.M.A) evaluated the quality of the included studies using the Newcastle–Ottawa Quality Scale (NOS) [20]. The NOS evaluates the studies’ quality according to three domains: selection of study population; comparability between study cases and controls; and exposure determination. The results from the NOS were converted into the Agency for Healthcare Research and Quality (AHRQ) standards as the following: (A) Good quality: 3 or 4 stars in selection domain, 1 or 2 stars in comparability domain, and 2 or 3 stars in outcome/exposure domain; (B) Fair quality: 2 stars in selection domain, 1 or 2 stars in comparability domain, and 2 or 3 stars in outcome/exposure domain; (C) Poor quality: 0 or 1 star in selection domain, 0 stars in comparability domain, or 0 or 1 star in outcome/exposure domain [20]. According to AHRQ standards, two of the included studies in our review have good quality [13, 15], and four studies have poor quality [7, 14, 16–18] (Table 3).
Table 3.
Quality assessment using the Newcastle–Ottawa scale for cohort studies
| Study ID | Year | Selection | Comparability | Exposure | AHRQ standards | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of the study | Study controls for age | Study controls for any additional factor | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Papolos et al | 2021 | ★ | ★ | ★ | ★ | – | – | ★ | ★ | ★ | Poor quality |
| Hong et al | 2020 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | Good quality |
| Lee et al | 2020 | ★ | ★ | ★ | ★ | – | – | ★ | ★ | ★ | Poor quality |
| Taleb et al | 2019 | ★ | ★ | ★ | ★ | ★ | – | ★ | ★ | ★ | Good quality |
| Tehrani et al | 2019 | ★ | ★ | ★ | ★ | – | – | ★ | ★ | ★ | Poor quality |
| Sebat et al | 2005 | ★ | ★ | ★ | ★ | ★ | – | ★ | – | – | Poor quality |
Results
Search results
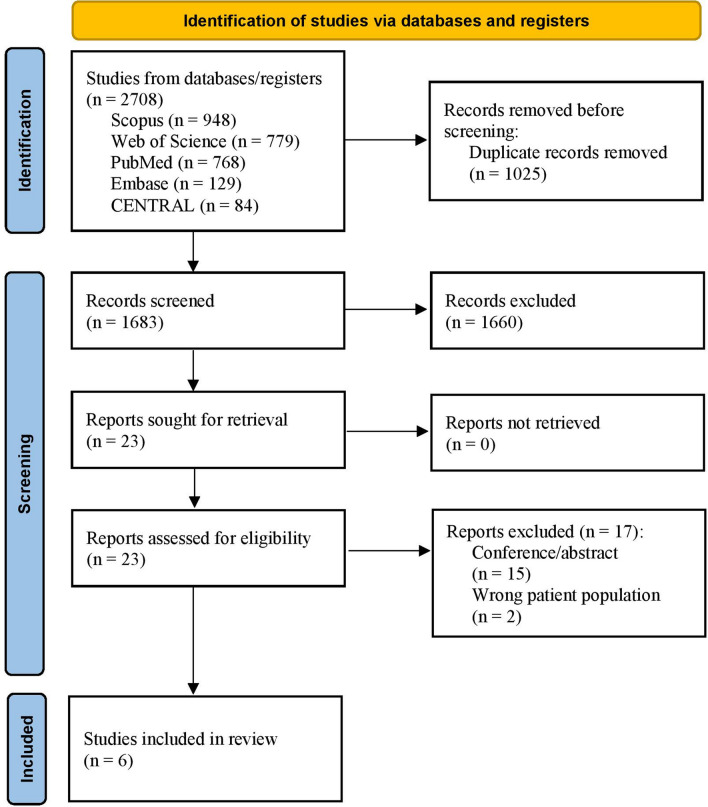
The search strategy over the different medical databases yielded 1683 after removing duplicates. Screening the title and the abstract yielded 23 studies after excluding 1660 papers because they were animal studies, out of the study criteria, and/or not primary studies. Twenty-three articles were screened for their full-text testing for eligibility. Six papers met the study criteria [7, 13–17] and were further included in the quality assessment, as shown in Fig. 1.
Fig. 1.
The PRISMA flow diagram
Baseline characteristics and outcomes
All the included studies are peer-reviewed cohort studies. Three of the studies are retrospective cohort studies [15–17], while the other three are retrospective-prospective cohorts. Five studies are from the USA [7, 13, 14, 16, 17] or Canada, and one study is from Korea [15]. The included studies encompass data from 2066 cardiogenic shock patients, with 1071 patients being treated by the shock team and 995 without the shock team’s intervention. The mean age of the participants is greater than 50 years old. These studies consist of 1433 male patients, 850 patients with acute myocardial infarction-related cardiogenic shock, 1195 patients with non-AMI CS, 541 patients who experienced cardiac arrest, and 293 patients with diabetes mellitus. Further details regarding baseline characteristics and baseline laboratory work, as well as cardiovascular risks, are summarized in Table 1.
Table 1.
The summary of the included studies
| Study ID | Country | Study design | The team | Group | No. of patients |
|---|---|---|---|---|---|
| Hong et al. (2020) | Republic of Korea | Retrospective cohort study |
Interventional cardiologist Critical care physician Cardiovascular surgeon Heart failure physician Pharmacist Nutritionist Perfusionists (formal intensive care registered nurses and received specific ECMO training) |
CS team | 124 |
| Control | 131 | ||||
| Lee et al. (2020) | Canada | Retrospective cohort study |
Interventional cardiologist Heart failure physician Cardiovascular surgeon Intensive Care Unit physician Coronary care unit |
CS team | 64 |
| Control | 36 | ||||
| Papolos et al. (2021) | United States and Canada | Retrospective cohort study |
Interventional cardiologist Critical care physician Cardiovascular surgeon Heart failure physician ECMO Service |
CS team | 546 |
| Control | 696 | ||||
| Taleb et al. (2019) | United States | Retrospective and prospective cohort study (control cohort) |
Interventional cardiologist Intensive Care Unit physician Cardiovascular surgeon Heart failure physician |
CS team | 123 |
| Control | 121 | ||||
| Tehrani et al. (2019) | United States | Retrospective and prospective cohort study (control cohort) |
Interventional cardiologist Critical care physician Cardiovascular surgeon Heart failure physician |
AMI | 82 |
| Acute Decompensated HF (ADHF) | 122 | ||||
| Control | NA | ||||
| Sebat et al. (2005) | United States | Retrospective and prospective cohort study (control cohort) |
Critical care physician Emergency physician Nurses and other health-care providers, respiratory therapist, clinical laboratory, radiology, pastoral care, and social services |
CS team | 10 |
| Control | 11 |
AMI Acute myocardial infarction, CS Cardiogenic shock, HF Heart failure
Sebat et al. excluded all patients who were not eligible for aggressive treatment or were suffering from non-survivable conditions that preceded the shock state, such as untreatable metastatic carcinoma, brain death, or ruptured thoracic aneurysm [14]. Similarly, Lee et al. excluded terminally-ill patients with a life expectancy of less than 6 months, as well as those who experienced cardiac arrest for more than 30 min [16]. While Hong et al. included patients with AMI-CS undergoing veno-arterial extracorporeal membrane oxygenation (VA-ECMO), they excluded stable patients who received prophylactic VA-ECMO before revascularization [15]. Taleb et al. also excluded postcardiotomy patients and those who required ECMO [13].
Efficacy of shock teams
Three studies reported the 30-day survival rate (Fig. 2), two of them (Hong et al. & Taleb et al.) found significantly higher survival rates among CS patients treated with the shock team [13, 15, 16]. Two studies investigated the in-hospital survival rate (Fig. 3), one of them (Taleb et al.) reported a higher survival rate in the CS team group [13], while the other (Lee et al.) reported no significant difference between both groups [16]. Hong et al. and Sebat et al. investigated the in-hospital mortality rate and reported lower rates with the implementation of the shock team [14, 15]. Sebat et al. also found trends toward lower mortality in subgroups with cardiogenic, hypovolemic, and septic shock, though these trends did not reach statistical significance. Notably, septic shock patients experienced the greatest reduction in mortality, with a 17.4% absolute reduction in the shock team group compared to controls [14]. Hong et al. and Papolos et al. reported the ICU mortality rate and found a lower mortality rate for the patients treated with shock team [15, 17]. Also, Hong et al. and Taleb et al. reported door-to-balloon time and found a shorter time to start treating patients with shock team [13, 15]. However, Hong et al. reported that NSTEMI Shock patients treated with a shock team spent more time to start the treatment [15]. Moreover, the utilization rate of MCS was documented in studies by Lee et al. and Papolos et al. (Fig. 4). The former demonstrated a higher rate of MCS utilization in the CS team group, whereas the latter presented contrasting results [16, 17]. Papolos et al. stratified patients by AMI-CS and non-AMI-CS subgroups and found that shock team centers used more pulmonary artery catheters (PACs) and advanced MCS in AMI-CS patients [17]. In non-AMI-CS patients, shock teams used more PACs but did not significantly increase advanced MCS utilization. Despite this, lower CICU mortality rates were observed at shock team centers for both AMI-CS and non-AMI-CS patients. Among patients who received PACs, those treated at shock team centers experienced lower mortality (21.2% vs. 26.4%) and less overall MCS use, while advanced MCS was more commonly employed [17].
Fig. 2.
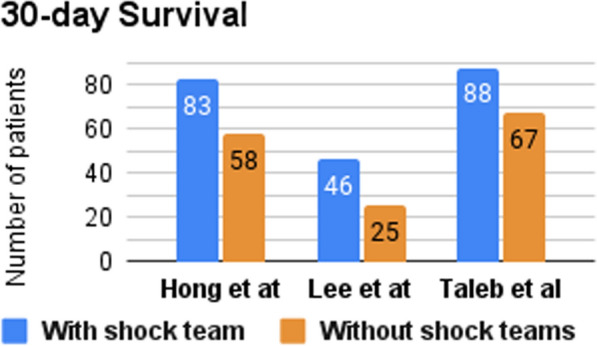
Number of patients who survived for ≥ 30 days
Fig. 3.
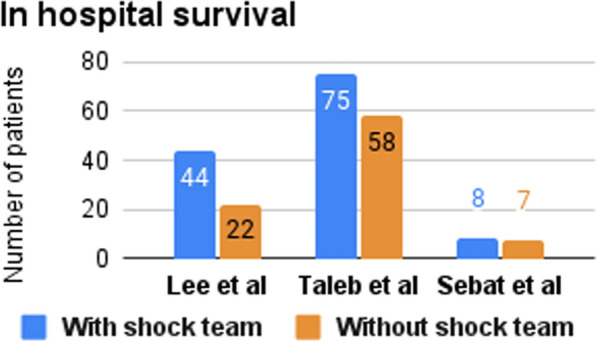
Number of patients who survived in hospital stay
Fig. 4.
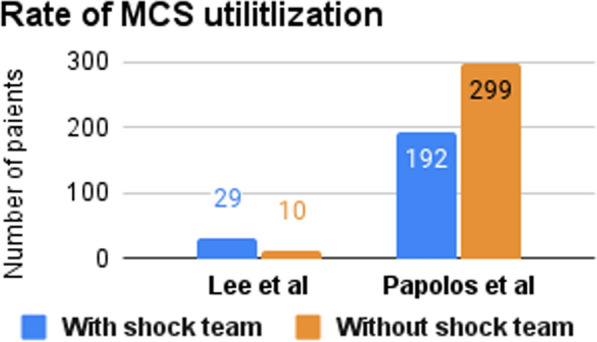
Rate of mechanical cardiac support utilization
Additionally, we summarized more details about the reported measures for CS patients’ management, such as mechanical ventilation, and renal replacement therapy in Table 2.
Table 2.
Baseline characteristics of the participants
| Study ID | Group | Demographics | Cardiogenic shock etiology | Cardiac Arrest | Baseline lab | Cardiovascular risks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Age, y, M (SD) | Male, n (%) | AMI-CS, n (%) | Non-AMI-CS, n (%) | Out-of-hospital, n (%) | In-hospital, n (%) | Baseline creatinine, mg/dL, M (SD) | Baseline lactic acid, mmol/L, M (SD) | Diabetes mellitus, n (%) | Baseline cardiac index, L/min/m2, M (SD) | Left ventricular ejection fraction, M (SD) | ||
| Hong et al. (2020) | CS team | 124 | 64.7 (11.5) | 95 (76.6) | 124 (100%) | 0 | 22 (17.7) | 81 (65.3) | 1.3 (0.5) | 5.03 (4.05) | 61 (49.2%) | NA | 31.5 (11.63) |
| Control | 131 | 63.4 (12) | 105 (80.2) | 131 (100%) | 0 | 17 (13) | 82 (62.6) | 1.3 (0.6) | 6.5 (5.7) | 67 (51.1%) | 34 (14.99) | ||
| Lee et al. (2020) | CS team | 64 | 53.9 (15.8) | 50 (78) | 7 (11) | 57 (89) | 13 (20) | 1.5 (0.7) | 3.6 (2.6) | NA | 1.65 (0.6) | 18.3 (9.9) | |
| Control | 36 | 60.1 (16.1) | 24 (67) | 6 (17) | 30 (83) | 8 (22) | 2 (1.5) | 3.1 (2.2) | 2.03 (1) | 21.7 (11.6) | |||
| Papolos et al. (2021) | CS team | 546 | 64.7 (15.6) | 351 (64.3) | 147 (26.9) | 399 (73.1) | 61 (12) | 67 (13.1) | 1.6 (0.9) | 3.77 (2.38) | NA | 1.9 (0.54) | CS/control ≥ 50:87 (17.09)/88 (13.79) 20–50: 267 (52.46)/255 (39.97) < 20: 55 (30.45%)/295 (46.24) |
| Control | 696 | 63 (13.4) | 477 (68.5) | 193 (27.7) | 503 (72.3) | 77 (11.8) | 83 (12.7) | 1.7 (0.9) | 2.7 (2.23) | 2.04 (0.67) | |||
| Taleb et al. (2019) | CS team | 123 | 57 (1) | 96 (78.04) | 75 (60.97) | 48 (39.02) | NA | 5.4 (0.5) | 37 (30.08) | 2.2 (0.2) | 24 (2) | ||
| Control | 121 | 59 (1) | 93 (76.85) | 85 (70.25) | 36 (29.75) | 5.9 (0.5) | 35 (28.92) | 2.2 (0.2) | 28 (2) | ||||
| Tehrani et al. (2019) | AMI | 82 | 64 (11) | 58 (70.73) | 82 (100) | 0 (0) | 9 (10.98) | 10 (12.2) | NA | 4.9 (4.3) | 46 (56.09) | < 1.8/ < 2.2 l/min/m2 without/with inotropes/vasopressors | NA |
| Decompensated HF | 122 | 58.4 (14) | 84 (68.85) | 0 (0) | 122 (100) | 6 (4.92) | 5 (4.1) | 4.3 (3.8) | 47 (38.52) | ||||
| Control | NA | NA | |||||||||||
| Sebat et al. (2005) | CS team | 11 | NA | ≥ 2.5 L/min/m2 | NA | ||||||||
| Control | 10 | ||||||||||||
AMI Acute myocardial infarction, CS Cardiogenic shock, HF Heart failure, NA Not available
Discussion
Definition of CS
Early identification and diagnosis of cardiogenic shock are critical to improving its poor outcomes. CS is pragmatically referred to as the state of impaired cardiac output and decreasing tissue perfusion. The first definition of CS was mentioned in the SHOCK trial [2] based on clinical and hemodynamic indices. Clinical indices include systolic blood pressure (SBP) < 90 mmHg for ≥ 30 min OR SBP ≥ 90 mmHg with support AND end-organ hypoperfusion (i.e., urine output (UOP) < 30 mL/h or cool extremities). Hemodynamic indices include a Cardiac Index (CI) of ≤ 2.2 L/min/m2 AND pulmonary capillary wedge pressure (PCWP) ≥ 15 mmHg. The IABP-SHOCK II trial [3] defined CS as the following: SBP < 90 mmHg for ≥ 30 min OR catecholamines to maintain SBP > 90 mmHg AND clinical pulmonary congestion AND impaired end-organ perfusion including altered mental status, cold/clammy skin, and extremities, UOP < 30 mL/h, or lactate > 2.0 mmol/L. A recent definition of CS was mentioned by the European Society of Cardiology (ESC) [21] as SBP < 90 mmHg with adequate volume AND clinical signs of hypoperfusion, including cold extremities, oliguria, mental confusion, dizziness, narrow pulse pressure, OR laboratory signs of hypoperfusion including metabolic acidosis, elevated serum lactate, and elevated serum creatinine. The Society for Cardiovascular Angiography and Intervention (SCAI) also proposed and validated a recent classification of CS into five stages ranging from ‘at risk’ to ‘extremis’ and defined CS according to the stage. For example, classic CS was described as a manifestation of hypoperfusion requiring medical or MCS intervention to restore perfusion AND relative hypotension [22, 23].
In our review, Hong et al. used the ECS definition of CS to identify their inclusion criteria [15], Lee et al. and Tehrani et al. used the SHOCK trial definition [16, 18], Lee et al. and Papolos et al. reported that most of their patient population fell into categories C “classic CS” and D “deteriorating” respectively according to the SCAI classification system [16, 17], while Taleb et al. and Sebat et al. didn’t report the criteria based on which CS was confirmed [13, 14].
Phenotypes of CS
Earlier, we mentioned that CS presents at different stages and phenotypes. The most common phenotype presents with low CI, elevated systemic vascular resistance (SVR), and high PCWP and is referred to as “cold and wet” CS [24]. Cold and wet CS is mainly AMI-CS. Another less common phenotype is called “cold and dry.” It presents with low CI, lower PCWP, and euvolemia. Cold and dry CS is mainly non-AMI CS. A third AMI-CS phenotype is called “wet and warm.” This wet and warm CS presents with low CI, systemic inflammation, increased PCWP, and low SVR; it also has a higher risk of mortality [25]. A fourth AMI-CS phenotype was reported in the SHOCK trial with SBP > 90 mmHg without vasopressors [26]. The last AMI-CS phenotype reported in the SHOCK trial is right ventricular (RV) CS [27]. Heart Failure (HF)-CS is another phenotype which relates to CS that develops as a complication of acute decompensated HF (ADHF) and is also mostly described as “wet and warm” [28]. Overall, the common characteristic between all phenotypes is low CI.
Multidisciplinary CS team
Given the different phenotypes, complexity, and high mortality of CS, new approaches to improving its outcomes started to be embraced. A cardiogenic shock team, or shock team, is one of the newly recognized approaches for managing CS and has shown favorable outcomes. The idea of creating a shock team was inspired by other teams developed to manage acute complex health problems, such as stroke teams and ST-elevation myocardial infarction (STEMI) teams. The shock team is a multidisciplinary team that gets activated to identify and manage patients who meet predefined criteria for CS. To date, there is no unified structure for the shock team. However, the most frequently reported members of the team in the studies included in our review are a cardiothoracic surgeon, an interventional cardiologist, a heart failure specialist, a critical care cardiologist, and an emergency physician. These members cover four main areas, including the intensive care unit (ICU), cardiac catheterization lab, cardiac surgery, and advanced heart failure [8, 9, 29]. Support from trained nurses, perfusionists, and respiratory therapists is also required. The team has a coordinating physician or a team leader who gets notified first once a suspected CS patient presents. The team leader, in turn, notifies the rest of the team members and activates the team as well as the supporting staff. Most of the time, the team leader is the on-call critical care cardiologist. To make the team more efficient, different departments should be aware of the shock team and the adopted local guidelines for CS identification and thus notify the team leader whenever a case of CS is suspected. The team leader responds to these notifications, assesses the case, and then activates the team [29]. If the shock is AMI-CS, the interventional cardiologist and the cardiothoracic surgeon start the revascularization procedures and provide large-bore vascular access for MCS and emergency cardiac surgeries as needed. The heart failure specialist and critical care cardiologists usually continue to care for the patients after the interventional procedures. For patients presenting with non-AMI CS, the critical care cardiologist, and heart failure specialist initiate a complete assessment with the interventional team members on standby if needed.
Although shock team members may have overlapping medical skills, every member has a unique skill that adds to the team’s ability to maximize the patient’s outcome. Therefore, all team members should be actively involved in decision-making and considerate of the long-term plan for patient management.
Multidisciplinary CS-team and clinical outcomes
CS patients treated with CS team showed an overall higher 30-day survival rate, reduced in-hospital and ICU mortality, shorter door-to-balloon time, faster initiation of therapies, including MCS and less need for mechanical ventilation and renal replacement therapy.
30-Day survival
Tehrani et al. looked at the outcomes of implementing a CS “shock team” for treating patients with CS. Their study consisted of 204 patients with CS who were monitored for 18 months. Notably, the 30-day survival of CS patients post-discharge increased from a baseline survival of 47% to 58% in the first year and 77% in the second year after the implementation of a shock team, and to 52% and 78% in AMI and ADHF patients, respectively (Table 2) [18].
Similarly, Taleb et al. also compared the outcomes of treating CS with and without designated CS teams. They found a decrease in 30-day all-cause mortality for CS patients treated with the shock team (Table 2).
In-hospital survival
Taleb et al. found an increase in in-hospital survival (61% vs. 47%) for patients treated by a shock team when compared with non-shock team (Table 2). Contrarily, Lee et al. reported no statistically significant difference in the short-term survival rates between the CS patients treated with shock team vs. control team. However, the overall survival showed an improvement of 67% over a follow-up duration of 240 days. These findings support the efficacy of implementing CS teams, and especially noteworthy are the profound improvements in survival [13, 16].
In-hospital mortality
Hong et al. reported significantly reduced in-hospital mortality (71% vs. 42%) among CS patients in the Shock team group vs. control group [16]. Sebat et al. also reported an improvement of in-hospital mortality with the shock team implementation despite the small population of CS patients included in their study (Table 2) [14].
ICU mortality
Papolos et al. reported that the duration of stay in a cardiac intensive care unit (CICU) and CS outcomes showed considerable improvements. Patients under the care of a CS team were in the CICU for an average of 4 days, compared to 5.1 days for patients treated without a designated CS team. Furthermore, there was a reduction in mortality for patients in CICUs with a CS team compared to those in CICUs without a CS team (23% vs 29%, respectively) (Table 2) [17].
Similarly, another study conducted by Hong et al. compared mortality rates for 255 AMI-CS patients before and after the development of a designated multidisciplinary team. They found a significant decrease in in-hospital mortality (54% vs. 33%) and CICU mortality (45% vs. 25%) after implementation and treatment by a multidisciplinary ECMO team (Table 2). Furthermore, the study also found a decrease in both all-cause mortality (58% vs. 35%) and readmission rates for heart failure (28% vs. 6%) under an ECMO team at a 6-month follow-up [15].
Likewise, a study by Lee et al. found improved outcomes after the implementation of a multidisciplinary ECMO team for patients who underwent in-hospital cardiac arrest [16]. Specifically, their study showed decreases in both in-hospital mortality (75% vs. 40%) and negative neurological outcomes (78% vs. 48%) for these patients after the implementation of the ECMO team. Interestingly, there was no significant difference in either in-hospital mortality or neurological outcomes for patients who experienced cardiac arrest outside of the hospital, regardless of whether the ECMO team was utilized or not. Lee et al. reason that during a cardiac arrest, it is often difficult to determine appropriate interventions, especially due to a lack of information in a real-time situation; hence, a multidisciplinary team of experts can help determine optimal strategies and timing for advanced support, such as ECMO to help improve outcomes in critically acute situations [16].
Lastly, no differences were found in ICU stay or rates of major complications such as bleeding, cerebrovascular events, hemolysis, or other vascular complications between the experimental or control groups [13]. This could be an area for further research, specifically targeting the management of complications related to CS.
Time to treatment (door-to-balloon time)
Along with the deleterious effects that can be attributed to delays in identification and intervention for CS, another important factor to consider is the time required for stabilizing a very sick patient with CS [8]. In the case of AMI-CS, less than 40% of these patients are treated within the recommended contact-to-device time (90 min) [30]. Furthermore, findings by Scholz et al. have demonstrated that a 10-min delay in primary PCI for a patient presenting with CS led to over three additional deaths out of every 100 patients treated with PCI [31]. Moreover, the time between the initial presentation of a CS patient and PCI intervention has a strong association with a negative outcome. As a result, door-to-support time became an emergent concept for managing AMI-CS. This is important to consider as studies have demonstrated that the timely deployment of MCS devices have led to better clinical outcomes [32, 33]. Providing early and effective MCS, especially in AMI-CS, helps unload the left ventricle, prevents or can even reverse end-organ damage, decreases myocardial wall tension, and, in turn, improves outcomes [10, 34]. Basir et al. reported that early implementation of MCS in patients with cardiogenic shock independently improved survival [6]. When MCS was initiated less than 1.25 h from shock onset, the survival of patients with CS was 66%, when initiated within 1.25–4.25 h, survival was 37%, and 26% when initiated after 4.25 h. Papolos et al. compared the outcomes of managing CS patients with shock teams versus without and found that facilities with a shock team had increased pulmonary arterial catheter use (60% vs. 49%) that were initiated in nearly half the time (0.3 days vs. 0.66 days) [17]. Furthermore, both Hong et al. and Sebat et al. reported a shorter door-to-balloon (door-to-support) time after implementing a multidisciplinary team approach for treating patients with STEMI [14, 15]. Sebat et al. also reported a significant reduction in the median time for interventions in patients with CS after adopting a multidisciplinary team approach [14]. The reported times in the CS team group were as follows: intensivist arrival, 2:00 h to 50 min (p < 0.002); ICU/operating room admission, 2 h 47 min to 1 h 30 min (p < 0.002); 2 L fluid infused, 3 h 52 min to 1 h 45 min (p < 0.0001); and pulmonary artery catheter placement, 3 h 50 min to 2 h 10 min (p 0.02). These shorter times for intervention were also associated with improved clinical outcomes, including mortality (28.2% in the CS team vs. 40.7% in the control group). Tehrani et al. reported that the decrease of 5 and 10 h in the time to implement MCS was associated with increased survival of 53.6% and 135.8%, respectively [18]. On the contrary, only Taleb et al. reported similar time-to-support for the CS team vs. the non-CS team [13]. This is still important to consider as it demonstrates there are no delays in the delivery of care when utilizing multiple experts within a shock team. Hence, the majority of studies we reviewed have reported a shorter time to support when applying a multidisciplinary team approach for treating CS.
Management of CS (medical & interventional)
Not only is the time-to-intervention by the shock team shorter, but the utilization of resources is also more efficient. Higher doses of vasopressors and delayed escalation of patients from medical treatment to more invasive interventions are associated with unfavorable outcomes [35, 36]. The short-term stabilizing effects of inotropes and vasopressors are countered by their adverse effects on end-organ hypoperfusion [37]. The CS team optimizes the use of these pharmacological agents with consideration for the use of MCS. Papolos et al. reported that the CS team used fewer inotropic agents and MCS (35% vs. 43%) yet utilized more advanced MCS (i.e., Impella, ECMO, ventricular assist devices) as their first line when MCS was needed [17]. Similarly, Tehrani et al. outlined a goal in their protocol adopted by the CS team to lower the use of vasopressors and inotropes, but increase the early use of MCS of the left ventricle and/or right ventricle as appropriate [7]. Lee et al. also reported similar usage of inotropes before and after the implementation of the CS team. However, the use of MCS was higher after the implementation of the team [16]. Hong et al. reported that the use of inotropes and vasopressors was lessened after the CS team’s implementation [15].
The decision to start invasive interventions is taken in a timely manner given the team’s structure and resources, which usually reflect positively on the outcomes. Tehrani et al. reported that every 1-h delay in therapy escalation in patients requiring MCS was associated with a 9.9% increased risk of death [7]. Starting pulmonary artery catheterization (PAC), also known as right heart catheterization (RHC), for hemodynamic monitoring can be beneficial for diagnosing and determining the severity and phenotype of the CS; this can be especially helpful in patients who are not responding to the initial therapy [25]. This also facilitates individualized, invasive therapy tailored to the patient’s unique presentation. Despite the lack of evidence supporting the use of PAC monitoring for all patients, many studies have shown a decrease in mortality with the use of PAC monitoring in CS patients [38, 39]. Tehrani et al. showed that using RHC was associated with a decrease in 30-day mortality with an odds ratio of 0.19 (0.09–0.40) and P < 0.01 [7]. The use of RHC was also reported more frequently by the CS team in the study reported by Lee et al. [16]. Despite the reported increase in the usage of PAC by Papolos et al., the overall usage of MCS was lower after the implementation of the CS team [17]. Additionally, the formation of CS teams appears to influence MCS decisions. For example, the usage of Impella increased after the implementation of CS Teams [13, 16]. Also, the use of IABP was noticed to be less after the implementation of CS Teams [13, 15, 17]. The type of MCS used by different teams might differ, but the rapid use and escalation of the MCS are noticed in all CS teams. MCS may also need to be initiated outside the tertiary CS centers for critical patients in a timely manner, which supports the idea of a “Hub and Spoke” model.
Furthermore, Papolos et al. reported only 41% of CS patients were treated with mechanical ventilation, and 11% required additional renal replacement therapy, compared to 52% and 19%, respectively, for patients without a CS team [17]. Hong et al. also reported less patients in the shock team group who needed respiratory support through mechanical ventilation than without the shock team (Table 2). This might be as a result of the improved oxygenation and end-organ perfusion status of CS patients as a result of the time-critical shock team management strategies, which also involve better utilization of the resources.
Hub and spoke
One approach that can be implemented is the “Hub and Spoke” model to improve the response to CS cases. Specifically, it is important to address the poor mortality rates for CS patients that is often worsened due to a lack of resources. Moreover, delays in diagnosis and mobilization of resources impede prompt management and intervention [8]. This is especially true in rural medical centers that may lack PCI and MCS capabilities, making the already underwhelming outcomes even worse [40, 41]. Hence, a “Hub” level I center that is comprised of a cardiogenic shock team with full capabilities of managing CS in a timely and efficient manner will lead to earlier access to PCI, coronary artery bypass graft (CABG), and MCS, along with expertise in the management of hemodynamics. Moreover, studies have shown that high-volume specialized and larger academic centers have better outcomes than lower-volume, smaller centers [40, 41]. This supports the implementation of specialized centers (hubs) and cardiogenic shock teams that are experienced in performing CS interventions and managing critically ill patients. Furthermore, this team-based model will promote open communication among providers, which is beneficial for appropriate patient selection and for escalating or de-escalating care for patients [29].
Although none of the studies included in our review discussed a potential “hub and spoke” model to follow, possibly due to the novelty of the concept, as well as the challenge of providing specifics of the model, which can vary widely based on the healthcare system and the region’s resources, SCAI have proposed a model based on the cardiogenic shock stage in their classification system. This model advises medical centers to identify as hubs or spokes according to their resources. Hubs would allow accepting CS patients from spokes at stage D “deteriorating” before further worsening into the most complex stage E “extremis” [22]. Further research/clinical studies is warranted to propose more models, and validate the current ones, as well as discussing their applicability and potential challenges.
Challenges facing multidisciplinary team application
According to the Critical Care Cardiology Trials Network, 14 out of 24 centers in North America do not have shock teams [17]. Major hurdles for incorporating a multidisciplinary team include a lack of resources that can range from staffing, education, and equipment. From the papers we have discussed, most of the cardiogenic shock teams include cross-collaboration among physicians from different specialties and subspecialties. This can be challenging as it requires a tertiary center that is well-funded and staffed, along with regular training to ensure the team is competent in dealing with diverse cases of cardiogenic shock [8]. By that same note, education to identify and manage CS is important for both physicians and non-physician personnel, yet it can also pose a challenge. In fact, a key takeaway from Sebat et al. were the improvements in shock outcomes after implementing education for non-physician personnel, which led to earlier interventions, prevention of multi-organ damage, and improved outcomes [14].
Furthermore, it is important to consider geographic differences in CS team implementation. Loccoh et al. studied the differences between rural and urban settings when addressing acute cardiac conditions (i.e., AMI and Heart Failure), and found that patients in rural areas underwent fewer medical procedures and had higher mortality rates (30-day and 90-day) compared to patients in urban settings [42]. We believe this highlights the additional hurdles that underserved and rural communities will face; namely, disparities in available specialists, services offered, modernized equipment, and under-resourced facilities will all pose a challenge in implementing a CS team [42, 43].
Additionally, Moghaddam et al. raised an important concern regarding expediency. In situations such as CS, where timing is imperative while making clinical decisions and interventions, any delays such as that relating to the involvement of multiple specialists can become detrimental if not organized and coordinated [8]. Considering these multiple factors, we believe that the expansion of CS teams remains a challenge, especially for healthcare centers in under-resourced areas.
Strengths
Our systematic review discusses the most up-to-date evidence regarding the definitions and phenotypes of CS, the efficacy of the shock team implementation particularly on CS outcomes, and the potential challenges facing the shock team application.
Limitations
Despite the promising results, our study has several limitations. Firstly, most of the evidence provided by the included studies in our systematic review is retrospective/observational, which increases the risk of bias. Prospective and retrospective cohort studies, like those in our review, are inherently susceptible to selection bias, information bias and confounding factors. Secondly, the quality of evidence according to the Newcastle–Ottawa scale for cohort studies (Table 3) was highlighted as “poor quality” in four of the six studies included in our review Three studies received this rating due to a lack of comparability between the exposure and control groups in baseline characteristics. The fourth study was rated as poor quality because of an inadequate follow-up period and a significant loss to follow-up (Table 4). Thirdly, heterogeneity among the included studies is a notable limitation. This heterogeneity manifests in the geographical diversity of the study populations (with studies conducted in the USA, Canada, and Korea) and the varying structures and protocols of the shock teams implemented alongside the variations in the reported clinical outcomes in each study. Such variations may affect the consistency and comparability of the clinical outcomes reported. Additionally, some of the clinical outcome data/numbers were not reported by the studies’ investigators. This has limited our ability to conduct a meta-analysis of the available evidence due to its insufficiency. Finally, the population size reported by some of the studies in our review is small, which impacts the representativeness and generalizability of our findings.
Table 4.
The reported studies’ outcomes data
| Study ID | Group | 30-day survival, n (%) | In hospital survival, n (%) | In hospital mortality, n (%) | ICU mortality, n (%) | Time to treatment (door-to-balloon time), min, M (SD) | Rate of MCS utilization, n (%) | Mechanical ventilation n (%) | Renal replacement therapy n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Hong et al. (2020) | CS team | 83 (66.9) | NA | 42 (33.9) | 38 (30.6) | STEMI: 90.5 (34.13)@NSTEMI: 918 (1530.16) | NA | 102 (82.3) | Continuous RRT 50 (40.3) |
| Control | 58 (44.3) | 71 (54.2) | 68 (51.9) | STEMI: 114.33 (62.22)@NSTEMI: 389.33 (478.24) | 124 (94.7) | 49 (37.4) | |||
| Lee et al. (2020) | CS team | 46 (72) | 44 (69) | NA | 29 (45) | Invasive ventilation: 41 (64) | Dialysis:16 (25) | ||
| Control | 25 (69) | 22 (61) | 10 (28) | Invasive ventilation:25 (69) | Dialysis: 17 (47) | ||||
| Papolos et al. (2021) | CS team | NA | 126 (23.1) | NA | 192 (35.2) | 223 (40.8) | New RRT: 58 (10.6) | ||
| Control | 200 (28.7) | 299 (43.0) | 363 (52.2) | New RRT: 131 (18.8) | |||||
| Taleb et al. (2019) | CS team | 88 (71.85) | 75 (61.0) | NA | 19 ± 5 h | NA | 81 (65.9) | NA | |
| Control | 67 (55.56) | 58 (47.9) | 25 ± 8 h | 81 (66.9) | |||||
| Tehrani et al. (2019) | AMI | 52 (63.4) | NA | Dialysis:27 (32.9)@Transplant: 0 (0.0) | |||||
| Acute Decompensated HF (ADHF) | 78 (63.9) | Dialysis: 34 (27.9) @Transplant: 5 (4.1) | |||||||
| Control | NA (47) | NA | |||||||
| Sebat et al. (2005) | CS team | NA | NA | 2 (0.2) | NA | NA | NA | NA | NA |
| Control | 4 (36.36) | ||||||||
AMI Acute myocardial infarction, CS Cardiogenic shock, HF Heart failure, NA Not available
Implications for clinical practice
The findings of our review suggest that the implementation of multidisciplinary CS teams can significantly improve clinical outcomes for patients with cardiogenic shock. Specifically, the use of shock teams has been associated with higher survival rates, faster initiation of treatment, and more effective management of complex cases. These benefits are likely due to the collaborative nature of shock teams, which facilitate the rapid and coordinated application of advanced therapies, such as MCS.
However, several important considerations must be addressed to optimize the integration of CS teams into clinical practice. Firstly, shock teams should be implemented in high-volume centers that frequently manage cardiogenic shock cases, as these facilities are more likely to benefit from the improved resource utilization and outcomes that shock teams offer. Secondly, healthcare systems must consider the financial and logistical requirements of maintaining a well-trained shock team, which could include additional staffing, specialized equipment, and continuous training. While these factors may represent a significant initial investment, the potential for improved survival and reduced length of ICU stay may offset these costs in the long term.
Implications for future research
Our review offers valuable insights for clinicians regarding the multifaceted roles, benefits, and challenges associated with CS teams in enhancing patient outcomes. Nevertheless, there are several key implications for future research that can significantly enhance the quality of evidence. Firstly, it is advisable to conduct a greater number of prospective cohort studies and randomized controlled trials (RCTs), involving larger participant populations. These endeavors will yield more compelling evidence of the team’s effectiveness by minimizing potential confounding factors and selection biases.
Secondly, delving into the extended impact of CS teams’ interventions on the long-term outcomes of CS patients will be invaluable. This exploration can provide a comprehensive understanding of the team’s influence on factors such as patient survival, quality of life, and readmission rates.
Thirdly, it is pertinent to assess the cost-effectiveness of establishing and maintaining CS teams, as these insights can be pivotal in guiding strategic decisions within hospital management.
Lastly, it is worth noting that the current body of evidence concerning the impact of CS teams on outcomes specifically related to Acute Decompensated Heart Failure with Cardiogenic Shock (ADHF-CS) patients is notably limited. Recent research has highlighted a rising prevalence of ADHF-CS when compared to cases of AMI-CS [42]. Consequently, further research in this realm is imperative to enhance our understanding of the distinct implications and benefits of CS teams for ADHF-CS patients.
Conclusions
Despite advancements in cardiac care, cardiogenic shock continues to have high mortality rates. Our systematic review found that the implementation of multidisciplinary cardiogenic shock teams has led to significant reductions in both in-hospital and all-cause mortality. Key findings suggest that these teams facilitate earlier diagnosis, prompt initiation of therapies, including mechanical circulatory support (MCS) when required, and reduce ICU stays. These benefits highlight the crucial role of CS teams in improving outcomes across a broad spectrum of CS patients. However, challenges remain, particularly in establishing and maintaining well-trained CS teams, especially in underserved and rural settings. Based on our findings, we recommend that healthcare institutions prioritize the development of CS teams to optimize patient outcomes. For clinical practice, it is essential to standardize protocols for CS management within multidisciplinary teams, ensuring timely and effective intervention. Future research should focus on evaluating the cost-effectiveness of these teams, exploring their long-term impact on patient outcomes, and investigating their efficacy in managing specific CS complications, particularly in acute decompensated heart failure. Addressing these gaps will provide further clarity on how to best integrate CS teams into diverse healthcare settings.
Supplementary Information
Acknowledgements
None.
Abbreviations
- ADHF
Acute Decompensated Heart Failure
- AMI
Acute myocardial infarction
- CS
Cardiogenic shock
- ECMO
Extracorporeal membrane oxygenation
- ESC
European Society of Cardiology
- ICU
Intensive care unit
- IABP
Intra-aortic balloon pump
- MCS
Mechanical circulatory support
- PAC
Pulmonary artery catheterization
- PCI
Percutaneous coronary intervention
- RHC
Right heart catheterization
- SCAI
Cardiovascular angiography and intervention
- STEMI
ST-elevation myocardial infarction
Author contributions
MA, BA, ASE, and AMA: Conceptualization, methodology. ASE, AMA, AJ, and AG: Data curation, writing—original draft preparation. ASE and AMA: Visualization, investigation. MA and BA: Supervision. MA and BA: Software, validation. ASE, AJ, AG, HR and MA: Writing—reviewing and editing.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohamed Abdelnabi and Ahmed Saad Elsaeidy are contributed equally and co-first authors.
References
- 1.Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J et al (2020) Epidemiology, pathophysiology and contemporary management of cardiogenic shock—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 22(8):1315–1341 [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD et al (1999) Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 341(9):625–634 [DOI] [PubMed] [Google Scholar]
- 3.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J et al (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367(14):1287–1296 [DOI] [PubMed] [Google Scholar]
- 4.Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M et al (2019) Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial. Circulation 139(3):395–403 [DOI] [PubMed] [Google Scholar]
- 5.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJ et al (2017) Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 69(3):278–87 [DOI] [PubMed] [Google Scholar]
- 6.Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS et al (2017) Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 119(6):845–851 [DOI] [PubMed] [Google Scholar]
- 7.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC et al (2019) Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 73(13):1659–1669 [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam N, van Diepen S, So D, Lawler PR, Fordyce CB (2021) Cardiogenic shock teams and centres: a contemporary review of multidisciplinary care for cardiogenic shock. ESC Heart Fail 8(2):988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusca SB, Caughron H, Njoroge JN, Cheng R, O’Brien CG, Barnett CF (2022) The shock team: a multidisciplinary approach to early patient phenotyping and appropriate care escalation in cardiogenic shock. Curr Opin Cardiol 37(3):241–249 [DOI] [PubMed] [Google Scholar]
- 10.Esposito ML, Kapur NK (2017) Acute mechanical circulatory support for cardiogenic shock: the “door to support” time. F1000Res 6:737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y, Nie SP, Wang X, Thomas A, Thompson E, Zhao GQ et al (2020) Role of Pulmonary Embolism Response Team in patients with intermediate- and high-risk pulmonary embolism: a concise review and preliminary experience from China. J Geriatr Cardiol 17(8):510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morey JR, Zhang X, Marayati NF, Matsoukas S, Fiano E, Oxley T et al (2021) Mobile interventional stroke teams improve outcomes in the early time window for large vessel occlusion stroke. Stroke 52(9):e527–e530 [DOI] [PubMed] [Google Scholar]
- 13.Taleb I, Koliopoulou AG, Tandar A, McKellar SH, Tonna JE, Nativi-Nicolau J et al (2019) Shock team approach in refractory cardiogenic shock requiring short-term mechanical circulatory support. Circulation 140(1):98–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebat F, Johnson D, Musthafa AA, Watnik M, Moore S, Henry K et al (2005) A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 127(5):1729–1743 [DOI] [PubMed] [Google Scholar]
- 15.Hong D, Choi KH, Cho YH, Cho SH, Park SJ, Kim D et al (2020) Multidisciplinary team approach in acute myocardial infarction patients undergoing veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care 10(1):83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee F, Hutson JH, Boodhwani M, McDonald B, So D, Roock SD et al (2020) Multidisciplinary code shock team in cardiogenic shock: a canadian centre experience. CJC Open 2(4):249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papolos AI, Kenigsberg BB, Berg DD, Alviar CL, Bohula E, Burke JA et al (2021) Management and outcomes of cardiogenic shock in cardiac ICUs with versus without shock teams. J Am Coll Cardiol 78(13):1309–1317 [DOI] [PubMed] [Google Scholar]
- 18.Tehrani B, Truesdell A, Singh R, Murphy C, Saulino P (2018) Implementation of a cardiogenic shock team and clinical outcomes (INOVA-SHOCK Registry): observational and retrospective study. JMIR Res Protoc 7(6):e160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29(372):n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, Robertson J, Peterson J, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis.
- 21.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37(27):2129 [DOI] [PubMed] [Google Scholar]
- 22.SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. | Journal of the American College of Cardiology [Internet]. [cited 2024 Aug 9]. Available from: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed]
- 23.SCAI clinical expert consensus statement on the classification of cardiogenic shock—Baran—2019—Catheterization and Cardiovascular Interventions—Wiley Online Library [Internet]. [cited 2024 Aug 9]. Available from: 10.1002/ccd.28329 [DOI] [PubMed]
- 24.Menon V, White H, LeJemtel T, Webb JG, Sleeper LA, Hochman JS (2000) The clinical profile of patients with suspected cardiogenic shock due to predominant left ventricular failure: a report from the SHOCK Trial Registry. J Am Coll Cardiol 36(3, Supplement 1):1071–1076 [DOI] [PubMed] [Google Scholar]
- 25.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK et al (2017) Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 136(16):e232–e268 [DOI] [PubMed] [Google Scholar]
- 26.Menon V, Slater JN, White HD, Sleeper LA, Cocke T, Hochman JS (2000) Acute myocardial infarction complicated by systemic hypoperfusion without hypotension: report of the SHOCK trial registry. Am J Med 108(5):374–380 [DOI] [PubMed] [Google Scholar]
- 27.Goldstein JA, Barzilai B, Rosamond TL, Eisenberg PR, Jaffe AS (1990) Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation 82(2):359–368 [DOI] [PubMed] [Google Scholar]
- 28.Acute heart failure | Nature Reviews Disease Primers [Internet]. [cited 2024 Aug 9]. Available from: https://www.nature.com/articles/s41572-020-0151-7
- 29.Doll JA, Ohman EM, Patel MR, Milano CA, Rogers JG, Wohns DH et al (2016) A team-based approach to patients in cardiogenic shock. Catheter Cardiovasc Interv 88(3):424–433 [DOI] [PubMed] [Google Scholar]
- 30.Kochar A, Al-Khalidi HR, Hansen SM, Shavadia JS, Roettig ML, Fordyce CB et al (2018) Delays in primary percutaneous coronary intervention in ST-segment elevation myocardial infarction patients presenting with cardiogenic shock. JACC Cardiovasc Interv 11(18):1824–1833 [DOI] [PubMed] [Google Scholar]
- 31.Scholz KH, Maier SKG, Maier LS, Lengenfelder B, Jacobshagen C, Jung J et al (2018) Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J 39(13):1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tongers J, Sieweke JT, Kühn C, Napp LC, Flierl U, Röntgen P et al (2020) Early escalation of mechanical circulatory support stabilizes and potentially rescues patients in refractory cardiogenic shock. Circ Heart Fail 13(3):e005853 [DOI] [PubMed] [Google Scholar]
- 33.Pieri M, Sorrentino T, Oppizzi M, Melisurgo G, Lembo R, Colombo A et al (2018) The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J Interv Cardiol 31(6):717–724 [DOI] [PubMed] [Google Scholar]
- 34.Meraj PM, Doshi R, Schreiber T, Maini B, O’Neill WW (2017) Impella 2 5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol 30(3):256–263 [DOI] [PubMed] [Google Scholar]
- 35.Kim DH (2020) Mechanical circulatory support in cardiogenic shock: Shock team or bust? Can J Cardiol 36(2):197–204 [DOI] [PubMed] [Google Scholar]
- 36.Bellumkonda L, Gul B, Masri SC (2018) Evolving concepts in diagnosis and management of cardiogenic shock. Am J Cardiol 122(6):1104–1110 [DOI] [PubMed] [Google Scholar]
- 37.Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS (1999) Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the abiomed BVS system. J Card Surg 14(4):288–293 [DOI] [PubMed] [Google Scholar]
- 38.Na SJ, Park TK, Lee GY, Cho YH, Chung CR, Jeon K et al (2017) Impact of a cardiac intensivist on mortality in patients with cardiogenic shock. Int J Cardiol 244:220–225 [DOI] [PubMed] [Google Scholar]
- 39.Damluji AA, Myerburg RJ, Chongthammakun V, Feldman T, Rosenberg DG, Schrank KS et al (2017) Improvements in outcomes and disparities of ST-segment-elevation myocardial infarction care: the Miami-Dade County ST-segment-elevation myocardial infarction network project. Circ Cardiovasc Qual Outcomes 10(12):e004038 [DOI] [PubMed] [Google Scholar]
- 40.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr, Prasad A (2019) Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol 124(4):491–498 [DOI] [PubMed] [Google Scholar]
- 41.Shaefi S, O’Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J et al (2015) Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc 4(1):e001462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loccoh EC, Maddox KEJ, Wang Y, Kazi DS, Yeh RW, Wadhera RK (2022) Rural-urban disparities in outcomes of myocardial infarction, heart failure, and stroke in the United States. J Am Coll Cardiol 79(3):267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallabhajosyula S, Patlolla SH, Dunlay SM, Prasad A, Bell MR, Jaffe AS et al (2020) Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail 13(2):e006661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.