Abstract
Bacterial infections are major causes of human mortality. The activation of coagulation pathways leading to the deposition of insoluble fibrin frequently accompanies bacterial infection, and much attention has focused upon the pathological attributes of infection-stimulated fibrin deposition. Nevertheless, here we present conclusive evidence that infection-stimulated fibrin deposition can perform critical protective functions during bacterial infection. Specifically, we demonstrate that coagulation-impaired fibrin(ogen)-deficient mice, in comparison with genetically matched control mice, display increased mortality upon peritoneal infection with the gram-positive facultative intracellular bacterium Listeria monocytogenes. To distinguish effects of fibrinogen from those of fibrin, we treat wild-type mice with warfarin, an anticoagulant that suppresses fibrin formation without impacting fibrinogen levels. Warfarin treatment exacerbates listeriosis, suggesting that fibrin is the key mediator of protection. With regard to the underlying protective mechanisms, we demonstrate that fibrin(ogen) suppresses anemia, reduces hemorrhagic pathology, and limits bacterial growth during listeriosis. Despite confirming a prior report that fibrin(ogen) promotes the peritoneal clearance of the extracellular bacterium Staphylococcal aureus, we demonstrate that fibrin(ogen) plays little role in controlling peritoneal numbers of L. monocytogenes bacteria or the dissemination of L. monocytogenes bacteria from the peritoneal cavity. Rather, fibrin(ogen) primarily limits the growth of these intracellular bacteria within hepatic tissue. While the pathological potential of excessive infection-stimulated fibrin deposition is well appreciated, our findings reveal that fibrin can function protectively, via multiple mechanisms, during bacterial infection.
Vascular trauma activates coagulant cascades, leading to the proteolytic cleavage of fibrinogen, which then polymerizes and deposits as insoluble mesh-like fibrin. In that setting, fibrin helps to stanch blood loss, thereby functioning protectively. Infection also frequently stimulates coagulation leading to fibrin deposition. However, infection-stimulated coagulation can function pathologically; fibrin contributes to tissue necrosis and organ dysfunction by obstructing the microvasculature (30) and prompts the formation of peritoneal abscesses and adhesions (1, 33, 39, 40). Moreover, septic infections often prompt disseminated intravascular coagulation, a life-threatening condition that significantly increases mortality rates (27, 30).
Despite the well-documented pathological attributes of infection-stimulated coagulation, accumulating evidence suggests that coagulation also functions protectively during infection. For example, we recently demonstrated that fibrin performs a critical protective function during infection by the obligate intracellular protozoan parasite Toxoplasma gondii (25). In that setting, fibrin has no discernible impact on pathogen growth or dissemination. Rather, fibrin suppresses an otherwise lethal hemorrhagic pathology that accompanies the robust immune response prompted by toxoplasmosis (25). Prior studies also indicate that fibrin can function protectively during peritoneal bacterial infections. There, it has been reported that fibrin promotes the clearance of bacteria from within the peritoneal cavity, either by helping to activate the microbicidal properties of phagocytes (19) or by physically trapping bacteria, thereby directly limiting dissemination (1, 17, 18, 39, 46).
Listeria monocytogenes is a ubiquitous, facultative intracellular, gram-positive bacillus that causes listeriosis, an often fatal disease in newborns, pregnant women, and the immunocompromised (15, 45). The experimental infection of rodents with L. monocytogenes, as first described by George Mackaness in 1962 (31), has become a prototypical model for the study of innate and acquired immunity against intracellular pathogens (29). In 1981, Nelson and colleagues demonstrated that pharmacologic anticoagulation of Listeria-infected rats with heparin dramatically increased bacteremia and mortality (12). While they suggested that the protective mechanism mediated by coagulation may relate to a bactericidal activity of platelets (12), the actual nature of this protective effect has yet to be defined in vivo. Given our demonstration that fibrin protects against hemorrhagic pathology during infection by the intracellular pathogen T. gondii (25), and given reports that fibrin constrains the peritoneal growth of certain extracellular bacteria (1, 17-19, 39, 46), we performed a detailed evaluation of roles for fibrin deposition during infection by L. monocytogenes, which is an intracellular bacterium. We demonstrate that fibrin both suppresses hemorrhagic pathology and restrains bacterial growth during listeriosis. However, in contrast to recent findings with an extracellular bacterium (19), we establish that fibrin primarily limits the growth of L. monocytogenes within hepatic tissue, rather than limiting its growth in and/or dissemination from the peritoneal cavity. Together, these findings establish that fibrin functions protectively during bacterial infection by multiple mechanisms.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Taconic. Fibrin(ogen)-deficient mice (41) (backcrossed seven generations to C57BL/6 mice) were generously supplied by Jay Degen (Children's Hospital Medical Center, Cincinnati, OH) and were bred at the Trudeau Institute Animal Breeding Facility. Six- to 10-week-old mice were used for all experiments. Animals were housed in a specific-pathogen-free facility. All animal experiments were reviewed and approved by the Trudeau Institute Animal Care and Use Committee.
Infections.
Stocks of L. monocytogenes (strain EGD, supplied by Robert North, Trudeau Institute) were prepared after passage through C57BL/6 mice (32). The 50% lethal dose (LD50) of this stock was 2 × 106 CFU when administered via the intraperitoneal (i.p.) route to wild-type C57BL/6 mice. Staphylococcus aureus was obtained from the American Type Culture Collection (ATCC 27217). The administered doses were determined by plating aliquots of the infectious inoculums on Trypticase soy agar and counting CFU after 24 to 36 h of incubation at 37°C. Sham-infected mice received phosphate-buffered saline. Where indicated, 2 mg/liter warfarin [3-(α-acetonylbenzyl)-4-hydroxycoumarin; Sigma] was added to drinking water beginning 3 days prior to infection and replenished every 48 h thereafter (25). On the indicated days after infection, mice received 500 U of heparin intravenously (i.v.) just prior to euthanasia by carbon dioxide asphyxiation, and numbers of viable bacteria in organs were measured by homogenizing tissues in phosphate-buffered saline and then determining CFU as described above. Numbers of viable bacteria in the peritoneal cavity were determined by flushing the cavity with 7 ml of saline containing 5 mM EDTA and lysing cell pellets with 0.2% saponin before determining CFU.
Measurements of fibrin and immune parameters.
Fibrin levels within tissue samples were measured quantitatively by Western blotting as previously described (25). Spontaneous ex vivo fibrin formation in whole blood was measured by a modification of that procedure. In brief, whole blood was drawn by cardiac puncture from carbon dioxide-asphyxiated mice. Immediately, 50-μl blood aliquots were transferred to polypropylene microcentrifuge tubes that had been prewarmed to 37°C. At the indicated times, fibrin formation was quenched by the addition of 750 μl of 10 mM sodium phosphate (pH 7.5) containing 5 mM EDTA, 100 mM ɛ-aminocaproic acid, 10 U/ml aprotinin, 10 U/ml heparin, and 2 mM phenylmethylsulfonyl fluoride. Then, samples were homogenized and processed for quantitative fibrin Western blotting (25). Gamma interferon (IFN-γ) protein levels in plasma were determined by sandwich enzyme-linked immunosorbent assay (BD Biosciences). Tissue levels of specific mRNA were measured by real-time PCR and normalized to levels of β2-microglobulin (25).
Measurements of red blood cell survival and reticulocyte numbers.
Hematocrits and red cell numbers were determined using a Coulter Counter (Beckman). Reticulocytes were measured essentially as previously described (44). In brief, 5 μl of whole blood was stained with 500 μl of thiazole orange solution (Retic-Count; BD Biosciences) for 1 h at room temperature, centrifuged at 800 × g for 5 min, and resuspended in 1% formaldehyde. The percentage of thiazole orange-stained red cells (i.e., reticulocytes) was then determined by flow cytometry using forward and side scatter to gate on total red cells. Finally, the total number of reticulocytes was obtained by multiplying the percentage of reticulocytes by the total number of red cells determined by Coulter Counter. Red cell survival was measured by biotinylating all circulating cells in vivo and then monitoring the loss of biotinylated red cells over time (23). Circulating cells were biotinylated the day prior to infection by i.v. administration of 1.2 mg of sulfo-N-hydroxysuccinimide-biotin (Calbiochem) dissolved in 10% dimethyl sulfoxide and given as two doses separated by 2 h. On the indicated days after infection, 5 μl of whole blood was analyzed by flow cytometry after staining with TER119 monoclonal antibody conjugated with fluorescein isothiocyanate (Santa Cruz Biotechnology, Inc.) and streptavidin conjugated with phycoerythrin (BD Pharmingen). The total number of biotinylated red cells was then obtained by multiplying the percentage of streptavidin-positive TER119-positive cells by the total number of red cells (as determined by Coulter Counter).
Statistics.
Statistical analyses were performed using the program Prism 4.0 (GraphPad Software, Inc.). Survival data were analyzed by the log rank test. Other data were analyzed by Student's t tests, analysis of variance, or Kruskal-Wallis tests, as appropriate.
RESULTS
Fibrin deposition accompanies listeriosis.
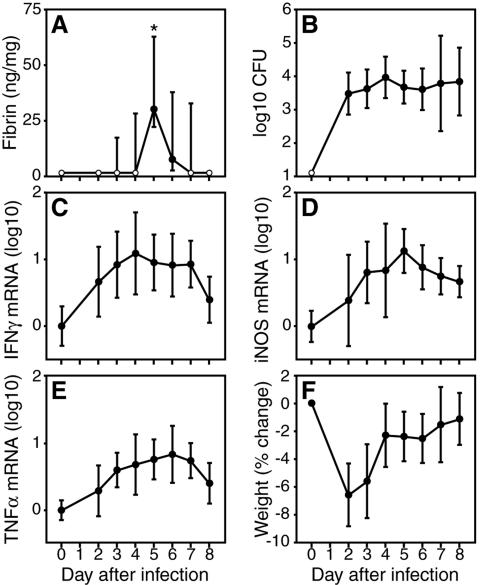
As previously reported for infection by T. gondii (25), we found that fibrin deposition accompanies the acute phase of infection by L. monocytogenes. Quantitative Western blotting revealed substantial accumulation of fibrin within Listeria-infected tissues, even during sublethal infections. For example, after i.p. inoculation of wild-type C57BL/6 mice with 1 × 105 CFU of L. monocytogenes (approximately 0.05 LD50), we detected transient fibrin deposition within liver tissue, peaking at day 5 of infection (Fig. 1A). Interestingly, levels of fibrin did not correlate strictly with bacterial burdens, as L. monocytogenes infected the liver before, during, and after this transient spike in fibrin deposition (Fig. 1B). Levels of fibrin deposition also failed to correlate strictly with the production of well-characterized mediators of the type 1 immune response prompted by listeriosis, such as IFN-γ, inducible nitric oxide synthase (iNOS), and tumor necrosis factor alpha (TNF-α) (Fig. 1C to E), or with general symptoms of infection, such as weight loss (Fig. 1F). We conclude that transient fibrin deposition accompanies the acute phase of sublethal listeriosis and that the kinetics of fibrin deposition do not correlate precisely either with bacterial burdens or with measures of infection-stimulated immunity.
FIG. 1.
Transient fibrin formation accompanies the acute phase of sublethal listeriosis. Wild-type C57BL/6 mice were i.p. inoculated with 1 × 105 bacteria (0.05 LD50) of L. monocytogenes strain EGD. On the indicated days, we measured hepatic fibrin deposition (A), hepatic bacterial burdens (B), hepatic IFN-γ mRNA (C), hepatic iNOS mRNA (D), hepatic TNF-α mRNA (E), and body weight change (G). Data are compiled from two independent experiments, and closed symbols depict the median ± the interquartile range (A) or the mean ± the standard deviation (B to E) from 10 mice per time point, except five mice for day 8. Open symbols depict values below the detection limit of our assays. mRNA levels are presented as log10 changes relative to day 0. Infection by L. monocytogenes stimulated highly significant increases in fibrin deposition on day 5 of infection (*, P < 0.001 using the Kruskal-Wallis test followed by Dunn's multiple-comparison test), which did not strictly correlate with bacterial burdens or levels of immunity.
Fibrin functions protectively during listeriosis.
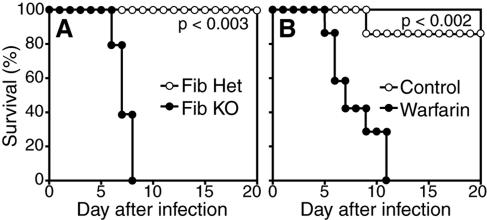
To evaluate the function of fibrin deposition during acute listeriosis, we infected gene-targeted fibrin(ogen)-deficient mice, which cannot produce fibrin, as they lack its precursor, fibrinogen (41). As shown in Fig. 2A, fibrin(ogen)-deficient mice succumbed to i.p. inoculation with 1 × 105 CFU of L. monocytogenes an infectious dose that all littermate control fibrin(ogen)-heterozygous mice survived (P < 0.003). The time at which fibrin(ogen)-deficient mice succumb to listeriosis coincides with the time at which fibrin levels peak in wild-type mice (Fig. 1A).
FIG. 2.
Fibrin functions protectively during listeriosis. (A) Fibrin(ogen)-deficient (Fib KO) mice were i.p. inoculated with 1 × 105 L. monocytogenes bacteria and monitored for survival. Fibrin(ogen)-deficient mice succumbed to bacterial infection significantly earlier than did littermate control fibrin(ogen)-heterozygous (Fib Het) mice (P < 0.003; five mice per group). (B) Wild-type C57BL/6 mice were pharmacologically anticoagulated with warfarin and then i.p. inoculated with 7.5 × 105 L. monocytogenes bacteria. Warfarin-treated mice succumbed to bacterial infection significantly earlier than did untreated mice (P < 0.002; seven mice per group). Results similar to those shown in panels A and B were observed in two or more independent experiments.
Since fibrin(ogen)-deficient mice lack both fibrinogen and fibrin, we next sought to distinguish their roles during listeriosis. Warfarin is an orally available pharmaceutical used clinically for the long-term anticoagulation of thrombosis-prone humans. Mice treated with warfarin possess normal levels of circulating fibrinogen but generate fibrin far less efficiently than untreated mice (Table 1). Like fibrin(ogen)-deficient mice, warfarin-treated wild-type mice succumbed to doses of L. monocytogenes, that most control mice survived (Fig. 2B; P < 0.002). In comparison with fibrin(ogen)-deficient mice, higher doses of L. monocytogenes were required to demonstrate the increased sensitivity of warfarin-treated mice, consistent with their capacity to generate detectable, albeit greatly reduced, levels of fibrin (Table 1). Overall, the increased susceptibility of both fibrin(ogen)-deficient and warfarin-treated mice strongly suggests that fibrin is a key determinant of survival during listeriosis.
TABLE 1.
Impaired capacity to produce fibrin in warfarin-treated mice
| Treatment and Timea (min) | Fibrin formationb |
|---|---|
| Nonec | |
| 10 | >250, >250, >250, >250, >250 |
| 30 | >250, >250, >250, >250, >250 |
| 90 | >250, >250, >250, >250, >250 |
| Warfarind | |
| 10 | 0, 0, 0, 0, 0 |
| 30 | 0, 0, 0, 60, >250 |
| 90 | 0, 50, >250, >250, >250 |
| Fib KOe | |
| 10 | 0, 0, 0, 0, 0 |
| 30 | 0, 0, 0, 0, 0 |
| 90 | 0, 0, 0, 0, 0 |
| Heparinf | |
| 10 | 0, 0, 0, 0, 0 |
| 30 | 0, 0, 0, 0, 0 |
| 90 | 0, 0, 0, 0, 0 |
Time at which further spontaneous fibrin formation was quenched by addition of EDTA-heparin-containing extraction buffer.
Ex vivo fibrin formation in whole blood collected by cardiac puncture after euthanasia by carbon dioxide asphyxiation. Data from five individual animals/group are presented as nanograms of fibrin per 10 μl of whole blood.
Wild-type C57BL/6 mice.
Wild-type C57BL/6 mice treated with warfarin for 8 days.
Fib KO, fibrin(ogen)-deficient mice.
C57BL/6 mice treated with 500 U of heparin i.v. just prior to euthanasia.
Fibrin protects against hemorrhage during listeriosis.
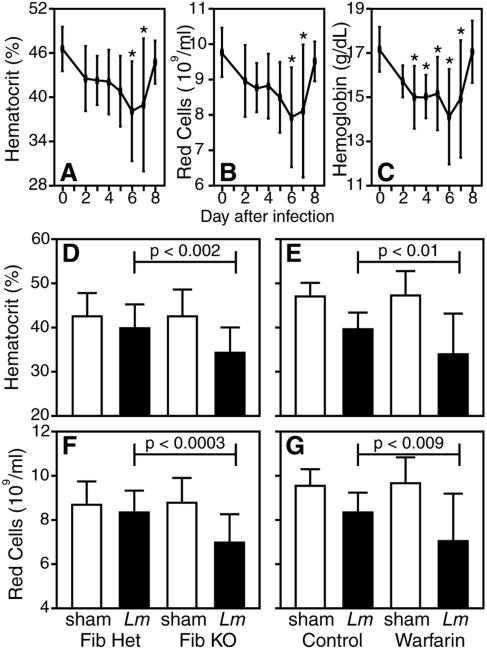
Having recently suggested that failure to adequately control hemorrhagic pathology accounts for the increased susceptibility of fibrin-deficient mice to infection by the protozoan parasite T. gondii (25), we evaluated whether listeriosis prompts signs of hemorrhage that might be exacerbated by fibrin deficiency. Wild-type mice exhibited symptoms of mild anemia during the acute phase of sublethal Listeria infection, evidenced by reduced hematocrits, circulating red cell numbers, and hemoglobin levels (Fig. 3A to C). The infection-stimulated anemia peaked at day 6 after Listeria inoculation, thus correlating kinetically with the time at which fibrin-deficient mice succumbed to listeriosis (Fig. 2). In comparison with control mice, fibrin(ogen)-deficient mice displayed significantly greater anemia at day 5 after Listeria inoculation (Fig. 3D and F; P < 0.002 and 0.0003 for hematocrits and red cell numbers, respectively). Likewise, Listeria-infected, warfarin-treated mice displayed exacerbated anemia in comparison with infected control mice (Fig. 3E and G; P < 0.01 and 0.009 for hematocrits and red cell numbers, respectively). These findings suggest that fibrin protects against anemia during acute listeriosis.
FIG. 3.
Fibrin protects against infection-stimulated anemia. (A to C) Wild-type C57BL/6 mice were i.p. inoculated with 1 × 105 L. monocytogenes bacteria. On the indicated days, we measured hematocrits (A), circulating red cell numbers (B), and hemoglobin levels (C). Data were compiled from two independent experiments and depict the mean ± the standard deviation of 5 to 10 mice per time point. Infection by L. monocytogenes stimulated significant decreases in hematocrits, red cell numbers, and hemoglobin levels on the indicated days after infection (*, P < 0.05 by one-way analysis of variance using Dunnett's multiple-comparison test). (D to G) Fibrin(ogen)-deficient or warfarin-treated mice were i.p. inoculated with approximately 1 × 105 L. monocytogenes bacteria. Five days later, we measured hematocrits (D, E) and red cell numbers (F, G). Data were compiled from four independent experiments and depict the mean ± the standard deviation (14 to 24 mice per group). In comparison with control mice infected in parallel, the hematocrits and red cell numbers in fibrin(ogen)-deficient and warfarin-treated mice were significantly reduced by day 5 of infection (P < 0.01 for all conditions). Fib KO, fibrin(ogen) deficient; Fib Het, fibrin(ogen) heterozygous.
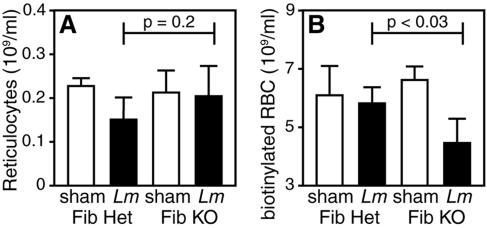
Listeria-stimulated anemia could result from either decreased red cell production or increased red cell loss. Red cell production can be measured by flow cytometric assays using RNA-binding dyes, as newly generated red cells (i.e., reticulocytes) possess greater levels of RNA than mature red cells (44). Using such an assay to measure circulating reticulocyte numbers during the acute phase of listeriosis, we observed significant decreases in reticulocyte numbers within control fibrin(ogen)-heterozygous mice (P < 0.02) but not within fibrin(ogen)-deficient mice (Fig. 4A). The trend toward higher reticulocyte numbers in the fibrin(ogen)-deficient mice, in comparison with control mice, may reflect a compensatory increase in red cell production in fibrin(ogen)-deficient mice owing to their exacerbated anemia (Fig. 3). Regardless, while red cell production is moderately suppressed during acute Listeria infection in fibrin-sufficient mice, decreased red cell production cannot account for the exacerbated anemia in fibrin-deficient mice.
FIG. 4.
Fibrin protects against infection-stimulated red cell loss. Fibrin(ogen)-deficient (Fib KO) and fibrin(ogen)-heterozygous (Fib Het) mice were injected i.v. with sulfo-N-hydroxysuccinimide-biotin and i.p. inoculated with 1 × 105 L. monocytogenes bacteria the following day. Five days later, blood was harvested and numbers of reticulocytes (A) and biotinylated red cells (RBC) (B) were determined as described in Materials and Methods. In comparison with fibrin(ogen)-heterozygous mice that were infected in parallel, reticulocyte numbers did not differ significantly in fibrin(ogen)-deficient mice (P = 0.2). However, numbers of biotinylated red cells were reduced significantly in fibrin(ogen)-deficient mice (P < 0.03). Data are depicted as the mean ± the standard deviation of six mice for each condition.
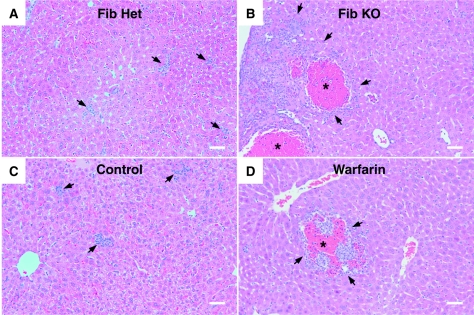
Given that the exacerbated anemia in Listeria-infected fibrin(ogen)-deficient mice could not be ascribed to decreased red cell production, we next evaluated whether listeriosis affected red cell survival. In vivo pulse-chase biotinylation procedures enable measurement of the extent of mature red cell loss from the circulation over time (23). Applying such methods to the murine listeriosis model, we found that fibrin(ogen)-deficient mice, in comparison with littermate control mice, displayed significantly greater loss of circulating red cells during infection (P < 0.03, Fig. 4B). Increased red cell loss could theoretically result from red cell lysis, red cell phagocytosis, and/or hemorrhage. Direct histologic evaluation of infected liver tissue revealed clear evidence of hemorrhagic pathology in Listeria-infected, fibrin-deficient mice (Fig. 5). As for survival and anemia, the phenotype of fibrin(ogen)-deficient mice was more pronounced than that of warfarin-treated mice, presumably reflecting the residual fibrin-generating capacity of warfarin-treated animals (Table 1). Notably, control uninfected fibrinogen-deficient or warfarin-treated mice never displayed hemorrhagic pathology (not shown). Together, our observations of both increased red cell loss and hemorrhagic histopathology in fibrin-deficient mice during acute listeriosis strongly suggest that Listeria infection prompts hemorrhage and that fibrin functions protectively during this bacterial infection, at least in part, by suppressing hemorrhagic pathology.
FIG. 5.
Fibrin protects against infection-stimulated hemorrhagic pathology. Micrographs depict representative histology of liver tissue harvested at 5 days after inoculation of fibrin(ogen)-heterozygous (Fib Het) mice with 1 × 105 L. monocytogenes bacteria (A), fibrinogen-deficient (Fib KO) mice with 1 × 105 L. monocytogenes bacteria (B), C57BL/6 mice with 1 × 106 L. monocytogenes bacteria (C), and warfarin-treated C57BL/6 mice with 1 × 106 L. monocytogenes bacteria (D). Control animals (A and C) displayed limited hepatic damage and multiple small inflammatory infiltrates (arrows). Fibrin-deficient animals (B and D) displayed large areas of hepatic necrosis accompanied by large numbers of mixed inflammatory cells (arrows) and moderate to severe hemorrhage (asterisks), which was more pronounced in fibrin(ogen)-deficient compared with warfarin-treated mice. Photomicrographs of hematoxylin-and-eosin-stained tissues were obtained with an Olympus BX50 microscope equipped with an UplanFL lens (10×, 0.3 aperture), a Canon EOS D30 camera, and Canon Remote Capture 2.7 software. Image processing was completed with Photoshop 7.0 software using equivalent gamma adjustments, color equalization, and normalization between individual photos. Bars = 40 μm.
Fibrin limits hepatic bacterial growth during listeriosis.
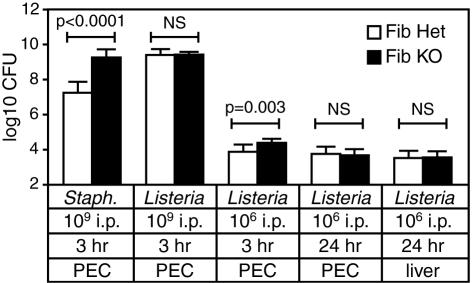
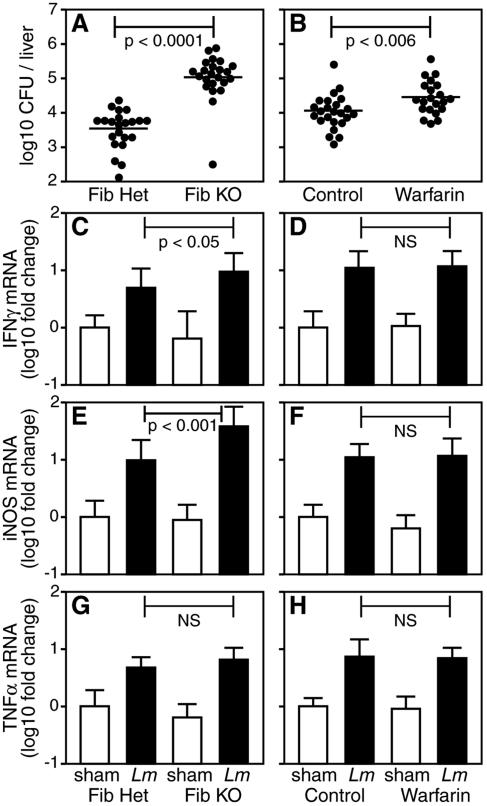
It was recently reported that mice expressing a mutant form of fibrin(ogen) exhibit reduced bacterial clearance at early time points after i.p. inoculation with 1 × 109 CFU of the extracellular bacterium S. aureus (19). Consistent with that report, we found that numbers of recoverable peritoneal bacteria were more than 100-fold higher in fibrin(ogen)-deficient mice, compared with littermate control mice, at 3 h after their inoculation with 1 × 109 CFU of S. aureus (Fig. 6). However, parallel inoculations with a similar dose of L. monocytogenes failed to reveal significant differences in the numbers of recoverable bacteria between fibrin(ogen)-deficient and control mice (Fig. 6). Peritoneal bacterial burdens were significantly higher in the fibrin(ogen)-deficient mice 3 h after their inoculation with 1 × 106 CFU of L. monocytogenes, a dose approximating that at which we observed differential survival of fibrin(ogen)-deficient and control mice (Fig. 2 and 6). However, this increase was quite modest (about threefold) and no longer evident by 24 h postinfection (Fig. 6). Seeding of L. monocytogenes in peripheral organs, such as the spleen and liver, was also evident by 24 h postinfection, but the levels of dissemination to these organs were unaffected by fibrin(ogen) deficiency (Fig. 6 and data not shown). In striking contrast, by day 5 of infection fibrin(ogen)-deficient mice exhibited hepatic bacterial burdens that were 10- to 100-fold higher than those of littermate control mice (Fig. 7A; P < 0.0001). Fibrin(ogen)-deficient mice also tended to display increased bacterial burdens in splenic tissue, although this finding was not statistically significant in all experiments (not shown). Warfarin-treated mice also displayed significantly greater hepatic bacterial burdens than control animals at day 5 of infection (Fig. 7B; P < 0.006). Again, the magnitude of the difference was less dramatic in warfarin-treated mice than in fibrin(ogen)-deficient mice, consistent with the residual fibrin-generating capacity of the warfarin-treated mice (Table 1). Thus, while dissemination of L. monocytogenes to hepatic tissue is not appreciably affected by fibrin deficiency, the growth of L. monocytogenes within liver tissue is markedly increased in mice lacking fibrin.
FIG. 6.
Fibrin does not restrain dissemination of L. monocytogenes from the peritoneal cavity. Fibrinogen-deficient and littermate control fibrinogen-heterozygous mice (five to eight per group) were i.p. inoculated with the indicated doses of S. aureus (Staph.) or L. monocytogenes. After 3 or 24 h, bacterial CFU were determined in peritoneal exudate cells (PEC) and liver tissue. Data were compiled from two independent experiments. In comparison with control animals, numbers of CFU were significantly higher in peritoneal exudate cells from fibrin(ogen)-deficient mice 3 h after inoculation with a high dose of S. aureus (P < 0.0001) but not after inoculation with a high dose of L. monocytogenes. Dissemination of L. monocytogenes to hepatic tissue was not significantly affected by fibrin(ogen) deficiency. NS = not significant. Fib KO, fibrin(ogen) deficient; Fib Het, fibrin(ogen) heterozygous.
FIG. 7.
Fibrin restrains hepatic burdens of L. monocytogenes. (A) Fibrinogen-deficient (Fib KO) and littermate control fibrinogen-heterozygous (Fib Het) mice (22 or 23 per group) were i.p. inoculated with approximately 1 × 105 L. monocytogenes bacteria. Five days later, CFU counts were significantly higher in the livers of fibrin(ogen)-deficient mice (P < 0.0001). (B) Untreated and warfarin-anticoagulated mice (21 to 25 per group) were i.p. inoculated with approximately 1 × 105 L. monocytogenes bacteria. Five days later, liver CFU counts were significantly higher in warfarin-treated mice (P < 0.006). Data for panels A and B were compiled from four independent experiments. All datum points are shown, and the bar depicts the mean of each group. (C to H) Hepatic mRNA levels encoding IFN-γ (C, D), iNOS (E, F), and TNF-α (G, H) were assayed on day 5 after sham infection (8 or 9 mice per group) or i.p. inoculation with approximately 1 × 105 L. monocytogenes (Lm) bacteria (14 to 19 mice per group). Data for panels C to H were compiled from three independent experiments and presented as the mean ± the standard deviation of log10-fold change relative to sham-infected control mice. Levels of immune mediators were never significantly suppressed in fibrin(ogen)-deficient or warfarin-anticoagulated mice. Rather, levels of IFN-γ and iNOS were significantly elevated in fibrin(ogen)-deficient mice compared with littermate controls. NS = not significant.
Intact type 1 immunity in fibrin-deficient mice.
Fibrin(ogen) reportedly possesses inflammatory and immunomodulatory properties (see reference 43 and references therein). Thus, we investigated whether diminished production of type 1 immune mediators could account for the elevated hepatic bacterial burdens in fibrin-deficient mice. We found that levels of hepatic mRNA encoding IFN-γ, iNOS, and TNF-α were not significantly decreased in fibrin(ogen)-deficient and warfarin-treated mice, compared with the relevant control mice, at 5 days after inoculation with L. monocytogenes (Fig. 7C to H). Rather, levels of IFN-γ and iNOS were significantly elevated in the fibrin(ogen)-deficient mice, presumably reflecting their elevated bacterial burdens (Fig. 7A). Additional analyses likewise failed to discern reduced hepatic production of mRNA encoding the chemokines monocyte chemoattractant protein 1 (CCL2), macrophage inflammatory proteins 1a (CCL3) and 1b (CCL4), RANTES (CCL5), monokine induced by IFN-γ (CXCL9), or IFN-γ-inducible protein 10 (CXCL10) in fibrin-deficient mice (not shown). Analyses of peritoneal and/or hepatic levels IFN-γ, iNOS, and TNF-α at day 1 or 2 postinfection also failed to reveal evidence of impaired type 1 immunity in fibrin(ogen)-deficient mice. Together, these observations suggest that impaired immunity is an unlikely explanation for the increased bacterial burdens in Listeria-infected, fibrin-deficient mice.
DISCUSSION
Scientists have long debated whether fibrin plays protective or pathogenic roles during bacterial infection. Early studies documented the accumulation of fibrin-rich matrices during peritoneal infections. Some researchers suggested that peritoneal fibrin physically restrains bacteria, thereby functioning protectively by limiting dissemination (1, 17, 18, 39, 46). Others failed to document such effects (33) and rather concluded that fibrin prompts the formation of pathological abscesses and adhesions and physically separates bacteria from host immune cells, thereby impairing bacterial clearance (1, 6, 33, 39, 40). These opposing conclusions caused significant controversy; some clinicians considered fibrin sufficiently harmful to warrant its surgical removal (24, 34, 39), while others believed fibrin sufficiently protective to warrant spraying fibrin-promoting solutions into the peritoneal cavity during abdominal surgery (16). In recent years, researchers have focused largely upon the deleterious functions of infection-stimulated fibrin deposition, which are clearly evident during advanced septic infections (13, 30).
Until recently, studies emphasizing fibrin's protective role during peritonitis relied upon surgical implantation of bacterium-laden fibrin matrices, treatment with relatively nonspecific anticoagulants, or administration of fibrinolytic factors. Some of these studies were highly artificial, while others were confounded by the potential coagulation-unrelated activities of the agents administered. Against that backdrop, we sought to apply a new tool to the study of roles for coagulation during bacterial infection. We reasoned that the production of gene-targeted fibrin(ogen)-deficient mice by Degen and colleagues (41) would enable decisive evaluations of the contributions of fibrin(ogen), be they positive or negative, to antibacterial defense. Indeed, we demonstrated that mice lacking fibrin(ogen) succumb to doses of L. monocytogenes that genetically matched control mice survive, thereby establishing that fibrin(ogen) performs important host-protective functions during listeriosis (Fig. 2). In addition, we found that mice treated with warfarin, a well-characterized pharmaceutical anticoagulant, likewise succumb to doses of L. monocytogenes that control mice survive (Fig. 2). As warfarin suppresses fibrin formation without affecting circulating levels of fibrinogen, our data suggest that fibrin, as opposed to fibrinogen, is the important determinant of survival in this model.
Recently, Degen and colleagues reported their independent evaluation of experimental peritonitis in gene-targeted fibrin(ogen)-mutant mice (19). That study employed an extracellular bacterium, S. aureus, and concluded that fibrin(ogen) aids bacterial clearance from the peritoneal cavity. Using mice lacking all fibrin(ogen), we readily confirmed its important role in the peritoneal clearance of S. aureus (Fig. 6). In contrast, we found that the peritoneal clearance of the intracellular bacterium L. monocytogenes was at most modestly and transiently affected by fibrin(ogen) deficiency (Fig. 6). Moreover, we documented that fibrin(ogen) deficiency does not affect the dissemination of L. monocytogenes from the peritoneal site of inoculation (Fig. 6). Rather, we found that fibrin(ogen) plays an important role in restraining the growth of L. monocytogenes within infected hepatic tissue (Fig. 7A). To our knowledge, this is the first demonstration that fibrin can restrain the growth of any intracellular pathogen.
Further studies are required to determine how fibrin restrains the growth of L. monocytogenes. Regarding the control of extracellular bacteria, Degen and colleagues suggested that fibrin(ogen) may be required for the full activation of phagocyte antimicrobial functions (19). We confirmed their observation that peritoneal neutrophils in Staphylococcus-infected, fibrin(ogen)-deficient mice appear excessively engorged with bacteria, apparently reflecting their failure to destroy phagocytosed S. aureus (not shown). Although recently questioned (28), a number of studies have suggested that neutrophils play important roles during the clearance of L. monocytogenes (8-11, 22, 35-37). While we cannot exclude the possibility that impaired neutrophil function accounts for the deficient clearance of L. monocytogenes from fibrin-deficient mice, multiple independent analyses have failed to reveal significant differences in the number, phenotype, or bacterial burdens of either peritoneal or hepatic neutrophils isolated from Listeria-infected fibrin(ogen)-deficient and control mice (not shown). Indeed, we have yet to discern any impairment of cellular recruitment, inflammation, or immunity in fibrin(ogen)-deficient mice during listeriosis (Fig. 7 and data not shown). Thus, it seems unlikely that the inefficient clearance of L. monocytogenes from hepatic tissue in fibrin(ogen)-deficient mice reflects fibrin(ogen)'s cell-adhesive, proinflammatory, or immunomodulatory properties (see references within reference 43). Rather, we ascribe the fibrin(ogen)-mediated control of bacterial growth within hepatic tissue to fibrin's physical attributes, which are well appreciated to trap circulating blood cells during the formation of a blood clot. Ongoing research efforts are aimed at determining whether fibrin physically restrains the movement of infected cells and/or restrains the cell-to-cell spread of intracellular bacteria.
Given that fibrin apparently limits bacterial infection by multiple mechanisms, it is not surprising that many bacteria produce proteins that facilitate fibrin degradation (5). Indeed, the bacterial products streptokinase and staphylokinase are used clinically as therapeutic “clot-busting” agents, as they are potent activators of human plasmin, a fibrin-degrading enzyme. Moreover, elegant genetic studies have demonstrated that host plasmin plays critical roles in promoting bacterial virulence (7, 21, 42). In addition to expressing factors that stimulate fibrin degradation, bacteria also appear to have usurped fibrin for their own advantage; they express a variety of fibrin(ogen)-binding receptors (20), and fibrin(ogen)-mediated bacterial clumping appears to activate bacterial quorum-sensing responses, thereby altering gene expression patterns and increasing virulence (38). Thus, the evolution of fibrin-mediated protective mechanisms apparently fostered the coevolution of bacterial responses that seek to escape and/or take advantage of host fibrin(ogen).
While hemorrhage is not commonly reported during listeriosis, many virulent infections prompt hemorrhage. Our studies revealed that fibrin deficiency predisposes to hemorrhagic pathology during infection by L. monocytogenes (Fig. 3 to 5), suggesting that fibrin functions to contain subclinical hemorrhage during listeriosis. Previously, we found that fibrin also suppresses hemorrhagic pathology during infection of mice by the protozoan parasite T. gondii, another intracellular pathogen that stimulates robust type 1 immunity (25). During infection by both L. monocytogenes and T. gondii, the time at which fibrin-deficient mice succumb to acute infection coincides with the time of peak levels of anemia in control animals. Together, these data suggest that fibrin plays a broader role than previously perceived in containing blood loss during infection.
In the context of T. gondii infection, we uncovered an important role for IFN-γ in the infection-stimulated hemorrhagic pathology (25). During listeriosis, it appears that that hemorrhagic pathology is not mediated by IFN-γ (not shown). During highly virulent infections that prompt overt hemorrhage, such as Yersinia pestis, Bacillus anthracis, and Ebola virus, it is often suggested that hemorrhage results from infection-stimulated thromboses, which may prompt rupture of the microvasculature, and/or from disseminated intravascular coagulation, which may globally suppress hemostasis by consuming platelets and coagulant factors (27, 30). However, such coagulation-mediated mechanisms are unlikely causes of the hemorrhagic pathology we observe in fibrin(ogen)-deficient and warfarin-treated mice because both are coagulation impaired. Rather, we suggest that infection and/or the immune response to infection likely prompts hemorrhage via mechanisms that have yet to be adequately described and whose precise identification may lead to novel therapies for hemorrhagic diseases. Paradoxically, such studies may suggest that immunosuppressive agents may be useful adjunctive therapies during infection by highly virulent hemorrhage-evoking pathogens.
Sepsis is a clinical condition defined by evidence of a systemic inflammatory response occurring during infection (3). In the United States, sepsis and its aggravated forms (severe sepsis, septic shock) are major causes of human mortality, accounting for nearly 10% of all deaths (2). A wide variety of microorganisms may prompt sepsis (4, 27), including L. monocytogenes (15, 45). While our data establish a protective role for coagulation during listeriosis, we do not dispute the capacity of coagulation, in particular disseminated intravascular coagulation, to function pathologically during sepsis. In fact, therapeutic administration of activated protein C, a natural anticoagulant protein, was recently shown to reduce septic mortality in humans (4), suggesting that some aspect of coagulation functions pathologically during sepsis (14). However, several robust anticoagulant therapies have failed to improve septic morbidity in humans (13, 30), and a gene polymorphism that increases coagulant activity (factor V Leiden) actually confers a significant survival advantage during severe sepsis (26). These apparent inconsistencies can be reconciled by hypothesizing that coagulation plays both protective and pathological roles during sepsis. In this report, we have demonstrated that fibrin both limits hemorrhagic pathology and contains bacterial growth during listeriosis. The accumulating evidence that coagulation functions protectively during infection strongly suggests that treatments for sepsis should strive to balance, rather than abrogate, coagulative responses.
Acknowledgments
This work was supported by Public Health Service grant HL72937 (S.T.S.) and funds from Trudeau Institute.
We are grateful to Robert North, Frances Lund, and Andrea Cooper for critical reading of the manuscript. We thank Tom Devecseri for assistance in the preparation of photomicrographs, and we are indebted to the employees of the Trudeau Institute Animal Breeding and Maintenance Facilities for dedicated care of the mice used in these studies.
Editor: J. L. Flynn
REFERENCES
- 1.Ahrenholz, D. H., and R. L. Simmons. 1980. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery 88:41-47. [PubMed] [Google Scholar]
- 2.Angus, D. C., W. T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M. R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29:1303-1310. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 1992. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874. [PubMed] [Google Scholar]
- 4.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, and C. J. Fisher, Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, M. D., and R. Lottenberg. 1997. Plasminogen activation by invasive human pathogens. Thromb. Haemostasis 77:1-10. [PubMed] [Google Scholar]
- 6.Ciano, P. S., R. B. Colvin, A. M. Dvorak, J. McDonagh, and H. F. Dvorak. 1986. Macrophage migration in fibrin gel matrices. Lab. Investig. 54:62-70. [PubMed] [Google Scholar]
- 7.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 8.Conlan, J. W., and R. J. North. 1991. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J. Exp. Med. 174:741-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., J. F. Brown, R. D. Wagner, and H. Steinberg. 1994. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect. Immun. 62:5161-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies, W. A., V. P. Ackerman, and D. S. Nelson. 1981. Mechanism for nonspecific immunity of Listeria monocytogenes in rats mediated by platelets and the clotting system. Infect. Immun. 33:477-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempfle, C. E. 2004. Coagulopathy of sepsis. Thromb. Haemostasis 91:213-224. [DOI] [PubMed] [Google Scholar]
- 14.Dhainaut, J. F., S. B. Yan, B. D. Margolis, J. A. Lorente, J. A. Russell, R. C. Freebairn, H. D. Spapen, H. Riess, B. Basson, G. Johnson III, and G. T. Kinasewitz. 2003. Drotrecogin alfa (activated) (recombinant human activated protein C) reduces host coagulopathy response in patients with severe sepsis. Thromb. Haemostasis 90:642-653. [DOI] [PubMed] [Google Scholar]
- 15.Doganay, M. 2003. Listeriosis: clinical presentation. FEMS Immunol. Med. Microbiol. 35:173-175. [DOI] [PubMed] [Google Scholar]
- 16.Dubrow, T., R. J. Schwartz, J. McKissock, and S. E. Wilson. 1991. Effect of aerosolized fibrin solution on intraperitoneal contamination. Arch. Surg. 126:80-83. [DOI] [PubMed] [Google Scholar]
- 17.Dunn, D. L., and R. L. Simmons. 1982. Fibrin in peritonitis. III. The mechanism of bacterial trapping by polymerizing fibrin. Surgery 92:513-519. [PubMed] [Google Scholar]
- 18.Echtenacher, B., K. Weigl, N. Lehn, and D. N. Mannel. 2001. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69:3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flick, M. J., X. Du, D. P. Witte, M. Jirouskova, D. A. Soloviev, S. J. Busuttil, E. F. Plow, and J. L. Degen. 2004. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 113:1596-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 21.Gebbia, J. A., J. C. Monco, J. L. Degen, T. H. Bugge, and J. L. Benach. 1999. The plasminogen activation system enhances brain and heart invasion in murine relapsing fever borreliosis. J. Clin. Investig. 103:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 23.Hoffmann-Fezer, G., H. Maschke, H. J. Zeitler, P. Gais, W. Heger, J. Ellwart, and S. Thierfelder. 1991. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann. Hematol. 63:214-217. [DOI] [PubMed] [Google Scholar]
- 24.Hudspeth, A. S. 1975. Radical surgical debridement in the treatment of advanced generalized bacterial peritonitis. Arch. Surg. 110:1233-1236. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerlin, B. A., S. B. Yan, B. H. Isermann, J. T. Brandt, R. Sood, B. R. Basson, D. E. Joyce, H. Weiler, and J. F. Dhainaut. 2003. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood 102:3085-3092. [DOI] [PubMed] [Google Scholar]
- 27.Kinasewitz, G. T., S. B. Yan, B. Basson, P. Comp, J. A. Russell, A. Cariou, S. L. Um, B. Utterback, P. F. Laterre, and J. F. Dhainaut. 2004. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit. Care 8:R82-R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaCourse, R., L. Ryan, and R. J. North. 2002. Expression of NADPH oxidase-dependent resistance to listeriosis in mice occurs during the first 6 to 12 hours of liver infection. Infect. Immun. 70:7179-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lara-Tejero, M., and E. G. Pamer. 2004. T cell responses to Listeria monocytogenes. Curr. Opin. Microbiol. 7:45-50. [DOI] [PubMed] [Google Scholar]
- 30.Levi, M., E. de Jonge, and T. van der Poll. 2004. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann. Med. 36:41-49. [DOI] [PubMed] [Google Scholar]
- 31.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 32.Mackaness, G. B. 1969. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J. Exp. Med. 129:973-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McRitchie, D. I., M. J. Girotti, M. F. Glynn, J. M. Goldberg, and O. D. Rotstein. 1991. Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J. Lab. Clin. Med. 118:48-55. [PubMed] [Google Scholar]
- 34.Muntean, V. E. 1987. Fibrin in peritonitis. Ann. Surg. 206:681-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.North, R. J., P. L. Dunn, and J. W. Conlan. 1997. Murine listeriosis as a model of antimicrobial defense. Immunol. Rev. 158:27-36. [DOI] [PubMed] [Google Scholar]
- 36.Rakhmilevich, A. L. 1995. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J. Leukoc. Biol. 57:827-831. [DOI] [PubMed] [Google Scholar]
- 37.Rogers, H. W., and E. R. Unanue. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 61:5090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothfork, J. M., S. Dessus-Babus, W. J. Van Wamel, A. L. Cheung, and H. D. Gresham. 2003. Fibrinogen depletion attenuates Staphylococcus aureus infection by preventing density-dependent virulence gene up-regulation. J. Immunol. 171:5389-5395. [DOI] [PubMed] [Google Scholar]
- 39.Rotstein, O. D. 1992. Role of fibrin deposition in the pathogenesis of intraabdominal infection. Eur. J. Clin. Microbiol. Infect. Dis. 11:1064-1068. [DOI] [PubMed] [Google Scholar]
- 40.Rotstein, O. D., and J. Kao. 1988. Prevention of intra-abdominal abscesses by fibrinolysis using recombinant tissue plasminogen activator. J. Infect. Dis. 158:766-772. [DOI] [PubMed] [Google Scholar]
- 41.Suh, T. T., K. Holmback, N. J. Jensen, C. C. Daugherty, K. Small, D. I. Simon, S. Potter, and J. L. Degen. 1995. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 9:2020-2033. [DOI] [PubMed] [Google Scholar]
- 42.Sun, H., U. Ringdahl, J. W. Homeister, W. P. Fay, N. C. Engleberg, A. Y. Yang, L. S. Rozek, X. Wang, U. Sjobring, and D. Ginsburg. 2004. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science 305:1283-1286. [DOI] [PubMed] [Google Scholar]
- 43.Szaba, F. M., and S. T. Smiley. 2002. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99:1053-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Hove, L., W. Goossens, V. Van Duppen, and R. L. Verwilghen. 1990. Reticulocyte count using thiazole orange. A flow cytometry method. Clin. Lab. Haematol. 12:287-299. [DOI] [PubMed] [Google Scholar]
- 45.Wing, E. J., and S. H. Gregory. 2002. Listeria monocytogenes: clinical and experimental update. J. Infect. Dis. 185(Suppl. 1):S18-S24. [DOI] [PubMed] [Google Scholar]
- 46.Zinsser, H. H., and A. W. Pryde. 1952. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann. Surg. 136:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]