Abstract
Chlamydiae are obligate intracellular pathogens that efficiently induce their endocytosis by susceptible eukaryotic host cells. Recently, a Chlamydia trachomatis type III secreted effector protein, Tarp, was found to be translocated and tyrosine phosphorylated at the site of entry and associated with the recruitment of actin that coincides with endocytosis. C. trachomatis Tarp possesses up to six direct repeats of approximately 50 amino acids each. The majority of the tyrosine residues are found within this repeat region. Here we have ectopically expressed distinct domains of Tarp in HeLa 229 cells and demonstrated that tyrosine phosphorylation occurs primarily within the repeat region, while recruitment of actin is mediated by the C-terminal domain of the protein. A comparison of other sequenced chlamydial genomes revealed that each contains an ortholog of Tarp, although Chlamydia muridarum, Chlamydophila caviae, and Chlamydophila pneumoniae Tarp lack the large repeat region. Immunofluorescence and immunoblotting using an antiphosphotyrosine antibody show no evidence of phosphotyrosine at the site of entry of C. muridarum, C. caviae, and C. pneumoniae, although each species similarly recruits actin. Ectopic expression of full-length C. trachomatis and C. caviae Tarp confirmed that both recruit actin but only C. trachomatis Tarp is tyrosine phosphorylated. The data indicate that the C-terminal domain of Tarp is essential for actin recruitment and that tyrosine phosphorylation may not be an absolute requirement for actin recruitment. The results further suggest the potential for additional, unknown signal transduction pathways associated specifically with C. trachomatis.
Chlamydiae are bacterial, obligate intracellular parasites that are responsible for a number of serious human diseases. Chlamydia trachomatis serovars A, B, Ba, and C are the etiologic agents of trachoma, the leading cause of preventable blindness worldwide. Serovars D to K are associated with sexually transmitted diseases, and serovars L1, L2, and L3 cause lymphogranuloma venereum, a more invasive sexually transmitted disease. Chlamydophila psittaci is a zoonotic agent that occasionally causes psittacosis in humans. Chlamydophila pneumoniae is a causative agent of community-acquired pneumonia that has also been associated with cardiovascular and other persistent diseases (30). All chlamydiae share several biological properties unique to the species, including a biphasic developmental cycle that involves cell types adapted for extracellular survival (elementary bodies [EBs]) and intracellular multiplication (reticulate bodies) (26). All species also share a unique interaction with the host cell in which they inhabit a parasitophorous vacuole, termed the inclusion, that is nonfusogenic with endocytic vesicles but is instead interactive with an exocytic pathway that delivers sphingomyelin and cholesterol from the Golgi apparatus to the inclusion (15).
Chlamydiae induce their internalization so efficiently that the process has been termed “parasite-specified phagocytosis” (5). Because entry into host cells is a critical step in the chlamydial developmental cycle and one potentially amenable to immune or chemotherapeutic intervention, there has been considerable effort to understand the molecular mechanisms mediating chlamydial endocytosis. Several chlamydial ligands and host receptors have been proposed (21), although there has been little consensus as to which are of primary importance. It is likely that many of these interactions do not function in all situations but may play a role on different cell types or anatomic sites. An efficient obligate intracellular pathogen such as chlamydia may utilize multiple means to gain entry into a host cell. Indeed, available evidence suggests that alternative means of entry, such as Fc-mediated endocytosis of opsonized EBs (37), can lead to productive infection and that the outcome of the initial interaction with the host is defined by the ability of the chlamydiae to synthesize protein to modify the inclusion membrane (32).
An initial electrostatic and reversible interaction mediated through heparan sulfate-like proteoglycans is thought to precede an irreversible host-dependent step that leads to internalization of EBs (36, 40). Recent efforts to understand the chlamydial entry process have employed chemically mutagenized CHO cells to distinguish two distinct stages in the chlamydial entry process (9, 17). The identity of the host gene mutated to block this secondary irreversible step was not determined in either study. The events associated with chlamydial entry are, however, beginning to be described. The first demonstrable host response is the recruitment of actin to the site of entry to form a pedestal-like structure at the site of EB attachment (8). This recruitment of actin is transient and culminates in the endocytosis of the EB into a membrane-bound vesicle that very quickly becomes devoid of known cellular markers of the endocytic pathway (31). Once internalized, the EB-containing vesicle remains noninteractive with host cytoskeletal components or vesicular trafficking pathways until such time as chlamydial transcription and translation are initiated and the inclusion membrane is modified by the insertion of chlamydial proteins (19, 32).
Recently, a chlamydial protein which is preexisting in EBs was found to be secreted via a type III-dependent mechanism into the host cell at the site of entry where it is tyrosine phosphorylated (11). This protein, termed Tarp for translocated actin-recruiting phosphoprotein, is temporally and spatially associated with the recruitment of actin and proposed to play a key role in mobilizing cellular signal transduction pathways that regulate actin recruitment and endocytosis of EBs. Tarp is notable in possessing several tyrosine-rich tandem repeats of approximately 50 amino acids each in length. The number of repeats differs between a C. trachomatis urogenital isolate, serovar D, with three repeat units (35) and an LGV strain with almost six full repeat units (11). The tandem repeat domain is absent from Chlamydia muridarum (C. trachomatis MoPn), Chlamydophila caviae (C. psittaci GPIC), and C. pneumoniae (22, 27, 28). In an effort to understand the role of the distinct domains of Tarp and the significance of tyrosine phosphorylation, we have examined the tyrosine phosphorylation patterns of Tarp from several chlamydial species as well as of ectopically expressed Tarp.
MATERIALS AND METHODS
Organisms and cell culture.
C. trachomatis serovars L2 (LGV-434) and D (UW3-Cx), C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae AR-39 were grown in HeLa 229 cells as previously described (6, 29, 39). EBs used for infections were purified by Renografin (E. R. Squibb and Sons, Princeton, NJ) density gradient centrifugation (6). Fluorescent CMTMR-labeled EBs were prepared as described previously (4, 8).
Antibodies.
Full-length L2 Tarp (anti-Tarp-F) was cloned into pRSET-B (Invitrogen), expressed as a His-tagged fusion protein, purified on a nickel column (Invitrogen), and used as an immunogen for production of specific polyclonal antibodies in female New Zealand White rabbits, as previously described (33). Antiphosphotyrosine monoclonal antibody (MAb) clone 4G10 and 4G10 coupled to agarose were from Upstate USA (Waltham, MA). Secondary antibodies for immunoblotting were horseradish peroxidase-conjugated anti-mouse or anti-rabbit (Cell Signaling Technology, Inc., Beverly, MA).
SDS-PAGE and immunoblotting.
Proteins were separated on 7.5 or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (25) and Coomassie stained (Bio-Rad Laboratories, Hercules, CA) for total protein or transferred to a 0.45-μm Trans-blot nitrocellulose membrane (Bio-Rad Laboratories). Immunoblots were developed using Super Signal West Pico chemiluminescence reagent (Pierce Biotechnology, Rockford, IL).
Cloning.
In-frame green fluorescent protein (GFP) fusion proteins for the full-length, N terminus (amino acids 1 to 155), repeat region (amino acids 120 to 431), and C terminus (amino acids 425 to 1005) of the CT 456 gene were amplified from Chlamydia trachomatis serovar L2 genomic DNA (QIAGEN genomic purification kit) in a Perkin-Elmer Cetus DNA thermocycler using Deep Vent DNA polymerase (New England BioLabs) and custom-synthesized oligonucleotide primers (Integrated DNA Technologies) engineered with KpnI and BamHI linkers. Full-length Tarp (CCA00170) from C. caviae was similarly amplified and expressed as an in-frame GFP fusion. Clones of amplified chlamydial DNA were first constructed in a Zero Blunt vector (Invitrogen) and subsequently transferred to the GFP expression vector pEGFP-C3 (Clontech). Miniprep DNA was isolated using the QIAGEN maxi prep kit according to the manufacturer's instructions and quantitated by UV spectrophotometry.
pTarpΔR was generated from full-length pTarp-FT (11) using the Quikchange II XL site-directed mutagenesis kit (Stratagene) and oligonucleotide sense (5′-TCCCCCGGGGATAGAAGTAGTTTCATTCTTGTTCCTAACGG-3′) and antisense (5′-TCCCCCGGGATCGCTTGATGAGGTAGAGCTAGTTTCTGAGC-3′) primers containing engineered SmaI restriction sites. The PCR-amplified product was SmaI digested and ligated to yield the mutant Tarp lacking codons 121 to 360. pTarp-FT and pTarpΔR were expressed in translocation-competent (YPIII pIB29MEKA) and null (YPIII pIB29MEKBA) Yersinia pseudotuberculosis. Tests in liquid media were performed as described previously (16) to confirm that both Tarp proteins were secreted by the type III system (data not shown). For translocation assays, yersiniae from overnight 26°C cultures in Ca2+-depleted heart infusion broth were diluted to an A620 of 0.1 into fresh media and cultivated at in the presence of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 2 h, bacteria were diluted into 37°C Hanks balanced salt solution containing 0.1 mM IPTG and semiconfluent HeLa monolayers were infected at a multiplicity of infection (MOI) of 10. Cultures with centrifuged for 5 min at 700 × g to initiate bacterium-host cell contact and then incubated for 4 h at 37°C in 5% CO2. Cultures were then washed with Hanks balanced salt solution, and cells were lysed by the addition of ice-cold H2O. Whole-culture material was trichloroacetic acid precipitated and resolved via SDS-PAGE, and Tarp and TarpΔR were specifically detected in immunoblots using Tarp-specific or phosphotyrosine-specific 4G10 antibodies.
Transfection and GFP expression.
HeLa 229 cells were seeded on 12-mm glass coverslips in 24-well plates to obtain a monolayer of approximately 50% confluence. Transfections of plasmid constructs were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. The transfection mixture was prepared as follows. DNA (1.0 μg) was diluted in 8 μl of PLUS reagent and 50 μl Optimem serum-free media (Invitrogen) and added to a solution of 2.5 μl Lipofectamine 2000 in 50 μl Optimem. After a 20-minute incubation at room temperature, the complexes were added to 1 well of a 24-well plate containing 400 μl of Optimem. The transfection cocktail was incubated at 37°C. After 4 hours, the Optimem with transfection cocktail was removed and fresh, antibiotic-free RPMI media with 10% fetal bovine serum was added. Expression from the transfection vectors was allowed to proceed for 24 hours at 37°C. Transfected HeLa 229 cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature and permeabilized for 5 minutes with 0.1% Triton X-100 plus 0.05% SDS in phosphate-buffered saline (PBS). Actin staining was performed using Texas Red X-phalloidin (Molecular Probes) in PBS-3% bovine serum albumin for 2 hours. Phosphotyrosine staining was performed using antiphosphotyrosine 4G10 mouse monoclonal antibody (Upstate Biotechnology) in PBS-3% bovine serum albumin for 1 hour followed by the secondary Alexa Fluor 594-conjugated goat anti-mouse immunoglobulin G (IgG) (Molecular Probes) for 1 hour. Fluorescent images were digitally acquired on a Nikon FXA microscope equipped with a Photometrics CoolSnap HQ camera supported by RS Image software (Roper Scientific, Inc.). Images were processed using Photoshop 6.0 (Adobe Systems).
Actin recruitment.
GFP-actin was expressed in HeLa 229 cells, and recruitment during chlamydial entry was monitored by spinning disk confocal microscopy as previously described (8).
RESULTS
Tyrosine phosphorylation of Tarp domains and recruitment of actin.
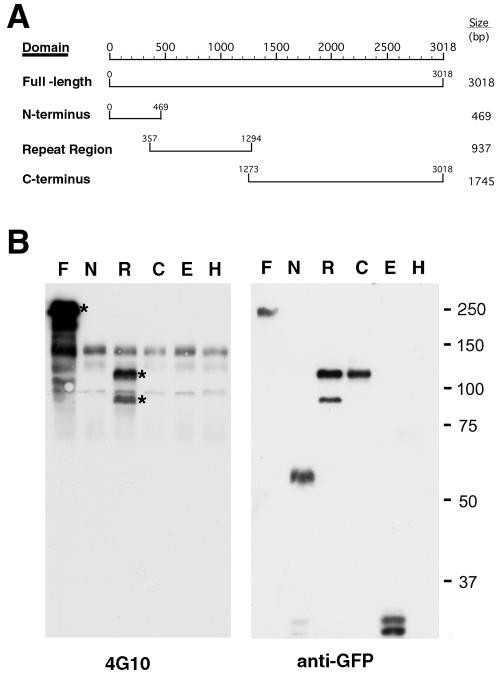
Full-length C. trachomatis L2 Tarp ectopically expressed as a GFP fusion in HeLa cells forms aggregates within the cytoplasm. GFP-Tarp is tyrosine phosphorylated when expressed in HeLa cells, suggesting that an unknown host kinase is responsible, and recruits actin as evidenced by staining with phalloidin or antiactin antibody (11). C. trachomatis L2 Tarp contains a domain with almost six nearly identical tandem repeats of approximately 50 amino acids each. Full-length Tarp and the N-terminal, repeat region, and C-terminal domains of Tarp (Fig. 1A) were cloned as GFP fusions and expressed in HeLa 229 cells. Tyrosine phosphorylation of the transfected full-length Tarp and repeat domain was observed by immunoblotting against the MAb 4G10 (Fig. 1B). The GFP fusions were then expressed in HeLa cells, and each was stained with MAb 4G10 or phalloidin (Fig. 2). Full-length Tarp and the C-terminal domains formed distinct aggregates within the cytoplasm of the transfected HeLa cells, whereas the N-terminal and repeat domains were soluble and showed diffuse staining throughout the cytoplasm and nucleus. Although aggregated full-length Tarp shows staining with both antiphosphotyrosine MAb 4G10 and phalloidin, the C-terminal domain stains with phalloidin but no antiphosphotyrosine staining is observed. No alterations of cytoskeletal structure were seen with either the N-terminal or repeat domains. However, diffuse staining of the repeat domain with the antiphosphotyrosine antibody was observed. Actin recruitment thus appears to be independent of tyrosine phosphorylation.
FIG. 1.
Ectopic expression of C. trachomatis GFP-Tarp domains indicates tyrosine phosphorylation of the repeat region of Tarp. (A) Schematic of the C. trachomatis L2 Tarp domains expressed as C-terminal GFP fusions. (B) Immunoblots of protein extracts from HeLa 229 cells transfected with the various enhanced GFP (EGFP) fusion constructs. F, full-length Tarp; N, N-terminal domain; R, repeat region; C, C-terminal domain; E, EGFP vector control; H, uninfected HeLa cells. Blots were probed with MAb 4G10 for tyrosine phosphorylation and anti-GFP to confirm expression and relative migration. Asterisks identify tyrosine phosphorylation of TARP and the repeat region. An apparent degradation product of the repeat region is also detected. Please note that the repeat region fusion migrates aberrantly on SDS-PAGE, as does native Tarp (11). The same Kpn-Bam insert was also cloned into pRsetB for expression in E. coli, and the product was confirmed as the Tarp repeat region by matrix-assisted laser desorption ionization-time of flight mass spectroscopy (data not shown). Background tyrosine-phosphorylated host protein bands seen in HeLa cells are apparent in all lanes probed with MAb 4G10.
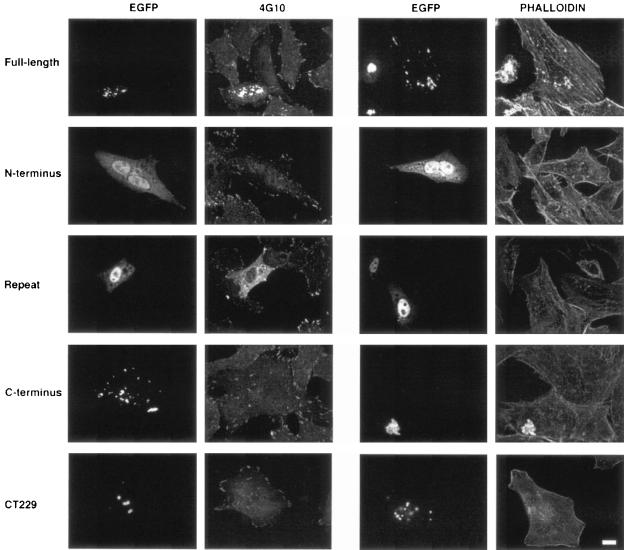
FIG. 2.
Immunofluorescent staining of C. trachomatis Tarp-enhanced GFP (EGFP) constructs for tyrosine phosphorylation. The constructs described in the legend to Fig. 1 were expressed in HeLa 229 cells and stained for tyrosine phosphorylation using MAb 4G10 with an Alexa Fluor 594-conjugated anti-mouse IgG secondary antibody or stained for actin recruitment using Texas Red phalloidin. Full-length Tarp and the C-terminal domain form aggregates in the host cell. The N-terminal domain and repeat region show diffuse localization throughout the cytoplasm and nucleus. CT229, an inclusion membrane protein (1, 34) expressed as a negative control, also forms aggregates within the cytoplasm. Bar, 10 μm.
Orthologs of Tarp among chlamydial species.
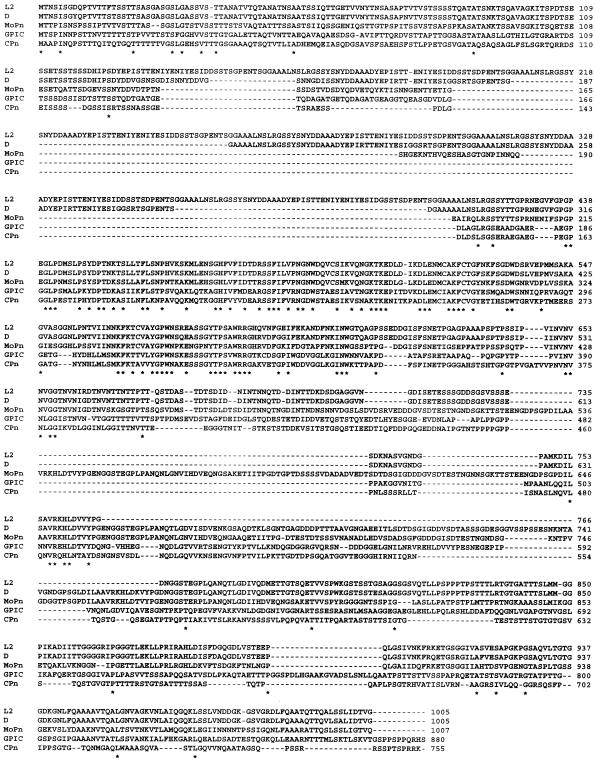
An alignment of Tarp orthologs from C. trachomatis serovars L2 and D, C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae (Cpn) is shown in Fig. 3. Total primary amino acid sequence identities with C. trachomatis L2 Tarp range from 94.3%, 59.0%, 43.0%, and 40.6% in C. trachomatis serovar D, C. muridarum, C. caviae, and C. pneumoniae, respectively (by BESTFIT alignment). In C. trachomatis L2, 26 of 31 tyrosine residues occur within the tandem repeat domain; for serovar D, 12 of 21 tyrosine residues reside within this domain. Note that the latter three species lack the tandem repeat domain. The highest degree of similarity occurs in the C-terminal domain downstream of the repeats in C. trachomatis.
FIG. 3.
Alignment of Tarp orthologs from C. trachomatis serovars L2 and D, C. muridarum, C. caviae, and C. pneumoniae. Sequences of C. trachomatis serovar L2 (LGV-434) CT456 (11) and serovar D (UW3-Cx) CT456 (35), Chlamydia muridarum (MoPn) TC0741 (27), Chlamydophila caviae (GPIC) CCA00170 (28), and Chlamydophila pneumoniae (CWL029) (CPn) CPn0572 (22) were aligned using Clustal W, version 1.82, multiple-sequence alignment software (http://www.ebi.ac.uk/clustalw). Asterisks indicate identities.
Actin recruitment by C. muridarum, C. caviae, and C. pneumoniae.
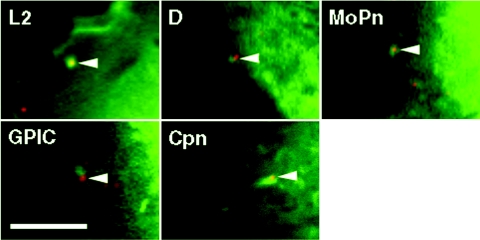
Internalization of C. trachomatis serovars L2 and D is associated with the localized recruitment of actin (7, 8, 11). To confirm that actin is recruited to the site of entry of the other chlamydial species, HeLa 229 cells expressing GFP-actin were infected with intrinsically fluorescent C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae (Cpn) EBs and actin recruitment was monitored by live cell imaging using a spinning disk confocal microscope (Fig. 4). All chlamydial species transiently recruited actin as has been demonstrated for C. trachomatis serovars L2 and D (8).
FIG. 4.
Actin is recruited to the site of entry by C. trachomatis, C. muridarum, C. caviae, and C. pneumoniae EBs. HeLa 229 cells expressing GFP-actin were infected with CMTMR-labeled (red) C. trachomatis serovars L2 and D, C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae (Cpn) EBs and examined by spinning disk confocal microscopy for recruitment of actin. All chlamydial species and serovars examined demonstrated robust but transient recruitment of actin to the site of entry. The figure is a composite of single frames taken from a time-lapse series for each strain or species. Bar, 5 μm.
Tyrosine phosphorylation of Tarp is specific to C. trachomatis.
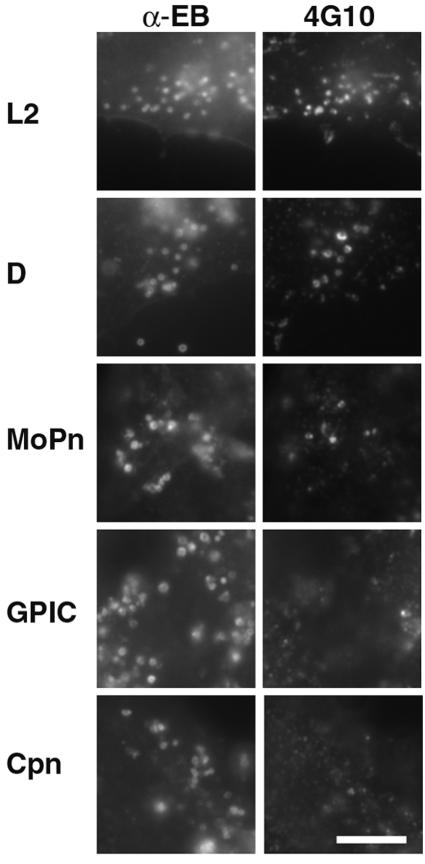
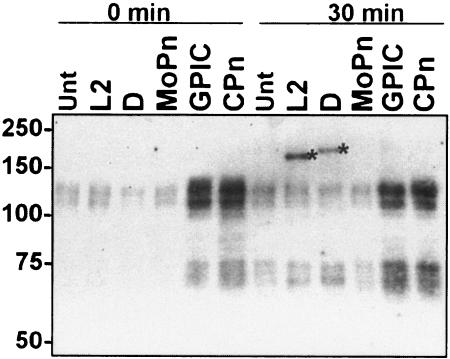
Because Tarp orthologs from C. muridarum, C. caviae, and C. pneumoniae lack the tyrosine-rich repeat domain, we examined these species for tyrosine phosphorylation of Tarp during the entry process. Tyrosine phosphorylation was not observed in association with C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae EBs by immunofluorescence with MAb 4G10, although C. trachomatis serovars L2 and D EBs showed colocalization as previously described (11) (Fig. 5). Similarly, no evidence of tyrosine phosphorylation of Tarp from C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae (Cpn) was observed by immunoblotting (Fig. 6). These results suggest that although chlamydial species other than C. trachomatis possess Tarp orthologs, these are not tyrosine phosphorylated as part of the entry process.
FIG. 5.
Immunofluorescent staining for tyrosine phosphorylation in association with C. trachomatis L2 and D, C. muridarum (MoPn), C. caviae (GPIC), and C. pneumoniae (Cpn) EBs during internalization by HeLa 229 cells. Cultures at 1 h postinfection were costained by indirect immunofluorescence for EBs using a rabbit polyclonal antiserum and tyrosine phosphorylation with MAb 4G10. Tyrosine phosphorylation is not seen in association with C. muridarum, C. caviae, and C. pneumoniae EBs but is readily observed with C. trachomatis serovars L2 and D. Bar, 5 μm.
FIG. 6.
C. muridarum, C. caviae, and C. pneumoniae Tarp is not tyrosine phosphorylated. Total protein lysates were collected from HeLa cells that were either mock-infected (Unt) or infected (MOI, ∼100) with C. trachomatis L2 or D, C. muridarum (MoPn), C. caviae (GPIC), or C. pneumoniae (CPn) for 1 h at 4°C (time = 0) or at 30 min post-temperature shift to 37°C. Equal volumes of parallel mock-infected or infected cultures were loaded on each lane. Immunoblots were probed with 4G10 and visualized by chemiluminescence. Asterisks indicate the positions of tyrosine-phosphorylated Tarp; molecular masses in kilodaltons are indicated on the left.
Tarp with a deletion of the repeat region is not tyrosine phosphorylated.
We have established that full-length C. trachomatis L2 Tarp is secreted from Y. pseudotuberculosis by a type III-dependent mechanism in liquid cultures (11). We expressed Tarp in Y. pseudotuberculosis translocation-competent MEKA or -deficient MEKBA to test whether this protein could additionally be targeted to host cells in a HeLa cell infection model (Fig. 7). For these experiments, we also constructed an in-frame deletion mutant (TarpΔR) lacking the Tarp-specific repeat region (codons 121 to 360). Both Tarp and TarpΔR were type III secreted from MEKA and MEKBA yersiniae in liquid cultures (data not shown). HeLa cells were infected at an MOI of 10 yersiniae (16), and whole-culture lysates were prepared. Both Tarp and TarpΔR were readily detectible in immunoblots probed with Tarp-specific antibodies. Tarp was detectible with phosphotyrosine-specific antibodies when expressed in MEKA but not in MEKBA, which lacks the translocator protein YopB. No signal was detected in material containing TarpΔR. These data indicate that a pool of Tarp is successfully translocated to host cells where it is subsequently phosphorylated. These data are consistent with the phosphorylation event occurring within the repeat region, since TarpΔR was not detected in the 4G10 immunoblot.
FIG. 7.
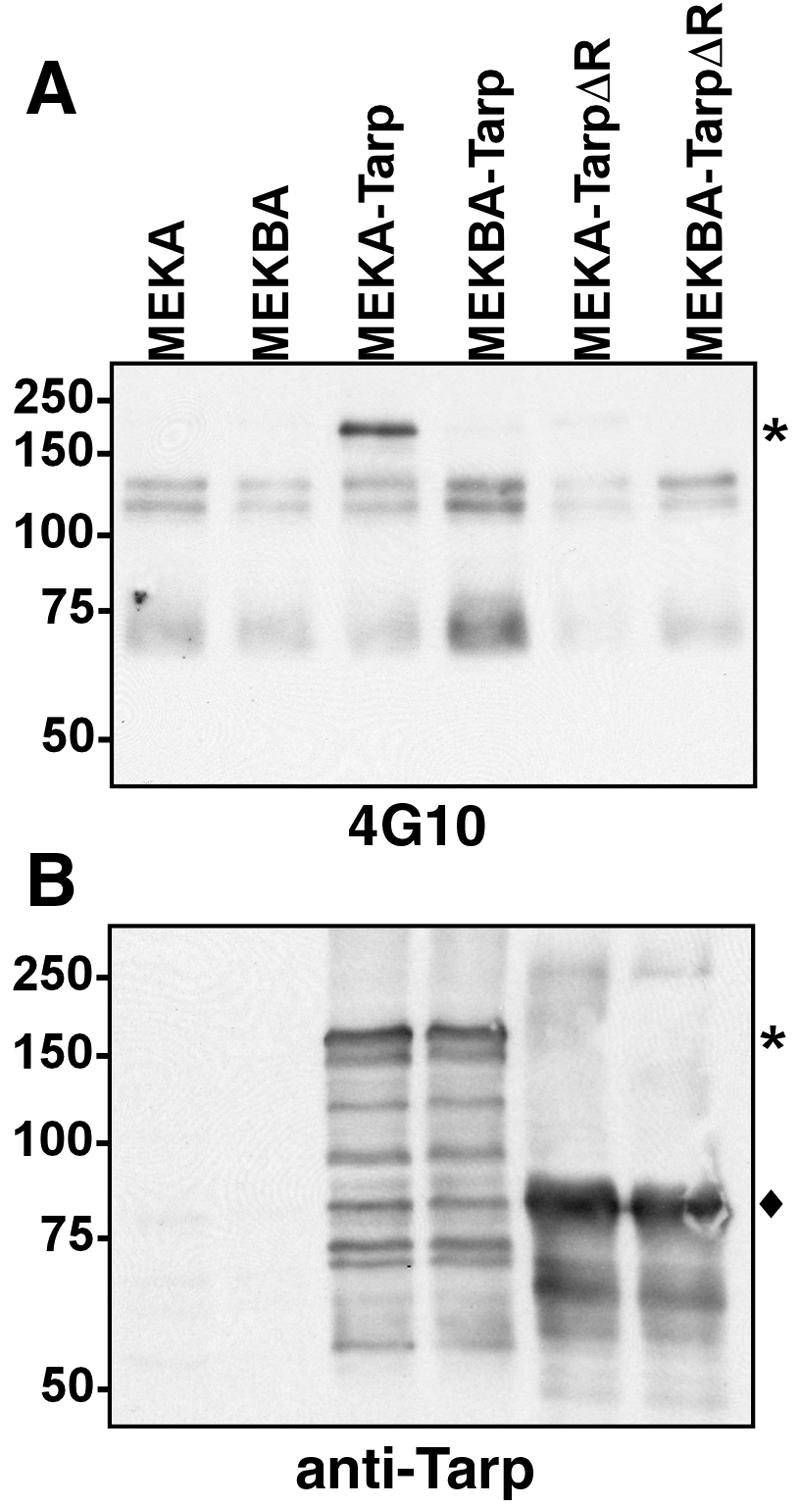
C. trachomatis Tarp secreted from Yersinia pseudotuberculosis is translocated via type III secretion into HeLa cells and tyrosine phosphorylated. Immunoblots of HeLa cells infected with Yersinia pseudotuberculosis MEKA (translocation competent) and MEKBA (translocation incompetent) vector controls or expressing C. trachomatis L2 full-length Tarp or Tarp with the repeat domain deleted are shown. Whole-cell lysates were probed for tyrosine phosphorylation with MAb 4G10 (A) or for Tarp expression with polyclonal rabbit anti-Tarp antibody (B). Asterisks indicate the positions of full-length Tarp; the diamond shows the position of the truncated product.
Actin recruitment by expressed C. caviae Tarp is independent of tyrosine phosphorylation.
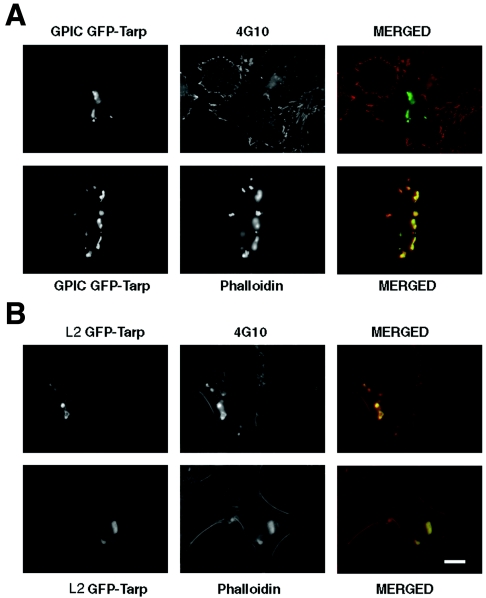
To confirm the lack of association of tyrosine phosphorylation with actin recruitment, C. caviae (GPIC) Tarp was ectopically expressed in HeLa 229 cells as a GFP fusion, as was C. trachomatis L2 Tarp. Like C. trachomatis Tarp-GFP, C. caviae Tarp-GFP cells appeared as aggregates within the cytoplasm and stained intensely with Texas Red phalloidin (Fig. 8). However, in contrast to C. trachomatis, MAb 4G10 did not visibly label expressed C. caviae Tarp-GFP, thus providing further evidence that C. caviae Tarp is not tyrosine phosphorylated yet still recruits actin.
FIG. 8.
Staining of C. caviae Tarp-enhanced GFP constructs for tyrosine phosphorylation and actin recruitment. Full-length C. caviae (GPIC) Tarp was expressed in HeLa 229 cells and stained for tyrosine phosphorylation using MAb 4G10 with an Alexa Fluor 594-conjugated anti-mouse IgG secondary antibody. Actin recruitment was detected using Texas Red phalloidin. Full-length C. trachomatis Tarp-enhanced GFP was expressed as a control. Bar, 10 μm.
DISCUSSION
Chlamydiae readily induce their uptake by eukaryotic host cells through mechanisms that are only now beginning to be deciphered. Recently, a chlamydial type III effector protein, Tarp, has been described as being translocated and exposed to the cytoplasm of the host cell where it is tyrosine phosphorylated by unknown kinases (11). Tyrosine-phosphorylated Tarp is temporally and spatially associated with the recruitment of actin that is required for endocytosis of EBs by cultured epithelial cells. C. trachomatis Tarp is characterized by a region of tandem repeats of approximately 50 amino acids each in serovars L2 and D. The number of repeats differ, however, with almost six complete copies in L2 and only three in serovar D. Although the tyrosine residues phosphorylated have not been identified, the majority of the tyrosines occur within the repeat region. Ectopic expression of full-length Tarp as well as of the N-terminal, repeat region, and C-terminal domains confirmed tyrosine phosphorylation of the full-length protein as well as of the isolated repeat region. This finding is supported by the absence of tyrosine phosphorylation on deletion mutants of Tarp secreted by Yersinia. Further analysis of the transfected domains of Tarp demonstrated that the C-terminal domain of the protein is associated with the recruitment of actin despite the inability to detect tyrosine phosphorylation of this domain. Orthologs of Tarp are present in the other chlamydial species but lack the tyrosine-rich repeat domain and show the greatest degree of similarity within the C-terminal domain (22, 27, 28). Consistent with the absence of the repeat region, tyrosine phosphorylation was undetectable on Tarp from C. muridarum, C. caviae, and C. pneumoniae, although each species similarly recruits actin to the site of entry. Collectively, the data suggest that tyrosine phosphorylation of Tarp may not be an absolute requirement for chlamydial entry.
The translocation of Tarp, its tyrosine phosphorylation, and its association with the recruitment of actin in conjunction with the formation of small pedestal-like structures are reminiscent of the pedestal formation induced by the Tir protein of enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC). Tir is similarly secreted into the host cytoplasmic membrane via a type III mechanism and is localized at the apex of the pedestal in association with the bacterium (24). For EPEC, phosphorylation of tyrosine 474 is an absolute requirement for pedestal formation (12, 23). EHEC Tir is not tyrosine phosphorylated, although EHEC also recruits actin to form phenotypically indistinguishable pedestals (13). The signal transduction pathways culminating in the recruitment of actin also appear to differ (18). The host adaptor protein, Nck, is bound by EPEC Tir and required for actin assembly (20). EHEC pedestals are formed independently of Nck but require additional bacterial effector proteins (12). The inability to detect tyrosine phosphorylation of Tarp from species other than C. trachomatis suggests that the conserved C-terminal domain of Tarp may be responsible for initiating actin recruitment regardless of tyrosine phosphorylation. In that case, tyrosine phosphorylation may initiate a signal transduction cascade specific to C. trachomatis and unrelated to actin recruitment. Alternatively, chlamydial species may signal actin recruitment through different mechanisms.
Previous studies have demonstrated tyrosine phosphorylation in response to C. trachomatis infection by both immunofluorescence and immunoblotting (2, 3, 14), although the tyrosine-phosphorylated proteins were not identified and the majority migrated on SDS-PAGE to a position corresponding to a molecular mass much less than that of Tarp. Subsequent studies described tyrosine phosphorylation of C. trachomatis EBs undergoing microtubule-dependent intracellular trafficking. Interestingly, and consistent with the results shown here, intracellular C. pneumoniae EBs showed distinctly less tyrosine phosphorylation by immunofluorescence and no infection-specific tyrosine phosphorylation by immunoblotting (10).
Distinct requirements for attachment and entry of different chlamydial strains and species are well recognized (21, 26). Despite these differences, all chlamydial species and strains examined show recruitment of actin to the site of entry and thus likely share at least some common mechanisms. Recently, differences in the cellular signal transduction pathways required for chlamydial species have been suggested. Several cellular signal transduction molecules have been identified as playing a role in the recruitment of actin to the site of EB entry. Rho family GTPases are now known to be recruited to the site of attachment and essential for internalization of EBs. Although direct side-by-side comparisons have not yet been performed, initial independent studies suggest that the requirements may be species specific. Rac is required for C. trachomatis internalization (7), while both Rac and Cdc42 are necessary for C. caviae entry (38). Additional host factors required for C. trachomatis entry include Wave2 and Arp2/3 (R. A. Carabeo, S. S. Grieshaber, C. A. Dooley, and T. Hackstadt, Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. D222, p. 231, 2004). Although Tarp has been proposed to play a role in actin recruitment, the actual molecular mechanisms remain unclear. It could potentially act as a guanine nucleotide exchange factor or indirectly activate Rac through unknown pathways. Alternatively, the role of Tarp could conceivably be unrelated to Rac activation. The absence of the repeat domain and lack of tyrosine phosphorylation by the non-C. trachomatis species indicate that any role of Tarp in actin nucleation may be independent of tyrosine phosphorylation and suggests the possibility of unrecognized phosphotyrosine-mediated signal transduction cascades specific to C. trachomatis.
Acknowledgments
We thank Janet Sager for excellent technical assistance and R. Heinzen, O. Steele-Mortimer, and T. Jewett for review of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Bannantine, J. P., R. S. Griffiths, W. Viratyosin, W. J. Brown, and D. D. Rockey. 2000. A secondary structure motif predictive of protein localization to the chlamydial inclusion membrane. Cell. Microbiol. 2:35-47. [DOI] [PubMed] [Google Scholar]
- 2.Birkelund, S., L. Bini, V. Pallini, M. Sanchez-Campillo, S. Liberatori, J. D. Clausen, S. Ostergaard, A. Holm, and G. Christiansen. 1997. Characterization of Chlamydia trachomatis l2-induced tyrosine-phosphorylated HeLa cell proteins by two-dimensional gel electrophoresis. Electrophoresis 18:563-567. [DOI] [PubMed] [Google Scholar]
- 3.Birkelund, S., H. Johnsen, and G. Christiansen. 1994. Chlamydia trachomatis serovar L2 induces protein tyrosine phosphorylation during uptake by HeLa cells. Infect. Immun. 62:4900-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boleti, H., D. M. Ojcius, and A. Dautry-Varsat. 2000. Fluorescent labelling of intracellular bacteria in living host cells. J. Microbiol. Methods 40:265-274. [DOI] [PubMed] [Google Scholar]
- 5.Byrne, G. I., and J. W. Moulder. 1978. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect. Immun. 19:598-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carabeo, R. A., S. Grieshaber, A. Hasenkrug, C. A. Dooley, and T. Hackstadt. 2004. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic 5:418-425. [DOI] [PubMed] [Google Scholar]
- 8.Carabeo, R. A., S. S. Grieshaber, E. Fischer, and T. Hackstadt. 2002. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 70:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carabeo, R. A., and T. Hackstadt. 2001. Isolation and characterization of a mutant Chinese hamster ovary cell line that is resistant to Chlamydia trachomatis infection at a novel step in the attachment process. Infect. Immun. 69:5899-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clausen, J. D., G. Christiansen, H. U. Holst, and S. Birkelund. 1997. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol. Microbiol. 25:441-449. [DOI] [PubMed] [Google Scholar]
- 11.Clifton, D. R., K. A. Fields, S. Grieshaber, C. A. Dooley, E. Fischer, D. Mead, R. A. Carabeo, and T. Hackstadt. 2004. A chlamydial type III translocated protein is tyrosine phosphorylated at the site of entry and associated with recruitment of actin. Proc. Natl. Acad. Sci. USA 101:10166-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVinney, R., J. L. Puente, A. Gauthier, D. Goosney, and B. Finlay. 2001. Enterohaemorrhagic and enteropathogenic Escherichia coli use different Tir-based mechanism for pedestal formation. Mol. Microbiol. 41:1445-1458. [DOI] [PubMed] [Google Scholar]
- 13.DeVinney, R., M. Stein, D. Reinschied, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawaz, F. S., C. van Ooij, E. Homola, S. C. Mutka, and J. N. Engel. 1997. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect. Immun. 65:5301-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields, K. A., and T. Hackstadt. 2002. The chlamydial inclusion: escape from the endocytic pathway. Annu. Rev. Cell Dev. Biol. 18:221-245. [DOI] [PubMed] [Google Scholar]
- 16.Fields, K. A., D. Mead, C. A. Dooley, and T. Hackstadt. 2003. Chlamydia trachomatis type III secretion: evidence for a functional apparatus during early-cycle development. Mol. Microbiol. 48:671-683. [DOI] [PubMed] [Google Scholar]
- 17.Fudyk, T., L. Olinger, and R. S. Stephens. 2002. Selection of mutant cell lines resistant to infection by Chlamydia trachomatis and Chlamydia pneumoniae. Infect. Immun. 70:6444-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goosney, D. L., R. DeVinney, and B. Finlay. 2001. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infect. Immun. 69:3315-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grieshaber, S., N. Grieshaber, and T. Hackstadt. 2003. Chlamydia trachomatis uses host cell dynein to traffic to the microtube organizing center in a p50 dynamitin independent process. J. Cell Sci. 116:3793-3802. [DOI] [PubMed] [Google Scholar]
- 20.Gruenheid, S., R. DeVinney, F. Bladt, D. Goosney, S. Gelkp, G. D. Gish, T. Pawson, and B. Finlay. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856-859. [DOI] [PubMed] [Google Scholar]
- 21.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 22.Kalman, S., et al. 1999. Comparative genomes of Chlamydia pneumoniae and C. trachomatis. Nat. Genet. 21:385-389. [DOI] [PubMed] [Google Scholar]
- 23.Kenny, B. 1999. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31:1229-1241. [DOI] [PubMed] [Google Scholar]
- 24.Kenny, B., R. DeVinney, M. Stein, D. J. Reinschied, E. A. Frey, and B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read, T. D., et al. 2000. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 28:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read, T. D., et al. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockey, D. D., E. R. Fischer, and T. Hackstadt. 1996. Temporal analysis of the developing Chlamydia psittaci inclusion by use of fluorescence and electron microscopy. Infect. Immun. 64:4269-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schachter, J. 1999. Infection and disease epidemiology, p. 139-169. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 31.Scidmore, M. A., E. R. Fischer, and T. Hackstadt. 2003. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect. Immun. 71:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scidmore, M. A., D. D. Rockey, E. R. Fischer, R. A. Heinzen, and T. Hackstadt. 1996. Vesicular interactions of the Chlamydia trachomatis inclusion are determined by chlamydial early protein synthesis rather than route of entry. Infect. Immun. 64:5366-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scidmore-Carlson, M. A., E. I. Shaw, C. A. Dooley, E. R. Fischer, and T. Hackstadt. 1999. Identification and characterization of a Chlamydia trachomatis early operon encoding four novel inclusion membrane proteins. Mol. Microbiol. 33:753-765. [DOI] [PubMed] [Google Scholar]
- 34.Shaw, E. I., C. A. Dooley, E. R. Fischer, M. A. Scidmore, K. A. Fields, and T. Hackstadt. 2000. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 37:913-925. [DOI] [PubMed] [Google Scholar]
- 35.Stephens, R. S., et al. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 36.Su, H., L. Raymond, D. D. Rockey, E. Fischer, T. Hackstadt, and H. D. Caldwell. 1996. A recombinant Chlamydia trachomatis major outer membrane protein binds to heparan sulfate receptors on epithelial cells. Proc. Natl. Acad. Sci. USA 93:11143-11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su, H., G. J. Spangrude, and H. D. Caldwell. 1991. Expression of Fc gamma RIII on HeLa 229 cells: possible effect on in vitro neutralization of Chlamydia trachomatis. Infect. Immun. 59:3811-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subtil, A., B. Wyplosz, M. E. Balana, and A. Dautry-Varsat. 2004. Analysis of Chlamydia caviae entry sites and involvement of Cdc42 and Rac activity. J. Cell Sci. 117:3923-3933. [DOI] [PubMed] [Google Scholar]
- 39.Wolf, K., E. Fischer, and T. Hackstadt. 2000. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect. Immun. 68:2379-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]