Abstract
Shiga toxin (Stx) derivatives, such as the Stx1 B subunit (StxB1), which mediates toxin binding to the membrane, and mutant Stx1 (mStx1), which is a nontoxic doubly mutated Stx1 harboring amino acid substitutions in the A subunit, possess adjuvant activity via the activation of dendritic cells (DCs). Our results showed that StxB1 and mStx1, but not native Stx1 (nStx1), resulted in enhanced expression of CD86, CD40, and major histocompatibility complex (MHC) class II molecules and, to some extent, also enhanced the expression of CD80 on bone marrow-derived DCs. StxB1-treated DCs exhibited an increase in tumor necrosis factor alpha and interleukin-12 (IL-12) production, a stimulation of DO11.10 T-cell proliferation, and the production of both Th1 and Th2 cytokines, including gamma interferon (IFN-γ), IL-4, IL-5, IL-6, and IL-10. When mice were given StxB1 subcutaneously, the levels of CD80, CD86, and CD40, as well as MHC class II expression by splenic DCs, were enhanced. The subcutaneous immunization of mice with ovalbumin (OVA) plus mStx1 or StxB1 induced high titers of OVA-specific immunoglobulin M (IgM), IgG1, and IgG2a in serum. OVA-specific CD4+ T cells isolated from mice immunized with OVA plus mStx1 or StxB1 produced IFN-γ, IL-4, IL-5, IL-6, and IL-10, indicating that mStx1 and StxB1 elicit both Th1- and Th2-type responses. Importantly, mice immunized subcutaneously with tetanus toxoid plus mStx1 or StxB1 were protected from a lethal challenge with tetanus toxin. These results suggest that nontoxic Stx derivatives, including both StxB1 and mStx1, could be effective adjuvants for the induction of mixed Th-type CD4+ T-cell-mediated antigen-specific antibody responses via the activation of DCs.
For the design of effective vaccines in the areas of immunology and infectious diseases, a primary focus of research is the development of effective and safe adjuvants, which instruct and control the selective induction of the appropriate type of antigen-specific immune response. Thus far, several bacterial enterotoxins, including the cholera toxin (CT) of Vibrio cholerae and the heat-labile enterotoxin (LT) of enterotoxigenic Escherichia coli, are known to be potentially strong adjuvants when given by the oral, nasal, or parenteral route (7, 9, 18, 52). As early as 1972, it was reported that CT acts as an adjuvant for antibody responses following intravenous administration (32). The mucosal administration of CT was shown to elicit antigen (Ag)-specific Th2-type CD4+ T-cell responses via high levels of interleukin-4 (IL-4) and IL-5 production, which in turn enhanced Ag-specific systemic immunoglobulin G1 (IgG1) and IgE and mucosal secretory IgA responses (28). In contrast, LT was shown to induce mixed CD4+ Th1- and Th2-type cells producing gamma interferon (IFN-γ), IL-4, IL-5, IL-6, and IL-10, with subsequent serum IgG1 and IgG2a and mucosal secretory IgA responses (47). Other bacterial toxins, such as pertussis toxin and a genetically detoxified derivative of pertussis toxin, PT-9K/129G, have also been shown to possess mucosal adjuvant activities (3, 11, 36). Pertussis toxin potentiates Th1 and Th2 responses to a coadministered antigen (37). The administration of a chimeric molecule composed of the gp120 V3 loop region of the MN strain of human immunodeficiency virus type 1 (HIV-1) and a nontoxic form of Pseudomonas exotoxin resulted in strong antigen-specific immune responses to an integrated HIV Ag (30).
It is interesting that in the case of Shiga toxin (Stx), oral administration confers immunogenicity but not adjuvanticity (43). Stx is produced by Stx-producing E. coli and is one of the major virulence factors for infectious diseases by Stx-producing E. coli. Stx is a holotoxin composed of an approximately 32-kDa A subunit in noncovalent association with a pentameric ring of identical nontoxic B subunits, each of which has a molecular mass of 7.7 kDa. The A subunit is the enzymatic component of the toxin and acts as a highly specific N-glycosidase enzyme, hydrolyzing the bond between ribose and a single adenine residue found on a prominent loop structure in the 28S rRNA component of eukaryotic ribosomes (10, 39). The B subunits mediate toxin binding to the membrane-neutral glycolipids glogotriaosylceramid and globotetraosylceramid (38). Stx toxins are classified into the following two groups: Stx1, which is identical to Shiga toxin at the amino acid sequence level, and Stx2, which is immunologically different from Stx1 (42).
In previous studies, we generated E167Q/R170L (mStx1), a double mutant of Stx1 which harbors amino acid substitutions in the RNA N-glycosidase active center which were derived by site-directed mutagenesis. mStx1 lacks RNA N-glycosidase activity, cytotoxicity, and mouse lethality (33). For the present study, we assessed the capability of mStx1 and StxB1 to provide activation signals to dendritic cells (DCs) and T cells and then addressed the issue of whether this capability of mStx1 and StxB1 is connected to in vivo adjuvanticity when these molecules are subcutaneously coadministered with a protein antigen. The results obtained in this study suggest that both mStx1 and StxB1 act as effective adjuvants for the induction of Ag-specific antibody (Ab) responses via DC activation.
MATERIALS AND METHODS
Mice.
C57BL/6 and BALB/c mice were purchased from SLC (Shizuoka, Japan) and were maintained and bred in the experimental animal facilities of Niigata University Graduate School of Medical and Dental Science, the Research Institute for Microbial Diseases, Osaka University, and the Institute of Medical Science, University of Tokyo, under pathogen-free conditions using microisolator cages. DO11.10 T-cell receptor (TCR)-transgenic mice, which recognize the OVA peptide 323-329 in association with I-Ad (31), were kindly provided by Kazuhiko Yamamoto (University of Tokyo, Tokyo, Japan). All mice were provided sterile food and water ad libitum and were maintained in our experimental animal facility. C57BL/6 and BALB/c mice of 8 to 12 weeks of age and DO11.10 Tg mice of 5 to 12 weeks of age were used for this study.
Bacterial toxins.
A mutant of Stx1 (mStx1) and native Stx1 (nStx1) were purified from E. coli MC 1061 strains M 23 and 87-27, respectively, according to a previously described method (14, 33). Purification steps included ion-exchange chromatography, chromatofocusing, and high-performance liquid chromatography as described previously (14). The B subunit of Stx1 (StxB1) was derived from Bacillus brevis pNU212-VT1B and was purified by the use of ion-exchange chromatography and gel filtration (5).
The amounts of endotoxin in the toxin preparations were measured with an Endospec-SP test (Seikagaku Co., Tokyo, Japan). The nStx1, mStx1, and StxB1 preparations contained 7.03 pg, 34.0 pg, and 3.05 pg of lipopolysaccharide (LPS) per 10 μg of protein, respectively. These ranges of LPS contamination have been shown to be ineffective for the stimulation of lymphoid cells (22, 50).
Culture conditions, treatment of BMDCs in vitro, and treatment of BMDCs with Stx1 derivatives.
For the generation of bone marrow-derived DCs (BMDCs),male C57BL/6 or BALB/c mice were sacrificed, and their bone marrow was isolated and then flushed from the femur and tibia (12). Erythrocytes were depleted with ammonium chloride. DCs were generated from bone marrow precursors as described previously (12). Following 6 days of incubation in the presence of an optimal dose of granulocyte-macrophage colony-stimulating factor (10 ng/ml), nonadherent cells were collected and used as a source of BMDCs.
BMDCs were cultured at 5 × 105 cells/ml in 24-well plates (Corning, Inc., Corning, N.Y.) in culture medium containing granulocyte-macrophage colony-stimulating factor (10 ng/ml) (12) in the presence or absence of an optimal dose of a Stx1 derivative (1 μg/ml) for 48 h at 37°C. Culture supernatants were collected and frozen at −70°C until assayed for the synthesis of cytokines, including tumor necrosis factor alpha (TNF-α) and IL-12 p70, by enzyme-linked immunosorbent assays (ELISAs) (AN′LYZA immunoassay kit; R&D Systems, Minneapolis, Minn.).
Fluorescence-activated cell sorting analysis.
BMDCs were analyzed 48 h after treatment with a variety of toxin derivatives since a preliminary time kinetics study showed that maximum levels of surface antigen expression were achieved and maintained between 24 and 48 h. Cells were analyzed by use of a FACScan cytometer (Becton Dickinson) using the following antibodies from BD Pharmingen and Beckman Coulter, Inc. (Fullerton, Calif.): fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD11c (clone HL3), biotin-conjugated anti-mouse CD80 (clone 16-10A1), biotin-conjugated anti-mouse CD86 (clone GL1), biotin-conjugated anti-mouse I-Ab (clone AF6-120.1), biotin-conjugated anti-mouse CD40 (clone 3/23), and phycoerythrin (PE)-conjugated streptavidin. BMDCs and splenic DCs were characterized with FITC-conjugated anti-mouse CD11b (Mac-1; M1/70), PE-conjugated anti-mouse CD11c (HL3), Cy-chrome-conjugated anti-mouse CD8α (53-6.7), allophycocyanin-conjugated anti-mouse CD4 (RM-4-5), FITC-conjugated hamster anti-mouse CD11c (HL3), and PE-conjugated anti-mouse CD45R/B220 (RA3-6B2).
Purification of TCR-transgenic T cells.
T cells were purified from the spleens of naive BALB/c mice expressing a transgenic α/β-TCR specific for peptide 323-329 of ovalbumin (OVA) (31) by magnetic bead-activated cell sorting (MACS) using a CD4+ T-cell purification system with CD4+-specific MACS beads (Miltenyi Biotech, Sunnyvale, Calif.). More than 90% of the resulting T-cell population was CD4+ and expressed the OVA-specific TCR transgene. These purified CD4+ T cells (2 × 106 cells/well) were then cultured in RPMI 1640 plus 10% fetal calf serum (FCS) with toxin derivative-treated DCs (5 × 105 cells/well) and 0.3 μM OVA peptide (ISQAVHAAHAEINEAGR-COOH; Peptide Institute, Inc., Minoh, Osaka, Japan) for 3 days at 37°C. In a preliminary experiment, three different amounts of toxin derivative-treated DCs were tested, and 5 × 105 cells/well consistently provided the most reproducible data. CD4+ T cells were then stimulated with 50 ng/ml phorbol myristate acetate (PMA; Sigma, St. Louis, Mo.) and 500 ng/ml ionomycin (Sigma) overnight. Culture supernatants from the different wells were tested for the synthesis of the cytokines IFN-γ, IL-4, IL-5, IL-6, and IL-10 by ELISAs (AN′LYZA immunoassay kit; R&D Systems).
Induction of T-cell proliferation.
BMDCs (1.7 × 104 cells/well) were incubated in round-bottomed 96-well plates (Corning) in the presence of 1 μg of Stx1 derivative for 48 h at 37°C and then were irradiated with 30 Gy of radiation. The plates were extensively washed with RPMI 1640 followed by complete RPMI 1640 containing 10% FCS, HEPES buffer (15 mM), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). OVA-specific transgenic T cells (5 × 104 cells/well) were added to the DC-coated wells. The plates were then incubated in the presence of 0.3 μM OVA peptide for an additional 3 days at 37°C. [3H]thymidine (0.5 μCi; Amersham Pharmacia Biotech, Buckinghamshire, England) was added to each well 18 h before harvesting, and incorporated radioactivity was then measured with an LS1701 scintillation counter (Beckman Coulter Inc., Hialeah, Fla.). The results are expressed as stimulation indexes (E/C), defined as the ratios between the amounts of [3H]thymidine incorporated into T cells incubated with toxin derivative-treated DCs and the amount of [3H]thymidine incorporated into T cells incubated with untreated DCs.
Isolation of splenic DCs.
Spleens were isolated from mice receiving subcutaneous administrations of Stx1 derivatives and were then suspended in RPMI 1640 medium containing 2% FCS, HEPES buffer (15 mM), l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The spleens were digested with collagenase D (400 Mandl units/ml; Roche, Indianapolis, Ind.) as previously described (45), and then the red blood cells were lysed with ammonium chloride-potassium lysing buffer. Briefly, the spleens were incubated with collagenase D (400 Mandl units/ml) and DNase I (15 μg/ml; Roche) for 35 min at 37°C in RPMI 1640 medium, and EDTA was added to a final concentration of 5 mM during the last 5 min of incubation. For DC enrichment, released cells were layered over a metrizamide gradient column (Accurate, Westbury, N.Y.) (14.5 g of metrizamide added to 100 ml of complete medium) and centrifuged, and the low-density fraction was collected as DCs (26). The enriched DCs were counted and then stained with appropriate monoclonal antibodies as described above for fluorescence-activated cell sorting analysis.
Immunization protocol.
A standard subcutaneous immunization protocol was used for this study (55). Mice were subcutaneously immunized on days 0 and 14 with a 100-μl aliquot containing 100 μg of ovalbumin (OVA; Sigma) alone or combined with an optimal dose of mStx1 (10 μg), StxB1 (10 μg), or nStx1 (50 ng) as an adjuvant. This dose of OVA has been shown to be optimal and is routinely used in our laboratory (55). The optimal doses of the Stx1 derivatives were determined in preliminary experiments. In the case of the native form, the dose was selected as the concentration which did not show in vivo toxicity. For an assay of protection against tetanus toxin, mice were subcutaneously immunized on days 0 and 14 with a 100-μl aliquot of tetanus toxoid (TT; 307 μg/ml, 900 limit of flocculation (Lf)/ml, 2,932 Lf/mg PN; provided by Y. Higashi, Osaka University, Biken Foundation, Osaka, Japan) alone or combined with mStx1 (1, 10, or 25 μg) or StxB1 (1, 10, or 25 μg) as an adjuvant.
Analysis of Ag-specific Ab isotype and IgG subclass responses.
Ag-specific Ab titers in serum were determined by ELISAs as described previously (28, 51). Briefly, plates were coated with OVA (1 mg/ml) or TT (5 μg/ml) and blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS). After the plates were washed, serial dilutions of serum were added in duplicate. Following incubation, the plates were washed and a peroxidase-labeled goat anti-mouse μ, γ, or α heavy chain-specific Ab (Southern Biotechnology Associates, Birmingham, Ala.) was added to appropriate wells. Finally, 3,3′,5,5′-tetramethylbenzidine (TMB) with H2O2 was added for color development. For IgG subclass analysis, biotinylated rat monoclonal anti-mouse γ1 (G1-7.3), γ2a (R19-15), γ2b (R12-3), and γ3 (R40-82) heavy chain-specific Abs (Pharmingen) and streptavidin-conjugated peroxidase (Vector Laboratories, Inc., Burlingame, Calif.) were employed. End-point titers were expressed as reciprocal log2 values of the last dilutions giving optical densities at 450 nm of ≥0.1 above the negative control.
Detection of Ag-specific AFCs.
For the elucidation of Ag-specific Ab-forming cells (AFCs), an enzyme-linked immunospot (ELISPOT) assay was employed as previously described in detail (51, 52). Splenic mononuclear cells were resuspended in complete medium. Ninety-six-well nitrocellulose-based plates were coated with 1 mg/ml of OVA diluted in PBS for the enumeration of Ag-specific AFCs. The wells were blocked with complete medium. Cells at various dilutions were added and incubated for 6 h at 37°C in 5% CO2 in moist air. Antigen-specific AFCs were detected with a peroxidase-labeled anti-mouse μ, γ, or α heavy chain Ab (Southern Biotechnology Associates) and then visualized by adding the chromogenic substrate 3-amino-9-ethylcarbazole (Moss Inc., Pasadena, Md.). Spots were counted with the aid of a dissecting microscope (SZH Zoom stereo microscope system; Olympus, Lake Success, N.Y.).
Analysis of OVA-specific CD4+ T-cell responses.
CD4+ T cells were purified from splenic cell suspensions by use of a magnetic bead-activated cell sorter system (Miltenyi Biotech) (51). Splenic mononuclear cells were initially applied to a nylon wool column (Polysciences, Warrington, Pa.) and incubated at 37°C for 1 h to remove adherent cells. Purified CD4+ T cells were then obtained by positive sorting using a magnetic bead separation system consisting of anti-CD4 monoclonal Ab (clone GK1.5)-conjugated microbeads (Miltenyi Biotech). Purified splenic CD4+ T cells (>98% pure) were cultured at a density of 4 × 106 cells/ml with OVA (1 mg/ml), T-cell-depleted, irradiated (30 Gy) splenic feeder cells (8 × 106 cells/ml), and recombinant IL-2 (rIL-2; 10 units/ml) (Pharmingen) in complete medium (51). These CD4+ T-cell cultures were incubated for 3 days at 37°C in 5% CO2 in air. For measurements of the levels of Ag-specific T-cell proliferation, 0.5 μCi of [3H]thymidine (Amersham Pharmacia Biotech) was added to individual cultures 18 h before termination, and the uptake of [3H]thymidine was determined in counts per minute (cpm) by use of a scintillation counter (55).
Tetanus toxin challenge.
Tetanus toxin was diluted in 0.5% gelatin-PBS, and an appropriate lethal dose (130 50% lethal doses [LD50s]) was given subcutaneously to each group of mice as described previously (15, 20). Mice were then monitored daily for paralysis and death.
Statistical analysis.
The results are presented as means ± 1 standard error (SE). Statistical significance (P < 0.05) was determined by Student's t test and by the Mann-Whitney U test of unpaired samples.
RESULTS
mStx1 and StxB1 up-regulate cell surface expression of costimulatory molecules and MHC class II molecules on BMDCs.
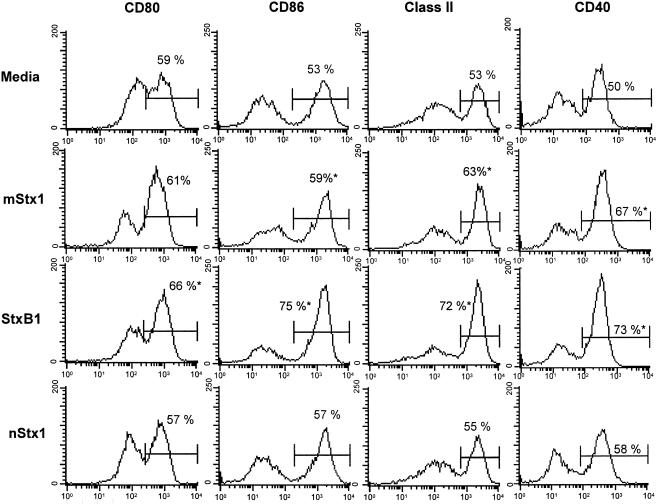
On day 6 of bone marrow-derived DC (BMDC) cultures, >90% of the cells were determined to be CD11c+ (data not shown). The CD11b, CD8α, CD4, and B220 cells identified among the BMDCs were characterized as having the CD11b+, CD8α−, CD4−, and B220− phenotypes, respectively (data not shown). These BMDCs were incubated with or without Stx1 derivatives for 48 h, and the expression of cell surface molecules was analyzed by flow cytometry. Even nonactivated BMDCs showed moderate expression of the costimulatory molecules CD80 (B7-1) and CD86 (B7-2) and of major histocompatibility complex (MHC) class II molecules. The addition of SxtB1 resulted in a moderate up-regulation of CD86, MHC class II, and CD40 expression on BMDCs. Furthermore, StxB1 enhanced the expression of CD80; however, the CD80 level was lower than that of CD86 (Fig. 1).
FIG. 1.
Effects of StxB1, mStx1, and nStx1 on the expression of CD80, CD86, CD40, and MHC class II by bone marrow-derived DCs (BMDCs). BMDCs were cultured with Stx1 derivatives (StxB1, 1 μg/ml; mStx1, 1 μg/ml; nStx1, 1 μg/ml) for 48 h since a preliminary study showed that the maximum levels of surface antigen expression occurred between 24 and 48 h. Cell surface Ag expression was analyzed by flow cytometry as described in Materials and Methods. The data are presented as histograms and are expressed as means of three independent experiments. The percentage within each panel indicates the number of cells staining strongly for the indicated marker. *, P < 0.05 compared with the control medium-treated culture. Data were obtained by using the CD11c+ gated cell fraction.
The expression of CD86, but not that of CD80, was up-regulated when BMDCs were exposed to mStx1. In contrast, nStx1 failed to enhance the expression of these activation molecules on BMDCs. The expression of CD40 on BMDCs was also up-regulated by treatment with mStx1 or StxB1 (Fig. 1). An increase in the expression of these activation molecules also occurred after the treatment of cells with an optimal concentration (1 μg/ml) of LPS (data not shown), a known activator of DCs (17). To exclude any effects of contaminating endotoxins, we incubated BMDCs, with or without Stx1 derivatives, after the pretreatment of Stx1 derivatives with 5 μg/ml polymyxin B. Polymyxin B did not affect Stx1 derivative-induced surface marker expression (data not shown).
Induction of cytokine synthesis by StxB1-treated BMDCs.
To analyze whether the observed phenotypic maturation (e.g., the expression of CD80, CD86, and MHC class II) was associated with cytokine production, we tested StxB1- and mStx1-treated BMDCs for an enhancement of TNF-α and IL-12 p70 synthesis. BMDCs incubated with StxB1 for 48 h produced modest amounts of TNF-α and IL-12 (Table 1), which was consistent with the observation of the expression of functional molecules of StxB1-treated BMDCs. In contrast, the incubation of BMDCs with nStx1 and mStx1 failed to invoke any increases in cytokine production. Thus, BMDCs activated by treatment with StxB1 exhibited the most enhanced capacity to secrete cytokines such as TNF-α and IL-12.
TABLE 1.
TNF-α and IL-12 p70 synthesis by StxB1-, mStx1-, or Stx1-treated murine BMDCsa
| Stimulator | TNF-α concn (pg/ml) | IL-12 concn (pg/ml) |
|---|---|---|
| mStx1 | 260 ± 81 | 90 ± 18 |
| StxB1 | 470 ± 92* | 260 ± 34* |
| nStx1 | 240 ± 42 | 76 ± 13 |
| media | 280 ± 92 | 82 ± 14 |
Culture supernatants were harvested and then analyzed for the production of secreted cytokines by the use of appropriate cytokine-specific ELISAs. The results are expressed as means ± SEM and were taken from a total of three separate experiments. *, P < 0.05 compared with a culture to which no stimulator was added.
Enhanced stimulation of T cells by Stx1 derivative-activated BMDCs.
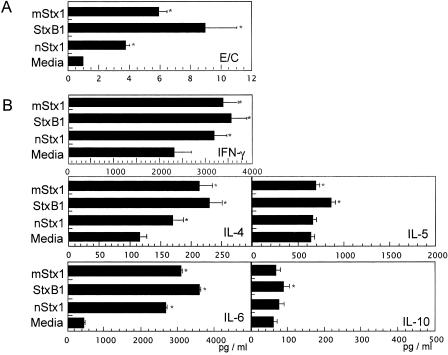
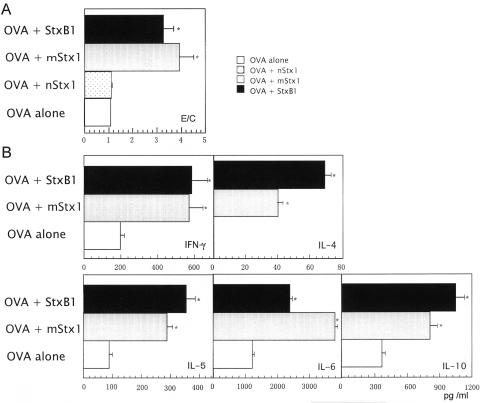
In the next experiment, we tested whether the activation of DCs by mStx1 or StxB1 translated to an increased functional ability of DCs to stimulate T-cell proliferation and subsequent Th1 (IFN-γ) and Th2 (IL-4, IL-5, IL-6, and IL-10) cytokine production. In this assay, Stx1 derivative-stimulated DCs were cocultured with an OVA-specific peptide and splenic T cells isolated from OVA Tg mice. Stx1 derivative-treated BMDCs promoted higher levels of OVA-specific CD4+ T-cell proliferation than did untreated DCs (Fig. 2A), with StxB1-treated BMDCs inducing the highest levels and mStx1-treated BMDCs inducing the next highest levels. In contrast, nStx1-treated BMDCs only weakly enhanced T-cell responses. Similarly enhanced T-cell proliferative responses were also noted when alloreactive responder T cells were cocultured with Stx1 derivative-treated BMDCs (data not shown).
FIG. 2.
Activation of OVA-specific CD4+ T-cell responses by Stx1 derivative-treated BMDCs. T-cell proliferation (A) and Th1 and Th2 cytokine production (B) by CD4+ T cells from DO10.11 Tg mice stimulated with Stx1 derivative-treated BMDCs were examined. BMDCs pretreated with 1 μg/ml Stx1 derivative were washed and then cocultured with purified CD4+ T cells (106/ml) from DO11.10 Tg mice in the presence of 0.3 μM OVA323-329 peptide for 3 days. An aliquot of cell culture was subjected to DNA synthesis by the addition of [3H]thymidine during the last 18 h of incubation. For cytokine analysis, another aliquot of CD4+ T cells was harvested and then treated with 50 nM PMA and 500 nM ionomycin overnight. No or little cytokine release was detected for CD4+ T cells without PMA and ionomycin. The results are expressed as mean E/C ± standard errors of the means (SEM) for triplicate cultures. *, P < 0.05 compared with the control medium-treated culture. The count for the control culture was 6,880 ± 380 cpm. The results of the T-cell proliferation assay (A) are expressed as mean E/C (experimental, stimulated value/control, nonstimulated value) ± SEM of triplicate cultures.
To determine whether the observed increase in OVA-specific CD4+ T-cell proliferation induced by StxB1- or mStx1-treated BMDCs was associated with Th1 and Th2 cytokine production, we harvested culture supernatants and subjected them to IFN-γ-, IL-4-, IL-5-, IL-6-, and IL-10-specific ELISAs. StxB1-treated DCs promoted an increased synthesis of cytokines such as IFN-γ, IL-4, IL-5, IL-6, and IL-10 (Fig. 2B) most effectively, followed by those treated with mStx1 and nStx1 (Fig. 2B). However, it should be pointed out that Stx1 derivatives significantly enhanced only IL-6 synthesis, prompting little or no release of the other cytokines. Among the Stx1 derivatives, StxB1 possessed the most potent immunoenhancing activity for an increase of T-cell proliferation and subsequent Th1 and Th2 cytokine production through the activation of BMDCs.
In vivo effects of mStx1 and StxB1 on the up-regulation of costimulatory molecules and MHC class II on splenic DCs.
It was important to examine whether in vivo administration of the Stx1 derivatives could modulate DC function. Thus, the expression of costimulatory molecules and MHC class II on splenic DCs was analyzed by flow cytometry 12 h and 48 h after Stx1 derivatives were subcutaneously administered to healthy mice (Table 2). As shown above for nonactivated BMDCs, the splenic DCs isolated from healthy mice also expressed CD11b+ (data not shown). Nonactivated splenic DCs were also found to naturally express moderate levels of the costimulatory molecules CD80 and CD86, MHC class II, and CD40 (Table 2), whose levels were up-regulated after the administration of StxB1. Interestingly, the up-regulation of CD80 expression was observed as early as 12 h after the administration of StxB1, with the expression of CD86, CD40, and MHC class II appearing 48 h after administration. After a subcutaneous injection of mStx1 into mice, the expression of CD40 and MHC class II was up-regulated. In contrast, as seen with BMDCs, nStx1 failed to enhance the expression of any of these costimulatory molecules, except for MHC class II, on splenic DCs.
TABLE 2.
Characterization of CD80, CD86, CD40, and MHC class II expression by mStx1-, StxB1-, or nStx1-treated splenic DCs
| Stimulator | % of the highest intensity of the expressed molecule
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD80
|
CD86
|
CD40
|
MHC class II
|
|||||
| 12 h | 48 h | 12 h | 48 h | 12 h | 48 h | 12 h | 48 h | |
| mStx1 | 43.0 ± 3.9 | 16.5 ± 9.3* | 43.6 ± 2.9* | 45.3 ± 2.5 | 58.3 ± 5.8* | 37.6 ± 1.7 | 48.8 ± 3.1 | 65.9 ± 7.5* |
| StxB1 | 55.8 ± 2.6* | 33.9 ± 1.6* | 20.0 ± 4.3* | 75.3 ± 14.3* | 37.6 ± 1.4* | 63.3 ± 4.4* | 45.1 ± 2.2* | 70.4 ± 6.3* |
| nStx1 | 33.2 ± 1.4* | 15.7 ± 8.6* | 17.4 ± 10.5* | 30.2 ± 7.5* | 27.4 ± 3.0* | 9.7 ± 9.1* | 42.5 ± 4.7* | 57.3 ± 2.7* |
| PBS | 42.4 ± 1.3 | 50.9 ± 6.6 | 49.6 ± 1.7 | 49.2 ± 6.6 | 34.7 ± 0.5 | 37.2 ± 7.5 | 51.3 ± 2.6 | 49.2 ± 6.2 |
Twelve or 48 h after the subcutaneous administration of StxB1 (10 μg/mouse), mStx1 (10 μg/mouse), or nStx1 (50 ng/mouse), mice were sacrificed for the preparation of splenocytes. The cells were then analyzed by flow cytometry. The data are means ± SEM are representative of three independent experiments.
P < 0.05 compared with mice administered PBS.
Enhancement of Ag-specific Ab responses by subcutaneous immunization of mice with OVA and mStx1 or StxB1.
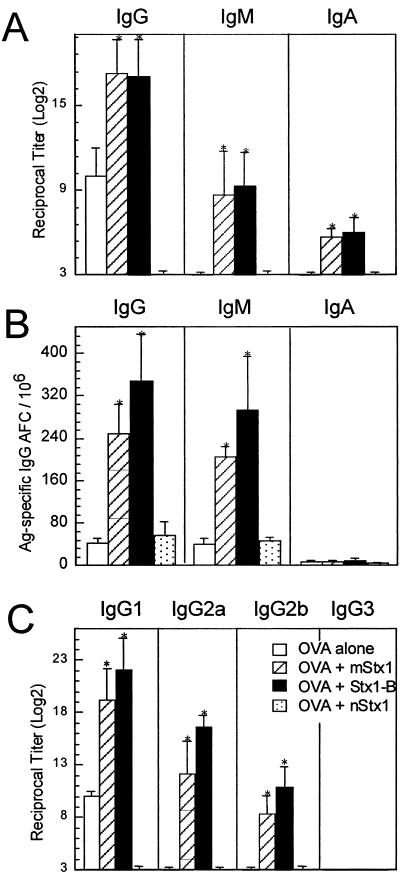
To examine in vivo the immunoenhancing activities of Stx1 derivatives, we subcutaneously immunized mice with an optimal dose of OVA in the presence or absence of the toxin derivatives. The coadministration of 10 μg of mStx1 or StxB1 resulted in high levels of OVA-specific IgG, IgM, and IgA (Fig. 3A). In contrast, 50 ng of nStx1 did not support the generation of any isotype of anti-OVA Ab. As one might expect, when mice were immunized with OVA alone, antigen-specific Ab responses were not induced (Fig. 3A). An analysis of antigen-specific IgG antibody-forming cells (AFCs) in the spleens of mice immunized with OVA plus Stx1 derivatives confirmed the results obtained for the characterization of OVA-specific Ab titers in sera. Thus, significant numbers of OVA-specific IgG AFCs were detected in the spleens of mice subcutaneously immunized with OVA plus mStx1 or StxB1 as an adjuvant (Fig. 3B). In contrast, obvious OVA-specific IgG AFCs were not seen in the spleens of mice given OVA alone or OVA plus nStx1 (Fig. 3B). A subsequent analysis of the OVA-specific IgG subclasses revealed that the major antigen-specific IgG subclass response was IgG1, followed by IgG2a, after the coadministration of mStx1 or StxB1 (Fig. 3C). These findings demonstrate that Stx1 derivatives, especially nontoxic forms of StxB1 and mStx1, are potent immunoenhancing molecules in vivo.
FIG. 3.
Induction of OVA-specific antibody responses by coadministered Stx1 derivatives. Mice were subcutaneously immunized with OVA plus mStx1, nStx1, or StxB1. Specifically, C57BL/6 mice were subcutaneously immunized with 100 μg of OVA plus 10 μg of the Stx1 mutant (E167R/R170L; mStx1) (hatched bars), 50 ng of nStx1 (dotted bars), or 10 μg of StxB1 (black bars) as an adjuvant or with OVA alone (white bars) on days 0 and 14. OVA-specific serum IgG, IgM, and IgA Ab (A) and splenic OVA-specific antibody-forming cell (AFC) (B) responses were determined by ELISAs and ELISPOT assays, respectively. Furthermore, OVA-specific IgG subclass Ab responses (C) were also analyzed by ELISAs. Serum samples were collected on day 21 and examined for OVA-specific Abs and OVA-specific IgG subclass Ab responses by ELISAs. Mononuclear cells were isolated from the spleens of subcutaneously immunized mice on day 21 and examined by Ag-specific ELISPOT assays. *, P < 0.05 compared with mice immunized with OVA alone. The results are expressed as means ± SEM from a total of three separate experiments, each of which used five or six mice per group.
Induction of OVA-specific CD4+ Th1- and Th2-cell responses after immunization with OVA and Stx1 derivatives.
When CD4+ T cells isolated from the spleens of mice subcutaneously immunized with OVA plus mStx1 or StxB1 were restimulated with OVA in vitro, increased proliferative responses were seen (Fig. 4A). In contrast, essentially no Ag-specific CD4+ T-cell proliferation occurred in splenic CD4+ T cells isolated from mice given OVA alone or OVA plus nStx1 (Fig. 4A). These results further demonstrate that mStx1 and StxB1 are potent adjuvants for the induction of OVA-specific CD4+ T cells in vivo.
FIG. 4.
Analysis of OVA-specific CD4+ T-cell responses induced by coadministered Stx1 derivatives. OVA-specific CD4+ Th-cell proliferative responses (A) and Th1 and Th2 cytokine synthesis (B) by CD4+ T cells isolated from the spleens of mice subcutaneously immunized with OVA plus 10 μg of mStx1 (shaded bars), 50 ng of nStx1 (dotted bars), or 10 μg of StxB1 (black bars) or with OVA alone (white bars) were examined. Purified splenic CD4+ T cells were cocultured at a density of 2 × 106 cells/ml with 1 mg/ml of OVA and with T-cell-depleted, irradiated splenic feeder cells (4 × 106 cells/ml) in complete medium containing rIL-2 (10 U/ml) for 3 days for proliferation assays and 5 days for cytokine synthesis measurements. A control culture consisting of the splenic CD4+ T cells of naïve mice, feeder cells, and rIL-2 (10 U/ml) resulted in the incorporation of 230 ± 42 cpm of [3H]thymidine. Culture supernatants were harvested and then analyzed for the synthesis of secreted cytokines by the use of appropriate cytokine-specific ELISAs. The minimum detection levels for the individual cytokines detected were as follows: IFN-γ, 9.4 pg/ml; IL-4, 7.8 pg/ml; IL-5, 15.6 pg/ml; IL-6, 15.6 pg/ml; and IL-10, 15.6 pg/ml. The results are expressed as means of the stimulation indexes ± SEM or pg/ml ± SEM from a total of three experiments using five or six mice per group. *, P < 0.05 compared with mice immunized with OVA alone. The results for OVA-specific CD4+ T-cell proliferative responses (A) are expressed as E/C (experimental, stimulated value/control, nonstimulated value).
In a subsequent experiment, Th1 (IFN-γ) and Th2 (IL-4, IL-5, IL-6, and IL-10) cytokine production by antigen-specific CD4+ T cells was analyzed at the protein level (Fig. 4B). Increased levels of both Th1 and Th2 cytokines were noted in cultures containing splenic CD4+ T cells from mice subcutaneously immunized with OVA plus mStx1 or StxB1 (Fig. 4B), while nStx1 enhanced the production of only selected Th2 cytokines, including IL-5, IL-6, and IL-10, but not IL-4 and IFN-γ production (data not shown). Splenic CD4+ T cells from mice given OVA alone produced low levels of IFN-γ, IL-5, IL-6, and IL-10 but did not produce IL-4. Taken together, these results show that the subcutaneous administration of OVA plus StxB1 or mStx1 as an adjuvant induces antigen-specific Th1 (e.g., IFN-γ)- and Th2 (e.g., IL-4)-type cytokine responses, which in turn account for the generation of OVA-specific IgG2a and IgG1 Ab responses, respectively, in serum.
Induction of neutralizing antibody responses to tetanus toxin by subcutaneous immunization with the toxoid vaccine and Stx1 derivatives.
Since the subcutaneous administration of OVA plus mStx1 or StxB1 elicited Ag-specific IgG and IgM Ab responses, we next determined whether vaccine Ag-specific Abs supported by the Stx1 derivatives were protective. Initially, we determined whether the subcutaneous administration of tetanus toxoid (TT) with mStx1 or StxB1 could induce TT-specific Ab responses. Mice subcutaneously immunized with TT plus >10 μg of mStx1 or StxB1 showed significant TT-specific serum IgM, IgG, and IgA Ab responses. In contrast, low Ab responses were detected after immunization with TT alone (Fig. 5A). In the next experiment, we determined if these Abs were also protective. Mice given TT plus Stx1 derivatives or TT alone were challenged with a lethal dose (130 LD50s) of tetanus toxin and then monitored for paralysis and death. As expected, subcutaneous immunization with TT plus Stx1 derivatives provided complete protection. In contrast, TT alone provided no protection in mice against the paralysis and death that normally occur within 2 days of the administration of tetanus toxin (Fig. 5B). These findings indicate the effectiveness of TT-specific IgG Abs in serum induced by subcutaneously coadministered Stx1 derivatives.
FIG. 5.
Induction of TT-specific serum IgG, IgM, and IgA antibody responses by coadministered Stx1 derivatives (A) and protection against fatal challenge with tetanus toxin (B). Mice were subcutaneously immunized with TT plus mStx1, nStx1, or StxB1. Specifically, C57BL/6 mice were subcutaneously immunized with 100 μl of TT plus 1 μg of Stx1 mutant (E167R/R170L; mStx1) or 1 μg of StxB1 (hatched bars), 10 μg of Stx1 mutant or 10 μg of StxB1 (dotted bars), or 25 μg of Stx1 mutant or 25 μg of StxB1 (black bars) as an adjuvant or with TT alone (white bars) on days 0 and 14. Serum samples were collected on day 21 and examined for TT-specific Ab responses by ELISA. One week after the last immunization, mice were challenged on day 21 by the subcutaneous injection of 130 LD50s of tetanus toxin in 0.5 ml of PBS including 0.2% gelatin. *, P < 0.05 compared with mice immunized with OVA alone. The results are expressed as means ± SEM from a total of three separate experiments, each of which used five or six mice per group.
DISCUSSION
B7-1 and B7-2 have been shown to be essential costimulatory molecules for the initial activation of CD4+ T cells (21, 23, 24). With our experiments, we sought to determine the effect of Stx1 derivatives on the expression of such costimulatory molecules. Immature BMDCs and splenic DCs were used to help map the early events occurring after the administration of Stx1 derivatives and to determine the extent of the ability of those derivatives to initiate primary T-cell responses. Stx1 derivatives provided two different types of immunoregulation signals to DCs. First, StxB1 and mStx1 were shown in our in vitro and in vivo studies to enhance the activation of BMDCs and splenic DCs by augmenting MHC class II, CD80, CD86, and/or CD40 expression. Since previous research has already shown that the strong expression of MHC and costimulatory molecules on antigen-presenting cells is associated with a high level of T-cell activation (4, 6), StxB1 and mStx1, with their demonstrated ability to enhance the expression of MHC and/or costimulatory molecules, must lead to an enhanced CD4+ T-cell response. Second, certain Stx derivatives were shown to induce TNF-α, which has been shown to play a role in the immune regulation of B lymphocytes and the maturation of DCs (34, 49). Among the derivatives, StxB1 proved to be the most potent inducer of TNF-α. With the two distinct immunoenhancing signals noted above, nontoxic forms of Stx1 derivatives have been demonstrated by our study to be strong candidates as adjuvants to enhance antigen-specific T-cell and B-cell immune responses.
Our in vitro studies demonstrated that the maturation of in vitro BMDCs was enhanced by Stx1 derivatives, especially StxB1. Our results also showed that T-cell proliferation and cytokine production by Ag-specific CD4+ T cells were augmented by StxB1-treated BMDCs. To examine whether the series of immunoenhancing events triggered by Stx1 derivative-treated BMDCs in vitro also reflected the in vivo situation, we also performed a series of in vivo experiments. Our findings revealed that DC maturation occurred after the administration of the Stx1 derivatives. Furthermore, we demonstrated that a mutant form of Stx1 (E167Q/R170L; mStx1) and the B subunit of Stx1 (StxB1) show potential as novel adjuvants for the induction of antigen-specific systemic Th and B-cell immune responses. The subcutaneous coadministration of nontoxic StxB1 or mStx1 as an adjuvant with a protein Ag resulted in the induction of high IgG anti-OVA Ab responses in serum. When these two distinct forms of nontoxic Stx1 derivatives were subcutaneously coadministered, the derivatives elicited both CD4+ Th1- and Th2-type responses via the mixed production of Th1 (IFN-γ) and Th2 (IL-4, IL-5, IL-6, and IL-10) cytokines. The Stx1 derivative supported mixed (Th1 and Th2) cytokine synthesis, reflecting the generation of OVA-specific IgG1, followed by IgG2a, in the systemic compartment. Similarly, Stx1 derivative molecules were seen to support the generation of Ag-specific Th1- and Th2-type CD4+ T cells in vitro. In addition to Stx1 derivatives, pertussis toxin (37) and LT (47) have been shown to potentiate a similarly mixed Th1- and Th2-type response.
In a separate study, LT-treated BMDCs, like StxB1-treated cells, were shown to enhance CD80, CD86, MHC class II, and CD40 expression (unpublished data). It has been shown that the up-regulation of CD80, CD86, MHC class II, or TNF-α and/or IL-12 production most closely correlates with the adjuvant activity of toxin-based immunomodulatory molecules (8, 53). Although many details of the molecular mechanisms behind the enhancement of Th1- and Th2-type responses by Stx derivatives remain to be elucidated, the present study has demonstrated that the Stx derivatives (e.g., StxB1 and mStx1) can be grouped as Th1- and Th2-inducing adjuvants. For an investigation of the molecular mechanisms underlying the adjuvanticity of mStx1 and StxB1, one possible experiment would be to examine and compare the Th1- and Th2-type pathway induced by mStx1 and that promoted by StxB1. The induction of signaling molecules such as T-bet, GATA-3, c-Maf, and SLAT by mStx1 and StxB1 could be compared, since these molecules have been shown to be associated with Th1- or Th2-cell differentiation (19, 27, 46).
Our results revealed that 50 ng of nStx1 did not induce serum IgG Ab responses to a coadministered Ag, indicating that nStx1 does not possess adjuvant activity. This observation is consistent with a previous study that showed that nStx1 does not possess adjuvant activity when given orogastrically (43). In contrast, all mice given 10 μg of mStx1 or StxB1 as an adjuvant generated systemic antigen-specific IgG responses (Fig. 4). Since a lower concentration of nStx1 was used in the in vivo experiment, one can consider that the administration of a higher dose may lead to the induction of the antigen-specific immune responses seen with mStx1 and StxB1. To this end, we subcutaneously coadministered higher doses of nStx1 (e.g., 100 to 150 ng) to mice, and all of those mice died (data not shown). These findings further demonstrate that nontoxic forms such as mStx1 and StxB1 possess adjuvant activities when administered at high doses, while the adjuvanticity of the native form cannot be assessed at these high doses due to its toxicity. In addition, mStx1 and StxB1 do not have the damaging side effects of nStx1. Previous studies have reported that Stxs induce necrosis via their RNA N-glycosidase activity (39) but that, in contrast, the Stx1 mutant and StxB1 (2) are much less toxic or nontoxic in terms of their inhibitory effects on protein synthesis, their cytotoxicity, and their lethality to mice compared with native forms of Stx1 (2, 33). nStx1 may signal the induction of cell death instead of immune enhancement. In contrast, the nontoxic forms, mStx1 and StxB1, provide appropriate activation signals for the induction of CD4+ Th1- and Th2-type responses via the expression of CD80, CD86, and MHC class II on DCs, leading to the generation of IgG1 and IgG2a Ab responses to the coadministered Ag.
It is well established that CT is an effective adjuvant for the induction of antigen-specific mucosal IgA and systemic IgG and IgA Ab responses to coadministered protein Ags (9). CT preferentially induces Ag-specific Th2-type CD4+ T-cell responses via the high-level synthesis of IL-4 and IL-5 (28). However, the enterotoxin possesses ADP-ribosyltransferase activity, which causes severe diarrhea and is thus unsuitable for use in humans (41). Therefore, several studies have investigated the potential adjuvant effect of the B subunit as a nontoxic derivative of CT. Highly purified recombinant CT-B has been shown to be ineffective as an adjuvant compared with the holotoxin for the induction of Ag-specific IgA and IgG immune responses (25, 54). However, it should be noted that two recent studies provided contradictory results, showing that nasally coadministered recombinant CT-B provided mucosal adjuvant activity (13, 48). It is interesting that StxB1 possesses adjuvant activity. Since the membrane ligand molecules of StxB1 (e.g., globotriaosylceramid [Gb3] and globotetraosylceramide [Gb4]) are completely different from those of CT-B and LT-B (e.g., GM1), biological stimulation signals provided by StxB1 via Gb3 and Gb4 could be more effective than those transmitted by CT-B and GM1. Several cell surface receptors, including Ig, transferrin receptor, FcγR, and DEC-205, can mediate endocytosis and effective antigen presentation (16, 29, 35). Furthermore, glycosphingolipids, including GM1, have also been implicated as sites for the delivery of immunity-enhancing signals (40). Our present findings suggest that Gb3 may also mediate the effective endocytosis of and antigen presentation by DCs. Therefore, the A subunits of the toxins may not be necessary for immunoenhancing activity, unlike those of other known AB5 toxins such as CT and LT. In this study, StxB1 possessed a costimulatory molecule-enhancing activity, while CT-B fails to induce either CD80 or CD86 expression on B cells or macrophages (1, 53). To elucidate the relationship between the increased expression of costimulatory molecules and the binding of StxB1 to its receptor, Gb3, we examined whether the signals for the enhancement of costimulatory molecules by StxB1 can be blocked by treatment of the receptors for StxB1. After a treatment of Gb3, costimulatory molecule expression was blocked (unpublished data). This means that the signaling pathway via the receptor for StxB1 plays a role in the enhancement of costimulatory molecule expression. In addition, it should be noted that it is possible that the A subunit of Stx1 is more responsible for toxicity than for adjuvanticity.
The nStx1 treatment of BMDCs was associated with some increase in OVA-specific CD4+ T-cell proliferative as well as Th1/Th2 cytokine responses (Fig. 2). However, nStx1 did not induce any up-regulation of CD80, CD86, or MHC class II and did not enhance the secretion of TNF-α or IL-12 (Fig. 1 and Table 1). When the nStx1-treated BMDCs were treated with CD80- and/or CD86-blocking antibodies, the levels of CD4+ T-cell proliferation and Th1/Th2 cytokine secretion were not altered. However, when mStx1- or StxB1-treated BMDCs were similarly treated with blocking antibodies specific for CD80 and CD86, the antigen-specific CD4+ T-cell responses were inhibited (unpublished results). These findings suggest that the ability of nStx1 to stimulate the proliferation and cytokine secretion of T cells does not stem from the up-regulation of costimulatory molecules.
Another interesting biological characteristic of nStx1 is that its in vivo administration resulted in the down-regulation of all of the surface molecules associated with lymphocyte stimulation on splenic DCs (Table 2). To this end, nStx2 has been shown to reduce the number of splenic CD4+ and B220+ cells when it is administered to mice (44). Thus, nStx1 may exert at least two negative influences on lymphocytes, namely, it can down-regulate costimulatory molecule expression or even result in death. A separate study lent support to this view by showing that approximately 25% of BMDCs underwent apoptosis and necrosis after exposure to nStx1 in vitro, while no such cell death was seen after exposure to nontoxic Stx1 derivatives such as mStx1 and StxB1 (data not shown).
In summary, our findings have provided new evidence that nontoxic Stx1 derivatives (e.g., mStx1 and StxB1) can effectively induce costimulatory molecules (CD80 and CD86) and/or MHC class II on DCs. This study has further shown that nontoxic forms of Stx1 derivatives, including mStx1 and StxB1, possess adjuvant activity and can elicit Ag-specific CD4+ Th1- and Th2-type responses for the subsequent induction of antigen-specific IgG1 and IgG2a Ab responses following subcutaneous immunization with a protein Ag. The nontoxic Stx derivatives can be considered promising new candidates for effective and safe adjuvants.
Acknowledgments
We appreciate the constructive comments regarding this work by members of the Division of Immunology and Medical Zoology of Niigata University Graduate School of the Medical and Dental Sciences and the Department of Mucosal Immunology of the Research Institute for Microbial Diseases, Osaka University.
This study was supported by grants from the Ministry of Health, Labor and Welfare; the Ministry of Education, Science, Sports and Culture; and CREST, JST; and the Research Institute of Oral Sciences, Nihon University School of Dentistry at Matsuda, Japan. M.O. was supported by the Japan Human Health Science Foundation.
All experiments described herein were approved by the local authorities. All procedures were done in agreement with National Institutes of Health guidelines for the handling of laboratory animals.
Editor: J. T. Barbieri
REFERENCES
- 1.Agren, L. C., L. Ekman, B. Lowenadler, and N. Y. Lycke. 1997. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 158:3936-3946. [PubMed] [Google Scholar]
- 2.Austin, P. R., and C. J. Hovde. 1995. Purification of recombinant Shiga-like toxin type I B subunit. Protein Expr. Purif. 6:771-779. [DOI] [PubMed] [Google Scholar]
- 3.Birnabaum, S., and M. Pinto. 1976. Local and systemic opsonic adherent, hemagglutinating and rosette forming activity in mice induced by respiratory immunization with sheep red blood cells. Z. Immunitatsforsch. Exp. Klin. Immunol. 151:69-77. [PubMed] [Google Scholar]
- 4.Bluestone, J. A. 1995. New perspectives of CD28-B7-mediated T cell costimulation. Immunity 2:555-559. [DOI] [PubMed] [Google Scholar]
- 5.Byun, Y., M. Ohmura, K. Fujihashi, S. Yamamoto, J. McGhee, S. Udaka, H. Kiyono, Y. Takeda, T. Kohsaka, and Y. Yuki. 2001. Nasal immunization with E. coli verotoxin 1 (VT1)-B subunit and a nontoxic mutant of cholera toxin elicits serum neutralizing antibodies. Vaccine 19:2061-2070. [DOI] [PubMed] [Google Scholar]
- 6.Cella, M., F. Sallusto, and A. Lanzavecchia. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10-16. [DOI] [PubMed] [Google Scholar]
- 7.Clements, J. D., N. M. Hartzog, and F. L. Lyon. 1988. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6:269-277. [DOI] [PubMed] [Google Scholar]
- 8.Cong, Y., C. T. Weaver, and C. O. Elson. 1997. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J. Immunol. 159:5301-5308. [PubMed] [Google Scholar]
- 9.Elson, C. O., and W. Ealding. 1984. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J. Immunol. 132:2736-2741. [PubMed] [Google Scholar]
- 10.Endo, Y., K. Tsurigi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 11.Herzenberg, L. A., T. Tokuhisa, and D. R. Parks. 1982. Epitope-specific regulation. II. A bistable, Igh-restricted regulatory mechanism central to immunologic memory. J. Exp. Med. 155:1741-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaka, M., Y. Yasuda, S. Kozuka, T. Taniguchi, K. Matano, J. Maeyama, T. Komiya, K. Ohkuma, N. Goto, and K. Tochikubo. 1999. Induction of systemic and mucosal antibody responses in mice immunized intranasally with aluminium-non-adsorbed diphtheria toxoid together with recombinant cholera toxin B subunit as an adjuvant. Vaccine 18:743-751. [DOI] [PubMed] [Google Scholar]
- 14.Ito, H., T. Yutsudo, T. Hirayama, and Y. Takeda. 1988. Isolation and some properties of A and B subunits of Vero toxin 2 and in vitro formation of hybrid toxins between subunits of Vero toxin 1 and Vero toxin 2 from Escherichia coli O157:H7. Microb. Pathog. 5:189-195. [DOI] [PubMed] [Google Scholar]
- 15.Jackson, R. J., K. Fujihashi, J. Xu-Amano, H. Kiyono, C. O. Elson, and J. R. McGhee. 1993. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect. Immun. 61:4272-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, W., W. J. Swiggard, C. Heufler, M. Peng, A. Mirza, R. M. Steinman, and M. C. Nussenzweig. 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375:151-155. [DOI] [PubMed] [Google Scholar]
- 17.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 18.Katz, J. M., X. Lu, S. A. Young, and J. C. Galphin. 1997. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J. Infect. Dis. 175:352-363. [DOI] [PubMed] [Google Scholar]
- 19.Kuo, C. T., and J. M. Leiden. 1999. Transcriptional regulation of T lymphocyte development and function. Annu. Rev. Immunol. 17:149-187. [DOI] [PubMed] [Google Scholar]
- 20.Kweon, M. N., M. Yamamoto, F. Watanabe, S. Tamura, F. W. Van Ginkel, A. Miyauchi, H. Takagi, Y. Takeda, T. Hamabata, K. Fujihashi, J. R. McGhee, and H. Kiyono. 2002. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J. Infect. Dis. 186:1261-1269. [DOI] [PubMed] [Google Scholar]
- 21.Lanier, L. L., S. O'Fallon, C. Somoza, J. H. Phillips, P. S. Linsley, K. Okumura, D. Ito, and M. Azuma. 1995. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immunol. 154:97-105. [PubMed] [Google Scholar]
- 22.Larsson, R., D. Rocksen, B. Lilliehook, A. Jonsson, and A. Bucht. 2000. Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation. Infect. Immun. 68:6962-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenschow, D. J., S. C. Ho, H. Sattar, L. Rhee, G. Gray, N. Nabavi, K. C. Herold, and J. A. Bluestone. 1995. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181:1145-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 25.Lycke, N., T. Tsuji, and J. Holmgren. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277-2281. [DOI] [PubMed] [Google Scholar]
- 26.Macatonia, S. E., S. C. Knight, A. J. Edwards, S. Griffiths, and P. Fryer. 1987. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166:1654-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madrenas, J. 2003. A SLAT in the Th2 signalosome. Immunity 18:459-461. [DOI] [PubMed] [Google Scholar]
- 28.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 29.McCoy, K. L., M. Noone, J. K. Inman, and R. Stutzman. 1993. Exogenous antigens internalized through transferrin receptors activate CD4+ T cells. J. Immunol. 150:1691-1704. [PubMed] [Google Scholar]
- 30.Mrsny, R. J., A. L. Daugherty, C. M. Fryling, and D. J. FitzGerald. 1999. Mucosal administration of a chimera composed of Pseudomonas exotoxin and the gp120 V3 loop sequence of HIV-1 induces both salivary and serum antibody responses. Vaccine 17:1425-1433. [DOI] [PubMed] [Google Scholar]
- 31.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+,CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 32.Northrup, R. S., and A. S. Fauci. 1972. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J. Infect. Dis. 125:672-673. [DOI] [PubMed] [Google Scholar]
- 33.Ohmura, M., S. Yamasaki, H. Kurazono, K. Kashiwagi, K. Igarashi, and Y. Takeda. 1993. Characterization of non-toxic mutant toxins of Vero toxin 1 that were constructed by replacing amino acids in the A subunit. Microb. Pathog. 15:169-176. [DOI] [PubMed] [Google Scholar]
- 34.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fc gamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, M., A. Bacon, R. Rappuoli, M. Pizza, I. Cropley, G. Douce, G. Dougan, M. Marinaro, J. McGhee, and S. Chatfield. 1995. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect. Immun. 63:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan, M., L. McCarthy, R. Rappuoli, B. P. Mahon, and K. H. Mills. 1998. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int. Immunol. 10:651-662. [DOI] [PubMed] [Google Scholar]
- 38.Samuel, J. E., L. P. Perera, S. Ward, A. D. O'Brien, V. Ginsburg, and H. C. Krivan. 1990. Comparison of the glycolipid receptor specificities of Shiga-like toxin type II and Shiga-like toxin type II variants. Infect. Immun. 58:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena, S. K., A. D. O'Brien, and E. J. Ackerman. 1989. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28S RNA when microinjected into Xenopus oocytes. J. Biol. Chem. 264:596-601. [PubMed] [Google Scholar]
- 40.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 41.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockbine, N., L. Marques, J. Newland, H. Smith, R. Holmes, and A. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suckow, M. A., D. F. Keren, J. E. Brown, and G. T. Keusch. 1994. Stimulation of gastrointestinal antibody to Shiga toxin by orogastric immunization in mice. Immunol. Cell Biol. 72:69-74. [DOI] [PubMed] [Google Scholar]
- 44.Sugatani, J., T. Igarashi, M. Shimura, T. Yamanaka, T. Takeda, and M. Miwa. 2000. Disorders in the immune responses of T- and B-cells in mice administered intravenous verotoxin 2. Life Sci. 67:1059-1072. [DOI] [PubMed] [Google Scholar]
- 45.Swiggard, W., M. D. Noncas, M. D. Witmer-Pack, and R. M. Steinman. 1991. Enrichment of dendritic cells by plastic adherence and EA resetting, p. 3.7.1-3.7.11. In J. E. Coligan (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.
- 46.Szabo, S. J., S. T. Kim, G. L. Costa, X. Zhang, C. G. Fathman, and L. H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655-669. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi, I., M. Marinaro, H. Kiyono, R. J. Jackson, I. Nakagawa, K. Fujihashi, S. Hamada, J. D. Clements, K. L. Bost, and J. R. McGhee. 1996. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 173:627-635. [DOI] [PubMed] [Google Scholar]
- 48.Tochikubo, K., M. Isaka, Y. Yasuda, S. Kozuka, K. Matano, Y. Miura, and T. Taniguchi. 1998. Recombinant cholera toxin B subunit acts as an adjuvant for the mucosal and systemic responses of mice to mucosally co-administered bovine serum albumin. Vaccine 16:150-155. [DOI] [PubMed] [Google Scholar]
- 49.Trevejo, J. M., M. W. Marino, N. Philpott, R. Josien, E. C. Richards, K. B. Elkon, and E. Falck-Pedersen. 2001. TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. USA 98:12162-12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ultrich, J. T., J. L. Cantrell, G. L. Gustafson, J. A. Rudbach, and J. R. Hiernant. 1991. The adjuvant activity of monophosphoryl lipid A. CRC Press, Boca Raton, Fla.
- 51.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 52.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto, M., H. Kiyono, S. Yamamoto, E. Batanero, M. N. Kweon, S. Otake, M. Azuma, Y. Takeda, and J. R. McGhee. 1999. Direct effects on antigen-presenting cells and T lymphocytes explain the adjuvanticity of a nontoxic cholera toxin mutant. J. Immunol. 162:7015-7021. [PubMed] [Google Scholar]
- 54.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. Van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]